The use of mechanical circulatory support (MCS) in the pediatric population has evolved significantly in the past 20 years, but its management still poses several challenges. We aim to describe patient characteristics, outcomes, and morbidity associated with different modalities of MCS, in a tertiary center.
MethodsRetrospective analysis of data from all the children who underwent MCS between 2002 and 2018 at a pediatric cardiology unit.
ResultsBetween 2002 and 2018, 22 devices were implanted in 20 patients. Patients were divided into three groups: Group A (n=11) extracorporeal membrane oxygenator (ECMO); Group B (n=8) pulsatile paracorporeal ventricular assist device (VAD) and group C (n=3) paracorporeal continuous flow VAD.
The median age was similar in groups A and B (18 and 23 months, respectively), and higher in group C (13 years). ECMO patients were cannulated mainly as a bridge to recovery (post cardiotomy- 8) while group B and C patients were bridged to transplantation. The most frequent complications were bleeding (group A - 36%, group C - 66.6%) and thromboembolic events (group B - 50%, group C - 33.3%).
As for outcomes, in group A the majority of patients (54.5%) were weaned and 27.3% died. Half of group B and all of group C patients underwent transplantation.
ConclusionBleeding and thromboembolic events were the main complications observed. Group B showed the highest mortality, probably related to the low weight of the patients. Overall, outcomes and complications are related to the type of device and patient status and characteristics.
Nos últimos 20 anos ocorreu uma grande evolução no uso de dispositivos de assistência circulatória mecânica (ACM) na idade pediátrica embora persistam grandes desafios no seu manejo. O objetivo deste trabalho é descrever as características dos doentes, resultados e morbilidade associada a diferentes modalidades de ACM, num centro terciário.
MétodosAnálise retrospetiva de doentes que necessitaram de ACM entre 2002 e 2018 numa unidade de cardiologia pediátrica.
ResultadosVinte e dois dispositivos foram implantados em vinte doentes, entre 2002 e 2018. Grupo A (n=11) Extracorporeal Membrane Oxygenator (ECMO); Grupo B (n=8) Assistência ventricular paracorporal pulsátil (AVPP); Grupo C (n=3) Assistência ventricular paracorporal de fluxo contínuo (AVPFC).
A mediana de idades foi semelhante no grupo A e B (18 e 23 meses, respetivamente) e superior no grupo C (13 anos). A ACM foi maioritariamente usada como ponte para recuperação no grupo A (pós-cardiotomia -8) e ponte para transplante no grupo B e C. As complicações mais frequentes foram hemorragia (grupo A - 36%, grupo C - 66,6%) e eventos tromboembólicos (grupo B - 50%, grupo C - 33,3%).
No grupo A, a assistência foi removida após recuperação em 54,5% dos casos e 27,3% faleceram. Nos grupos B e C, metade e todos os doentes foram transplantados, respetivamente.
ConclusãoA hemorragia e os eventos tromboembólicos foram as principais complicações observadas. No grupo B verificou-se a taxa de mortalidade mais elevada, provavelmente relacionado com a reduzido peso dos doentes e tipo de ACM. Em suma, os resultados e as complicações dependem das características dos doentes e do tipo de assistência implantada.
There has been a remarkable evolution in pediatric mechanical circulatory support in recent decades. The first mechanical circulatory support used at a pediatric age was extracorporeal membrane oxygenator (ECMO), which is now a well established therapy for patients with severe refractory pulmonary or cardiac failure. According to the 2019 Exracorporeal Life Support Organization (ELSO) registry report, there were a total of 133 371 ECMO runs, with the majority being neonatal and pediatric.1 The majority of pediatric ECMO runs are cardiac, which reflects its increasing use after cardiac surgery.2
Before the availability of long-term ventricular assist devices (VAD) for pediatric patients, the only way to support end-stage heart failure (HF) pediatric patients waiting for transplant was ECMO. Although some patients were successfully bridged to transplant, overall survival was low, with less than half surviving to hospital discharge.3–5
The first durable pediatric device, the Berlin Heart EXCOR, was introduced in 1992. It is a paracorporeal pulsatile device with several chamber and cannula sizes available, enabling the support of a wide range of pediatric patients, including neonates and infants. Its use rapidly expanded, and the rate of complications and mortality improved progressively with experience, optimization of the circuit and patient care, and aggressive anticoagulation protocols.6 Compared to ECMO, it showed better results on bridging pediatric patients to transplant with survival rates of 75-86%.7,8 However, the number of children waiting for a heart transplant greatly surpasses the number of donors, resulting in long waiting times.9,10
Although pulsatile devices profoundly impacted pediatric HF management, the evolution in durable mechanical circulatory support has been towards intracorporeal continuous flow devices. These are currently the most used type of devices in the adult population and are used not only as a bridge to transplant but also as “destination therapy”. The encouraging results in adults prompted their introduction in the pediatric population, but these are still not available for smaller children, with their use limited to children with a body suface area >1.5 m2.11
Paracorporeal continuous flow devices, such as Rotaflow® (MAQUET Cardiovascular; Wayne NJ) and Centrimag® (Thoratec Corp; Pleasanton CA) are deployed when full cardiac support and ventricular decompression are needed. However, in an unstable patient needing mechanical circulatory support, ECMO is still preferred in many centers due to the familiarity with its use and the possibility of starting circulatory support promptly and without central cannulation.
Despite this, paracorporeal devices have several advantages such as not having an oxygenator, which increases the inflammatory response and consequently leads to a more difficult anticoagulation process,12 more efficient decompression of the ventricle, the possibility of using larger cannulas due to central cannulation and, finally, longer duration. It also allows for more time for decision-making in case of high uncertainty about prognosis, neurologic status, or cardiac recovery. Although it has been used mainly as a bridge to recovery, several centers use it as a bridge to a decision, to another device, or even to transplant,13–16 due to its safety profile and longer duration. This underlines the plasticity in the use of mechanical circulatory support (MCS) regarding the duration of its use. The first PediMACS report defined temporary and durable support according to device type (including the BerlinHeart EXCOR and the intracorporeal assist devices in the durable group, and the paracorporeal continuous flow devices such as Centrimag in the temporary group). This classification was later abandoned given the trend in the use of many devices defined as temporary for longer-term support strategies. It was replaced by categorization based on the design and flow characteristics of the devices.
Patients and methodsThis observational study presents a retrospective analysis of all patients who underwent mechanical circulatory support between 2002 and 2018 at a tertiary hospital's pediatric cardiology unit. Data on demographic characteristics, cardiac diagnosis, the aim of mechanical circulatory support, type of device used, patient's status before and after MCS (glomerular filtration rate, lactates, aspartate transaminase, and alanine aminotransferase levels before device implantation, and on day three and five after implantation), complications and outcomes were obtained from the patients’ medical records and underwent descriptive analysis. Categorical variables are presented using frequencies, and percentages and continuous variables are expressed as mean and medians.
ResultsA total of 22 devices were implanted in 20 patients between 2002 and 2018 (Table 1).
Demographic data and data during support period.
| Group A (ECMO) (n=11) | Group B (PPVAD) (n=8) | Group C (PCFVAD) (n=3) | |
|---|---|---|---|
| Age in years (mean/median) | 4.1/1.5 | 2.9/1.6 | 11.3/13 |
| Weight in kg (mean/median) | 17.8/10 | 10.9/10 | 41.4/47 |
| Female gender | 8 (72.7%) | 6 (75%) | 1 (33.3%) |
| Cardiac diagnosis | |||
| DCM | 3 | 8 | 3 |
| TOF | 3 | - | - |
| Interruption of aortic arch+VSD | 1 | - | - |
| VSD | 1 | - | - |
| PA with IVS, SP Fontan | 1 | - | - |
| DORV with MPGA | 1 | - | - |
| Single ventricle with MPGA+PS, SP Glenn | 1 | - | - |
| Comorbidities | |||
| Polymalformative syndrome | 1 | - | - |
| DiGeorge syndrome | 1 | - | - |
| Congenital AV block | 1 | 1 | - |
| Asthma | - | 1 | - |
| Epilepsy | - | 1 | - |
| Combined primary immunodeficiency disorder | - | - | 1 |
| Aim of MCS | |||
| Bridge to recovery | 9 (82%) | 0 | 0 |
| Bridge-to-bridge | 2 (18%) | 0 | 0 |
| Bridge to transplant | 0 | 8 (100%) | 3 (100%) |
| Time of cannulation | |||
| In theatre | 4 (36%) | - | - |
| Hours post-surgery (average) | 23 | - | - |
| Days after admission (average) | 9.6 | 26.3 | 22.7 |
| Average time of support (days) | 7.5 | 71.7 | 14.3 |
| Need for RRT | 4 (36%) | 2 (25%) | 0 (0%) |
| Extubation (%) | 0 (0%) | 5 (62,5%) | 2 (66.6%) |
| Time to ICU/Hospital discharge (days) | 19.2/39.2 | 90.4/99 | 38.5/73.5 |
| Complications (% of patients) | |||
| Bleeding | 36% | 25% | 66,6% |
| Infection | - | 50% | - |
| Stroke | 9% | 37.5% | 33.3% |
| Device related | 9% | 12.5% | 33.3% |
| Outcome | |||
| Wean | 6 (55%) | 0 (0%) | 0 (0%) |
| Transplant | 0 (0%) | 4 (50%) | 3 (100%) |
| Death | 3 (27%) | 4 (50%) | 0 (0%) |
| Bridge to bridge | 2 (18%) | 0 (0%) | 0 (0%) |
DCM: dilated cardiomyopathy; TOF: tetralogy of fallot; VSD: ventricular septal defect; PA: pulmonary atresia; IVC: intact ventricular septum; SP: status post; DORV: double outlet right ventricle; MPGA: malposition of the great arteries; PS: pulmonary stenosis; ICU: intensive care unit; RRT: fenal replacement therapy.
Eleven patients were on venous-arterial (VA) ECMO, three patients were on paracorporeal continuous flow VAD with a magnetically levitated pump system (Thoratec Centrimag - levitronix), and eight patients were on pulsatile paracorporeal VAD (Berlin Heart EXCOR and Thoratec PVAD). We divided the patients into three groups according to device type (group A - VA-ECMO; group B - pulsatile paracorporeal VAD, and group C - paracorporeal continuous flow VAD).
Two patients received more than one modality of mechanical circulatory support as they were bridged with VA-ECMO to another device. One was bridged to a pulsatile paracorporeal VAD and the other to a paracorporeal continuous flow VAD.
Group A (ECMO)Patient characteristicsThere were a total of 11 runs of VA-ECMO in eleven patients. The majority of patients were female (72.7%), with a median age of 18 months (age range four days to fourteen years) and a mean weight of 17.8 kg (median 10 kg). Three patients had comorbidities (Table 1) and the majority (72.7%) had congenital heart disease (diagnosis specified in Table 1).
Cannulation and period on supportThe indication for ECMO was failure to separate from bypass after cardiac surgery (four), refractory postoperative arrhythmia (three), and cardiogenic shock (four patients in total, two with dilated cardiomyopathy, one Fontan patient in the immediate postoperative period, and one Fontan patient with systolic dysfunction and heart failure). ECMO was started as a bridge to recovery in almost all patients, except for two who were bridged to another ventricular assist device (VAD).
The duration of ECMO support was 7.5 days on average (maximum time on ECMO was 21 days).
Four patients needed renal replacement therapy (RRT) (three peritoneal dialysis and one continuous venovenous hemofiltration). All except one required this therapy while on support. All patients remained intubated during support.
The mean glomerular filtration rate (GFR), lactate, aspartate transaminase (AST), and alanine aminotransferase (ALT) levels pre and post-device implantation are depicted in Table 2.
Mean values for glomerular filtration rate, lactates, aspartate transaminase and alanine aminotransferase pre- and post-extracorporeal membrane oxygenator implantation (Group A).
| Pre-implantation | Post-implantation (day 3) | Post-implantation (day 5) | |
|---|---|---|---|
| GFR (mL/min/1.73 m2) | 71.7 | 66.25 | 32.8 |
| Lactates (mg/dL) | 86.5 | 18.5 | 8.6 |
| AST (U/L) | 1376.6 | 2240.4 | 288.5 |
| ALT (U/L) | 374.1 | 833.8 | 177.0 |
GFR: glomerular filtration rate; AST: aspartate transaminase; ALT: alanine aminotransferase.
During the ECMO support, four (36.3%) of patients experienced no complications related to ECMO. Bleeding was the most frequent complication occurring in four (36.3%), with two requiring surgical revision. One of the patients developed intravascular disseminated coagulopathy, which was the cause of death. An ischemic cerebrovascular accident occurred in one patient (9%) and a circuit-related complication (thrombus on the cannula) also occurred in one (9%) patient.
Regarding the outcome, six (54.5%) were weaned off ECMO, two (18.2%) were bridged to another device, and three (27.3%) died. The cause of death was disseminated intravascular coagulopathy with uncontrollable hemorrhage, an ischemic stroke with hemorrhagic transformation, and one patient died after severe unrecoverable dysfunction after tetralogy of fallot repair.
Group B (PPVAD)Patient's characteristicsA total of eight patients aged between one month and twelve years (median of 23 months) received a pulsatile paracorporeal ventricular assist device. The majority of patients (75%) were female. All patients had DCM, with six (75%) of them in PediMACS 2 and two (25%) in PediMACS 1. A total of three patients presented comorbidities (see Table 1).
Cannulation and period on supportThe device was used as a bridge to transplant in all patients, with one patient bridged to the device with VA-ECMO. The average time on support was 71.7 days (7 to 125 days).
Five (62%) patients were extubated, on average 4.6 days after device implantation, and three (37.5%) were not extubated while on support due to multiple complications. Two patients needed RRT (peritoneal dialysis) before VAD implantation, but RRT was not needed after implantation in any patient.
The mean glomerular filtration rate (GFR), lactate, aspartate transaminase (AST), and alanine aminotransferase (ALT) levels pre- and post-device implantation are depicted in Table 3.
Mean values for glomerular filtration rate, lactates, aspartate transaminase and alanine aminotransferase pre- and post-paracorporeal pulsatile ventricular assist device implantation (Group B).
| Pre-implantation | Post-implantation (day 3) | Post-implantation (day 5) | |
|---|---|---|---|
| GFR (mL/min/1.73 m2) | 101.5 | 96.1 | 121.0 |
| Lactates (mg/dL) | 56.8 | 13.0 | 9.8 |
| AST (U/L) | 381.7 | 164.6 | 64.5 |
| ALT (U/L) | 330.5 | 120.7 | 75.6 |
GFR: glomerular filtration rate; AST: aspartate transaminase; ALT: alanine aminotransferase; ECMO: extracorporeal membrane oxygenator.
The most frequent complications were thromboembolic events and infection, both occurring in four (50%) patients. Of the patients experiencing thrombo-embolic events, three (75%) had a stroke, and one (25%) had a massive pulmonary embolism.
Bleeding requiring surgical revision occurred in two patients (25%) and one (12.5%) experienced a circuit thrombus needing replacement. Overall, the majority of patients (75%) experienced two or more device-related adverse events.
Regarding outcomes, half (four) of the patients underwent transplantation, and the other half died during support. The cause of death was sepsis in one patient and thromboembolic events in two (massive pulmonary embolism and stroke). The other patient died of sepsis after severe Steven-Johnson syndrome secondary to carbamazepine, a death not directly related to the device.
Group C (PCFVAD)Patient characteristicsA total of three patients received a Thoratec Centrimag-levitronix.
The median age on cannulation was 13 years and two patients (66.6%) were male. All patients had a DCM diagnosis, and one patient had comorbidities (Table 1).
Cannulation and period on supportAll patients received the device as a bridge to transplant, and one of the patients was bridged to the device with VA-ECMO.
All patients were in PediMACS 1 at the time of cannulation and the average duration of support was 14.3 days.
None of the patients needed RRT, and two patients were extubated (on the first and second-day post-implantation, respectively) while on support.
The mean GFR, lactate, aspartate transaminase (AST), and alanine aminotransferase (ALT) levels pre- and post-device implantation are illustrated in Table 4.
Mean values for glomerular filtration rate, lactates, aspartate transaminase and alanine aminotransferase pre- and post-paracorporeal continuous-flow ventricular assist device implantation (Group C).
| Pre-implantation | Post-implantation (day 3) | Post-implantation (day 5) | |
|---|---|---|---|
| GFR (mL/min/1.73 m2) | 120.6 | 143.6 | 142.3 |
| Lactates (mg/dL) | 13.3 | 8.0 | 9.7 |
| AST (U/L) | 321.6 | 212.3 | 71.7 |
| ALT (U/L) | 335.0 | 71.3 | 177.0 |
GFR: glomerular filtration rate; AST: aspartate transaminase; ALT: alanine aminotransferase.
Bleeding requiring surgical revision was the most frequent, occurring in two (66.6%) of the patients. A third (one) experienced infection and an ischemic cerebrovascular accident also occurred in one patient. All the complications occurred in two patients, and one patient had no complications during support. The patient who suffered a cerebrovascular accident was promptly managed with thrombus aspiration (interventional neuroradiology) and recovered fully with no sequelae.
In this group all patients were successfully bridged to transplant.
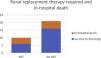
End-organ perfusion markers and need for RRTRegarding end-organ perfusion markers before and after implantation of devices (day three and day five), group A (ECMO) registered the higher values for pre-device AST, ALT, and lactates when compared to the other groups, with also a lower GFR which continued to decline until day five post-implantation. For both AST and ALT, the figures peaked on day three post-ECMO and decreased sharply on day five post-implantation. This pattern was not seen in the other two groups. There was a significant decrease in AST, ALT, and lactates from preimplantation to day five after implantation for groups B and C. Additionally, for both groups, GFR was higher compared with group A previous to device implantation and increased thereafter (Figure 1).
Comparison of mean values between ECMO, PPVAD and PCVAD for GFR, lactates, ALT and AST.
Table A Mean glomerular filtration rate in ml/min/1.73 m2 before and after implantation of devices; Table B Mean lactate in mg/dL before and after implantation of devices; Table C Mean alanine aminotransferase in U/L before and after implantation of devices.
Table D Mean aspartate transaminase in U/L before and after implantation of devices.
GFR: glomerular filtration rate; AST: aspartate transaminase; ALT: alanine aminotransferase.
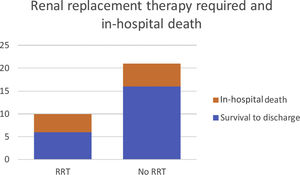
For patients who needed RRT, there was a higher proportion of non-survival when compared to the patients who did not need any RRT (Figure 2).
DiscussionThe type of MCS used at a pediatric age varies according to the patient's characteristics and the indication for cannulation. The use of ECMO is well established in patients requiring acute circulatory support in diverse scenarios. Almost all patients were put on ECMO as a bridge to recovery in our sample, most of them after cardiac surgery. Consequently, this group's low mean age reflects the early age at which congenital heart disease patients need corrective/palliative surgery. The end-organ perfusion state also reflects the setting in which these patients are cannulated to ECMO. All the parameters of end-organ perfusion measured in this study were worse in the ECMO group, highlighting the severity and acuteness of disease at the time of cannulation. A significant proportion of these patients had also previously undergone cardiopulmonary bypass, which in itself contributes to the derangement in these parameters. The strategy of using ECMO as a bridge to another device was used in two patients. The bridge-to-bridge strategy is sometimes used in acute cardiovascular collapse when immediate intervention and stabilization are needed. It enables an improvement in end-organ perfusion before implantation of a more durable device and gives the team time to make a decision when there is uncertainty regarding prognosis or candidacy for heart transplantation. In these cases, using VA-ECMO or a paracorporeal continuous flow device can be offered as a bridge to decision.8,16,17 Although the use of multiple MCS as a bridge to transplant is frequent, morbidity and survival until and post-transplant when compared to a single MCS strategy, are still not clear. De Rita et al.18 compared patients bridged to transplant with single or multiple devices and found that survival to recovery/transplantation and survival to discharge did not differ significantly between the two groups. These findings are in line with other reports, which also show no increase in mortality.7,19,20 However, another extensive and more recent study by Dipchand et al.21 shows that patients who had durable VAD as single support had lower mortality (12.9%) compared with patients who needed multiple MCS (27%). More studies are required to assess the real impact on mortality and morbidity for the use of multiple devices in the pediatric age group.
Regarding complications associated with the different types of devices, we report a favorable profile in the ECMO group with only one patient suffering a stroke and 36% experiencing major bleeding. The latter figure is in line with the 2017 ELSO registry report.1
In group B, we report a slightly higher percentage of adverse events such as stroke and infection and higher mortality than the 2019 PediMACS report.22 This can be explained firstly by our small sample, long study period, during which an improvement in the management of the devices took place, and finally, the small patient size of our sample (median weight was 10 kg), which is a known risk factor for higher mortality.23
In group C, the complication profile was also favorable, as only one patient had a stroke from which they recovered completely. Most importantly, all patients were successfully bridged to transplant.
There has been an increasing usage of paracorporeal continuous flow devices in the pediatric population. The appeal lies in the versatility and advantages over other short-term devices such as ECMO. The benefits are the absence of an oxygenator membrane that facilitates anticoagulation, a more effective decompression of the ventricles, the possibility of supporting both ventricles when used in a BiVAD strategy, and its low neurologic event rate. In the adult population, they have been widely used in acutely ill patients with cardiorespiratory failure (including pre- and postcardiotomy cardiogenic shock and post-transplant graft rejection) and as a bridge to recovery, decision, or durable VAD (paracorporeal pulsatile ventricular assist device or intracorporeal continuous flow device), with a low complication rate.24,25
While several studies report its successful use in the adult population, there is a lack of data concerning outcomes and complication rates in the pediatric population. The limited data available suggest positive outcomes and a low rate of complications.15,16,26 A large study involving this type of device in children, assessed its usefulness as a bridge to recovery, to a durable device, and to transplantation and found a positive outcome in 71% of the patients (17% transplanted, 30% recovered and 22% transitioned to durable VAD) and a duration of support as long as 227 days. Regarding neurologic events, there was a slightly lower rate when compared with the Berlin EXCOR cohort, but conclusions cannot be readily drawn due to the heterogeneity of the population in this study.27
The present study's limitations are its retrospective nature, heterogeneity, and the small size of the sample.
The outcomes and rate of complications are related to the type of device implanted and the underlying diagnosis, weight, and end-organ perfusion state of the patient at the time of implantation. Although there have been no significant changes in how and when we use pulsatile long-term VADs and VA-ECMO in recent years, there seems to be a more widespread use of paracorporeal continuous flow devices as they are versatile and can be used not only as a short-term but also as a medium to long-term option. Their adverse event profile, especially neurologic events, outcomes and survival require further assessment in more extensive studies.
To the best of our knowledge, this is the first report on MCS in children in a Portuguese sample. Reports such as ours are paramount as information on mechanical circulatory support in the pediatric age still lags behind that of adults. As stated above, some particular aspects require future research, including the use of ECMO as a bridge-to-bridge strategy and the use of short-term VADs as a medium to long-term option.
Conflicts of interestThe authors have no conflicts of interest to declare.
None.