Hypertrophic cardiomyopathy (HCM) is one of the most common inherited cardiac diseases, defined as a left ventricular wall thickness of ≥15 mm, in the absence of other causes of abnormal ventricular loading. A major hallmark of this disease is the presence of left ventricular outflow tract obstruction, which develops in up to three quarters of patients, referred to as obstructive hypertrophic cardiomyopathy. Current treatment is offered to symptomatic patients, based on the presence of documented left ventricular obstruction, aimed at reducing symptoms and disease progression. This is achieved through pharmacological empirical therapy, surgery, alcohol ablation and/or pacing. Mavacamten is a first-in-class allosteric inhibitor of cardiac myosin that promises to provide clinicians with targeted therapy for these patients. The aim of this review is to provide a general overview of the modern approach to the diagnosis and management of HCM, as well as to integrate all the current knowledge on mavacamten, in anticipation of a future change in the treatment algorithm of patients with HCM.
A miocardiopatia hipertrófica (MCH) é uma das doenças cardíacas hereditárias mais frequentes, sendo definida por um espessamento da parede ventricular esquerda ≥15 mm, na ausência de outras causas de sobrecarga ventricular. Um dos principais problemas associados a esta doença é a obstrução do trato de saída do ventrículo esquerdo, presente em até ¾ dos doentes, designando-se nesta circunstância por miocardiopatia hipertrófica obstrutiva. Atualmente, os principais objetivos da terapêutica destes doentes são a redução dos sintomas e a redução da progressão da doença. Estes objetivos são atingidos através de terapia farmacológica empírica, intervenção cirúrgica, ablação septal alcoólica e/ou pacing. O Mavacamten é o primeiro inibidor alostérico da miosina cardíaca, que vem permitir uma terapia mais dirigida para estes doentes. O objetivo desta revisão é fornecer uma visão geral da atual abordagem diagnóstica e de tratamento da MCH, bem como integrar todo o conhecimento atual sobre o Mavacamten, antecipando uma futura mudança no algoritmo de tratamento de doentes com MCH.
Hypertrophic cardiomyopathy (HCM) is a genetic cardiovascular disease with structural and functional abnormalities of the ventricular myocardium, defined by the presence of left ventricular hypertrophy (LVH) in the absence of other abnormal loading conditions that can lead to an increase in LV wall thickness (such as hypertension and valvular disease).1–3 It is a clinically significant disease with prevalence classically estimated at 1 in 500 (0.2%) in adults, making this disease one of the most common inherited cardiac diseases.1,2,4–6
The diagnosis of HCM in index patients requires a LV wall thickness of ≥15 mm in at least one myocardial segment, observed by any imaging technique.1,2 A major feature of this disease is the development of left ventricular outflow tract obstruction (LVOTO), defined as Doppler LV outflow tract gradient ≥30 mmHg, resulting in the disease known as hypertrophic obstructive cardiomyopathy (HOCM).1–3,7–11 Ever since the first descriptions, it has been evident that HOCM has a significant impact on quality of life and mortality, including an association with an increased risk of sudden cardiac death (SCD).1,2,12–15
The management of patients with HCM is aimed at reducing symptoms, complications, and disease progression, as well as improving overall functional capacity and prevention of SCD. This is achieved via pharmacological therapy (beta blockers (BBs), calcium antagonists and disopyramide), septal reduction therapies (SRT), and pacing. These therapies offer a generalist approach but fail to provide patients with a direct targeted therapy.1,2
Mavacamten is a novel selective allosteric inhibitor of cardiac myosin ATPase that seems to be promising in improving exercise capacity, LVOTO, New York Heart Association (NYHA) functional class, and health status in patients with HOCM, as demonstrated in the recently published EXPLORER-HCM trial.16,17 It is, therefore, pertinent to review current management strategies for HOCM patients, and the potential advantage of mavacamten therapy in the future.
Current diagnosis and management strategies are largely based on 2014 European Society of Cardiology (ESC) and 2020 American Heart Association/American College of Cardiology (AHA/ACC) guidelines.1,2 Data about mavacamten are based on early and more recently published studies with the MYK-461 molecule, which aimed to understand its biomechanism of action, as well as the safety and efficacy in HCM patients. When further information was needed regarding nomenclature, epidemiology, and genetics, the most recent published articles were considered. These articles were searched for in the PubMed web database, using the following search terms: “MYK-461”, “hypertrophic cardiomyopathy”, and “left ventricular outflow obstruction”.
Pathophysiology & etiologyTo understand the mechanism of action of mavacamten, as well as its role among other available therapies, it is important to analyze the underlying pathophysiological mechanism that leads to symptomatic HCM and LVOTO.
Cardiac muscle sarcomeres are composed of two different types of myofilaments – thick filaments composed primarily of the myosin protein (which includes two α- or β-myosin heavy chain units), and thin filaments composed primarily of the actin protein. Additionally, molecules such as tropomyosin, troponin, myosin ATPase and myosin-binding protein C play an important role in regulating contraction.
Sarcomere contraction occurs through the so-called cross-bridge cycle, in which the thick myosin filaments attach sequentially to the thin actin filaments, contract to move the thin actin filaments over them, and finally detach to restart the cycle and interact with a new actin molecule further down the line. It is the hydrolysis of ATP that allows the relaxed myosin filaments to attach to a new actin filament, thus promoting sarcomere contraction, while sequential relaxation can only occur when a new ATP molecule binds to the attached myosin molecule.
In most cases, HCM is a consequence of autosomal dominant (AD) sarcomere protein gene mutations, with major dominant mutations having been found in 11 different genes – the most common in the beta-myosin heavy chain (MYH7) and myosin-binding protein C (MYBPC3). These mutations lead to enhanced activity of cardiac myosin ATPase, resulting in an increase in sarcomere tension as more molecules are trapped in a contracting state, which in turn culminates in cardiomyocyte hyperdynamic contraction and impaired relaxation. Because cardiac fibroblasts respond to these excessive signals from the cardiomyocytes, increased production of extracellular matrix proteins is observed, further contributing to the fibrosis, hypertrophy and myofilament disarray observed in HCM. Studies on MYH7 and MYBPC3 carriers have shown that this cardiomyocyte hyperdynamic contraction state precedes LVH.1,2,18–23
In turn, HCM is associated with mechanical valvular abnormalities as well as abnormal coronary microcirculation. LVOTO can be due to myocardial hypertrophy, systolic anterior motion (SAM) of the mitral valve leaflets toward the interventricular septum, papillary muscle abnormalities, or other mitral valve abnormalities. HOCM patients can present with chest pain, dyspnea, syncope, and palpitations, explained by these different abnormalities, although several other mechanisms have also been described.1,2,24–27
Current management strategiesDiagnosisMany patients with HCM present with minor symptoms, if any, particularly in the absence of LVOTO. In the absence of clinical suspicion, patients are identified through the presence of a heart murmur during a routine examination, abnormal 12-lead electrocardiography (ECG) or echocardiogram performed for other reasons, or during familial screening.1,2
The basis of HCM diagnosis is the detection of a maximum ventricular wall thickness of ≥15 mm by any imaging technique – the most common being two dimensional (2D) transthoracic echocardiography (TTE), with cardiovascular magnetic resonance (CMR) as a viable alternative. Additionally, ventricular wall thickness of 13-14 mm can also be considered diagnostic in the presence of strong positive family history or a positive genetic test. The most common hypertrophied segments involve the interventricular septum, however ventricular wall thickness can be present in any other location.28 As such, a complete measurement of maximum diastolic ventricular wall thickness, utilizing a 2D TTE of all LV segments, is recommended.1,2
For the evaluation of LVOTO, both 2D and Doppler TTE at rest are recommended, standing and during Valsalva maneuvers, due to its dynamic nature. Classically, an LVOT gradient of ≥30 mmHg is considered the diagnostic threshold of LVOTO – however, a gradient threshold of ≥50 mmHg seems to be more reliable in identifying hemodynamically significant LVOTO. Furthermore, in symptomatic patients with a maximum provoked peak LVOT gradient of <50 mmHg, an exercise stress echocardiography is recommended to potentially identify exercise-induced LVOTO as well as mitral regurgitation.1,2
Therapeutic optionsThere is currently no definitive treatment for HOCM. As such, treatment options are targeted at reducing symptoms, improving functional capacity, and diminishing disease progression. This is achieved with drugs, SRT, and cardiac pacing. These treatment options are offered to patients with maximum provoked peak LVOT gradient of ≥50 mmHg, although ESC guidelines state that in selected patients, with gradients of ≥30 to <50 mmHg and no other explanation for their symptoms, these options may also be considered. Due to the dynamic nature of LVOTO and the absence of evidence regarding pharmacological therapies capable of reducing mortality, the success of therapy should be evaluated based on symptomatic relief and not on LVOT gradient.1,2
Non-vasodilating BBs are considered the first-line therapy. In the presence of intolerance or contraindications to BBs, non-dihydropyridine calcium channel blockers (NDH-CCB), such as verapamil and diltiazem, can be considered viable alternatives, with the caveat that their vasodilating properties can be a limitation to their use. Additionally, patients who are unresponsive to either BBs or NDH-CCB can benefit from combined therapy with disopyramide, a class IA antiarrhythmic drug. However, it is important to note that disopyramide can enhance atrioventricular node conduction, leading to rapid conduction in the onset of atrial fibrillation (AF), and thus should be avoided as monotherapy in these patients.29 Finally, low-dose diuretics can be a valid strategy to alleviate dyspnea or other congestive symptoms, taking caution to avoid hypovolemia.1,2
In patients with an LVOT gradient ≥50 mmHg, associated with an NYHA class ≥III or recurrent symptoms despite optimized medical therapy, SRT should be considered. Ventricular septal myectomy, sometimes associated with mitral valve surgery, provides a significant reduction in LVOT gradients and mitral regurgitation, resulting in diminished symptoms and improved functional capacity, with a low mortality rate and rare surgical complications. SAA achieves similar outcomes and procedural mortality rates. While septal myectomy can be preferred when additional mitral valve interventions are to be performed, SAA can be the preferred option in patients at a higher surgical risk.1,2,30–35
Future strategy: MavacamtenAs discussed previously, it is the cardiomyocyte hyperdynamic contraction and impaired relaxation state that ultimately lead to myocardial fibrosis, hypertrophy, and the myofilament disarray characteristic of HCM.18–23 It was in the pursuit of a molecule capable of inhibiting sarcomere power, and thus acting at the disease's pathophysiological core, that mavacamten (MYK-461) was designed. As a selective allosteric inhibitor of cardiac myosin ATPase, it works reducing the sarcomere hypercontractility observed in HCM.
In early 2016, the in vitro study by Green et al. revealed the dose-dependent ability of mavacamten to reduce myosin ATPase activity, based on murine myofibril (α-myosin) and bovine myofibril (β-myosin) models, with a reduction of ∼90% at maximum doses.36 Further characterization described its effect in diminishing the rate of phosphate release in the cross-bridge cycle, thus limiting the number of strongly bound myosin molecules. As such, mavacamten was able to reduce the percentage of bound actin-myosin cross-bridges, which are over-increased up to 80% in HCM patients as compared to 50% in normal individuals. Mavacamten reduced maximal muscle tension in cardiac muscle fibers from adult rat models, in a dose-dependent fashion. Following these results, in vivo tests were conducted on young mice, demonstrating the ability of mavacamten to reduce cardiac contractility without impairing skeletal muscle function. Furthermore, it was shown to reduce the development of LVH, myocardial disarray and fibrosis, and suppress hypertrophic and profibrotic gene programs in HCM mice models.
In late 2016, a new in vivo study by Stern et al. set out to assess mavacamten's ability to reduce LVOT pressure gradients in a HCM feline model.17 In this study, the felines were initially assessed through echocardiography for the presence of LVOTO and SAM, which were later provoked using adrenergic agonists after being anesthetized. This study showed mavacamten's ability at reducing LVOT pressure gradients and eliminating SAM in HCM animal models, while under adrenergic stimulation, thus demonstrating its potential role in the specific treatment of HOCM.
Further studies aimed to better understand the biochemical mechanism of action have been conducted. In late 2017, Kawas et al. demonstrated mavacamten's ability to act on multiple different steps along the cross-bridge cycle.37 Besides decreasing the rate of phosphate release from the myosin molecules, it also reduced the number of myosin heads interacting with the actin thin filaments and lowered the rate of myosin-binding to actin during the ADP-bound stage. Later, in 2018, Rohde et al. showed its capacity to stabilize an autoinhibited state of two-headed cardiac myosin, further exploring its mechanism of action.38,39 Lastly, in 2020 Sparrow et al. demonstrated that mavacamten was also able to act on reducing myocardial hypercontractility based on thin filament mutations, working on their altered sensitivity and dysregulation of Ca2+ flux.40
Phase 2 trialsAfter exclusion of possible major drug-drug interactions and a low clearance rate checked, long half-life time and high oral bioavailability, mavacamten was tested for the first time in a phase 2 trial – PIONEER-HCM (NCT02842242).41,42 This was an open-label, nonrandomized clinical trial with the aim to demonstrate and further characterize the effect of mavacamten on LVOT gradient. It was conducted via two sequential cohorts, composed of a 12-week treatment phase, followed by a 4-week washout phase. 21 symptomatic (NYHA II-III) patients with HOCM were separated into two cohort groups, where group A (n=11) received isolated oral doses of 10-20 mg/d of mavacamten, while group B (n=10) received 2-5 mg/d of Mavacamten. While participants in cohort A discontinued their HOCM background treatment (β-blockers, NDH-CCB and disopyramide) 14 days before initiating Mavacamten treatment, participants in cohort B maintained their HOCM treatment of β-blockers at their usual dosage. The primary endpoint was the change in post-exercise LVOT gradient at 12 weeks. Peak oxygen consumption (pVO2), resting LVOT gradient, left ventricle ejection fraction (LVEF) and dyspnea were registered as secondary endpoints.
The results were very promising with an average reduction in post-exercise LVOT gradient of ∼90 mmHg (mean change, 89.5 mmHg [95% confidence interval (CI), 138.3 to 40.7 mmHg]; p=0.008) in cohort A, and ∼25 mmHg (mean change, 25.0 mmHg [95% CI, 47.1 to 3.0 mmHg]; p=0.020) in cohort B. Additionally, a substantial improvement in exercise capacity (observed via a mean increase in pVO2 of 3.5 mL/kg/min in cohort A, and 1.7 mL/kg/min in cohort B), NYHA class and dyspnea scores was observed. Despite the open-label and small sample size limitations, it was concluded that mavacamten had the capacity to lower LVOTO, as well as to improve exercise capacity and diminish symptoms in HOCM patients. Mavacamten was shown to be generally well tolerated, presenting with mild (80%) and moderate (19%) adverse effects. The most common adverse effects were a reduction in LVEF and AF (one participant in cohort A discontinued the study after developing persistent AF). Another important study limitation was the exclusion of patients with NYHA class IV or under disopyramide therapy.
Following this success, an open-label extension study is currently being conducted – PIONEER-OLE (NCT03496168) to further characterize the safety and potential long-term adverse effects of mavacamten.
A second phase 2 trial took place, this time to investigate the safety and potential adverse effects of mavacamten on patients suffering from nonobstructive HCM (nHCM) – MAVERICK-HCM (NCT03442764).43 This was a double-blind, placebo-controlled study in patients suffering from symptomatic nHCM, as determined by a NYHA class of II-III. Selected patients had LVEF of ≥55% as well as NT-proBNP value of ≥300 pg/ml. The 59 participants were randomized (1:1:1) into two groups that received an adjusted dose to target mavacamten plasma levels of 200 ng/ml (n=19) or 500 ng/ml (n=21), and a third group that was treated with placebo (n=19). This was conducted over the course of 16 weeks, followed by a washout period of 8 weeks. Although the primary objective of this study was to determine the tolerability of mavacamten, several other exploratory assessments took place, including cardiac serum biomarkers, echocardiographic parameters, and a composite functional endpoint that combined NYHA class improvement with pVO2 increases. This last parameter will be a primary endpoint for the phase 3 EXPLORER-HCM trial.
Once again, a phase 2 trial with mavacamten proved to be a success, as the drug was well tolerated, with adverse effects being reported as mild (76%) or moderate (21%). The most common serious adverse effect was AF, occurring in both mavacamten (n=2; 5.1%) and placebo (n=2; 10.5%) groups. Additionally, five (12.5%) patients treated with mavacamten experienced a reversible, dose-dependent decrease in LVEF to ≤45%, leading to treatment discontinuation. Furthermore, the exploratory analysis of serum biomarkers also presented interesting results, as groups treated with mavacamten saw significant decreases in NT-proBNP (change from baseline: -435 pg/ml [mean decrease: 53%]) and high-sensitivity cardiac troponin I (hs-cTnI) (change from baseline: -0.008 ng/ml [mean decrease: 34%]) as compared to placebo (change from baseline: -6 pg/ml [mean decrease: 1%] and change from baseline: 0.001 ng/ml [mean increase: 4%], respectively). Both changes were observed at week 16, returning to baseline at week 24 after discontinuation of mavacamten therapy. As such, MAVERICK-HCM became the first study to effectively demonstrate a dose-dependent reduction in NT-proBNP by medical therapy in nHCM patients. Both these biomarkers are strongly associated with myocardial injury and have been used as prognosis tools in heart failure (HF) and HCM, with NT-proBNP associated with higher mortality and hs-cTnI with myocardial fibrosis, suggesting a potential added benefit of mavacamten therapy in these patients.44–46
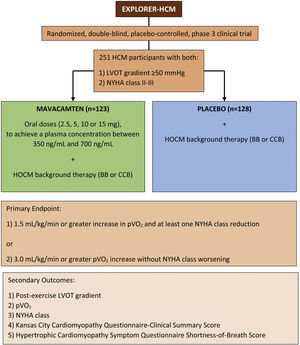
Phase 3 trialsRecently, the results of the mavacamten phase 3 trial EXPLORER-HCM (NCT03470545) have been published.16 This was a randomized, double-blind, placebo-controlled trial, which aimed to evaluate the efficacy and safety of mavacamten in the treatment of symptomatic HOCM patients (Figure 1). Two hundred fifty-one patients with a LVOT gradient of ≥50 mmHg and a NYHA class of II-III were enrolled into the study and randomized (1:1) into a mavacamten treatment group (n=123) and a placebo treatment group (n=128) for 30 weeks. Mavacamten was administered orally with doses of 2.5, 5, 10 or 15 mg, to achieve a plasma concentration between 350 ng/mL and 700 ng/mL. Patients were allowed to maintain their background HOCM therapy apart from disopyramide for safety reasons, resulting in 231 (92%) patients on BBs or NDH-CCB therapy. The study's primary endpoint was a composite clinical assessment: 1) a 1.5 mL/kg/min or greater increase in pVO2 and at least one NYHA class reduction; or 2) a 3.0 mL/kg/min or greater pVO2 increase without NYHA class worsening, both recorded at week 30 as compared to the baseline. Multiple secondary endpoints were also assessed at week 30: post-exercise LVOT gradient, pVO2, NYHA class improvement, Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score (KCCQ-CSS), and Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath subscore (HCMSQ-SoB). KCCQ is a well-validated tool that measures the quality of life impact of cardiovascular diseases and their treatments, while HCMSQ is a novel tool that evaluates the main symptoms of HCM.47,48 Additional pre-specified exploratory endpoints were assessed, including a complete response (defined as LVOT gradient <30 mmHg and NYHA class I), as well as serum concentrations of NT-proBNP and hs-cTnI.
EXPLORER-HCM produced very promising results with 37% (n=45) of mavacamten patients versus 17% (n=22) of those on placebo meeting the primary endpoint (difference +19.4% [95% CI, 8.7 to 30.1]; p=0.0005). Mavacamten patients outperformed on all the secondary endpoints, showing greater reductions in post-exercise LVOT gradient (-36 mmHg [95% CI, -43.2 to -28.1]; p<0.0001), greater increase in pVO2 (+1.4 mL/kg/min [0.6 to 2.1]; p=0.0006), and improved symptom scores (KCCQ-CSS +9.1 [5.5 to 12.7]; HCMSQ-SoB -1.8 [-2.4 to -1.2]; p<0.0001). Furthermore, a complete response was achieved in 27% (n=32) of mavacamten patients, as compared to less than 1% (n=1) in the placebo group (+26.6% [95% CI, 18.3 to 34.8]). Decreases in cardiac biomarkers were also observed, with a reduction in NT-proBNP 80% greater and hs-cTnI 41% greater in patients treated with mavacamten, as compared to placebo. Further subgroup analyses identified a difference in the primary endpoint based on concomitant background HOCM therapy: a greater effect was observed in patients not receiving BB therapy (n=29 on mavacamten, n=33 on placebo; difference +52.6% [95% CI, 32.9 to 72.2]), as compared to those on BB therapy (n=94 on mavacamten, n=95 on placebo; difference +8.7% [-3.6 to 21.1]).
The results of a further secondary analysis focusing on the health status of participants were also recently published.49 Here the primary endpoint was the KCCQ at baseline, weeks 6, 12, 18, 30 (end of treatment), and 38 (end of study). 75% (n=92) of mavacamten patients and 69% (n=88) of the patients assigned to placebo completed the KCCQ at baseline and at week 30. The change in KCCQ-OS score at week 30 was greater in the mavacamten group as compared to placebo (mean score 14.9 [standard deviation (SD) 15.8] vs 5.4 [SD 13.7]; difference +9.1 [95% CI, 5.5 to 12.8]; p<0.0001). Treatment with mavacamten also showed a higher proportion of patients with a very large change in KCCQ-OS (defined as ≥20 points), as compared to placebo, with 36% (n=33 of 92) on mavacamten and 15% (n=13 of 88) on placebo (estimated absolute difference of 21% [95% CI, 8.8 to 33.4] and a number needed to treat of five [95% CI, 3 to 11]).
Treatment with mavacamten revealed a good safety profile, with 11 serious adverse events being reported by 8% (n=10) of mavacamten patients versus 20 events reported by 9% (n=11) of the patients on placebo, with one patient on placebo experiencing sudden death. AF was reported by 2% (n=2) of mavacamten patients and 3% (n=4) of placebo patients, while seven patients on mavacamten and two on placebo showed a transient decrease in LVEF <50%. Finally, three patients on mavacamten and three on placebo met predefined criteria for QT interval changes. No serious heart failure-related adverse effects occurred. As such, mavacamten was shown to be effective in lowering LVOT gradient as well as improving symptoms and exercise performance, while attaining overall high tolerance with only moderate adverse effects.
With 251 patients being enrolled in 68 different cardiovascular centers across 13 countries, EXPLORER-HCM became the largest randomized clinical trial with HCM patients, showcasing that large clinical trials in these patients are feasible and able to demonstrate clinically significant results with the potential to alter current medical practices. Besides its high statistical power, its main strength was in the use of clinically significant endpoints, providing a clearer view of potential effects of mavacamten in clinical practice. In the absence of strong clinical cardiac endpoints, due to the low rate of events such as SCD, heart transplantation, and hospitalization, a functional endpoint capable of registering clinically significant results was needed. As such, a composite endpoint that combined an objective measurement of exercise capacity (pVO2) and subjective measurement of symptom burden (NYHA class) was created. NYHA class has been widely used to assess the symptomatic impact of HF, while pVO2 is associated with quality of life improvement and mortality reduction in HCM patients, with values of +1 mL/kg/min and +3 mL/kg/min showing to be clinically relevant thresholds.1,2,50,51
While a significant difference in the number of patients reaching the primary endpoint was achieved, only a little over one third (n=45; 37%) of mavacamten patients accomplished this. Most patients in both treatment arms maintained their BB therapy (n=94; 76% on mavacamten versus n=95; 74% on placebo), and further subgroup analysis demonstrated a greater effect on the primary endpoint in patients without this “background” therapy (difference 52.6% [95% CI, 32.9 to 72.2]) as compared to those under BB (difference 8.7% [95% CI, -3.6 to 21.1]). This is most likely due to the negative inotropic and chronotropic effects of BBs which lead to lower cardiac output and oxygen consumption. Notably, parameters of cardiopulmonary exercise testing (CPET) which act independent of heart rate changes, such as minute ventilation to carbon dioxide production (VE/VCO2), showed a greater improvement in the mavacamten group independent from BB therapy. As such, the concomitant use of BBs might have masked the true potential of mavacamten on exercise improvement capacity. Mavacamten in monotherapy might even be superior to BB therapy in the symptomatic treatment of patients with LVOTO.
Despite its significant achievements, the EXPLORER-HCM study had important limitations. With a participant mean age of 58.5 years (only 21% being younger than 50 years) and only 8.8% being not white, care should be taken when translating the study's results into these populations. Furthermore, severe symptomatic patients (NYHA class IV) and patients under disopyramide therapy were excluded from the study, further limiting the appreciation of mavacamten's effect on the real world of HOCM patients. Both populations will participate in the VALOR-HCM study, as will be further discussed.
Perhaps the greatest weakness of this study is the lack of a direct and thorough comparison of mavacamten treatment with the current standard therapy, both isolated and combined, and the use of a complex titration method which is not realistic for clinicians in daily practice. While mavacamten's beneficial effects with concomitant BB or NDH-CCB were clearly demonstrated, we are left wondering where this new drug can fit in the current treatment algorithm of HOCM patients. Further studies are needed to evaluate whether mavacamten should be used as double therapy with BB or NDH-CCB, as triple therapy with disopyramide, or simply as monotherapy in patients that do not achieve full symptomatic control with the standard HOCM therapy. Furthermore, the benefits of delaying more invasive therapies such as septal myectomy and SAA in favor of this new medical treatment are yet to be clarified.
Mavacamten was initially designed to treat HCM patients based on MYH7 mutations, but the genetic result was not considered an inclusion criterion in any of the conducted trials. Genetic testing was offered to participants, with 73% (n=90) of mavacamten and 78% (n=100) of placebo patients accepting it, resulting in 31% (n=28 of 90) of mavacamten and 22% (n=22 of 100) of placebo patients having an identified pathogenic or likely pathogenic gene variant. Although a direct comparison was not made, these patients appeared to have a better response to mavacamten treatment, as compared to patients with “negative” genetic testing. As such, it would be interesting to further explore the different responses to mavacamten in patients with known pathogenic gene variants, enabling more personalized treatment options in the future.
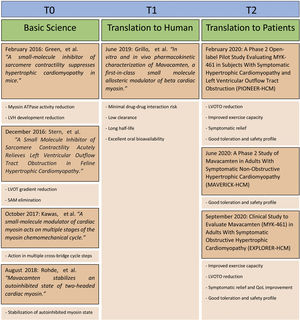
Finally, although mavacamten showed a favorable safety profile, its adverse effects were only evaluated over the course of 30 weeks. As such, long-term adverse effects and sustained efficacy remain to be evaluated. This is the aim of the long-term extension study MAVA-LTE currently underway, as discussed. A summary of the mavacamten clinical trials, with the respective endpoints and most significant results, is present in Table 1. A summary of mavacamten's translational pathways up to the current clinical results is presented in Figure 2.
Summary of the mavacamten clinical trials.
| Study | n | Inclusion criteria | Study endpoints | Significant results |
|---|---|---|---|---|
| PIONEER-HCM (Phase 2), published in February 2020 | 21 | LV Wall thickness ≥15 mm or ≥13 mm with a positive family history of HCM; age 18-70; BMI 18-37 kg/m2; LVEF ≥55%; Resting LVOT gradient ≥30 mmHg and post-exercise peak LVOT gradient ≥50 mmHg; NYHA ≥II. | Primary endpoints: change in post-exercise LVOT gradient from baseline to week 12, determined by echocardiography after treadmill stress.Secondary endpoints: post-exercise peak LVOT gradient response of < 30 mmHg; change in dyspnea numeric rating scale; pVO2; VE/VCO2; resting LVEF; LV fractional shortening; global longitudinal strain; post-exercise peak LVOT gradient from week 12 to week 16. | Cohort A: reduction in post-exercise LVOT gradient (mean change of -89.5 mmHg); reduction in resting LVEF (mean change of -15%); increase in pVO2 (mean change of 3.5 mL/kg/min).Cohort B: reduction in post-exercise LVOT gradient (mean change of -25.0 mmHg); reduction in resting LVEF (mean change of -6%); increase in pVO2 (mean change of 1.7 mL/kg/min).In both cohorts: Improved dyspnea scores, good toleration, with mild (80%), moderate (19%), and unrelated (79%) adverse events. |
| MAVERICK-HCM (Phase 2), published in June 2020 | 59 | LV Wall thickness ≥15 mm or ≥13 mm with a positive family history of HCM; age ≥18; body weight ≥45 kg; LVEF ≥55%; LVOT gradient <30 mmHg; NYHA II or III; NT-proBNP ≥300 pg/ml at rest. | Frequency and severity of treatment-emergent adverse events and serious adverse events, over 16 weeks. | Serious adverse events occurred in 10% of participants on mavacamten and in 21% participants on placebo; overall, mavacamten was deemed as well tolerated in most subjects. |
| EXPLORER-HCM (Phase 3), published in August 2020 | 251 | LVOT gradient of ≥50 mmHg; age ≥18; body weight ≥45 kg; LVEF ≥55%; NYHA II-III; oxygen saturation at rest ≥90%; able to perform an upright CPET and RER ≥1.0; adequate acoustic windows to transthoracic echocardiograms. | Primary endpoints: improvement in symptom severity from baseline to week 30 assessed by NYHA functional class and increase in exercise capacity from baseline to week 30 as assessed by measurement of pVO2 of ≥1.5 mL/min/kg; Or no worsening in NYHA functional class and increase in exercise capacity as a pVO2 of ≥3.0 mL/min/kg.Secondary endpoints: change in post-exercise LVOT gradient; NYHA class; pVO2; patient-reported outcomes (Kansas City Cardiomyopathy Questionnaire). | Greater percentage of mavacamten patients met the primary endpoint (+19.4%); greater reduction in post-exercise LVOT gradient (-36 mmHg); greater increase in pVO2 (+1.4 mL/min/kg); greater improvement in NYHA functional class (+34%); improved symptom scores. |
BMI: body mass index; CPET: cardiopulmonary exercise testing; HCM: hypertrophic cardiomyopathy; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association; pVO2: peak oxygen consumption; RER: respiratory exchange ratio; VE/VCO2: ventilation/carbon dioxide production.
A further substudy examined the effects of mavacamten versus placebo on cardiac structure and function through CMR imaging.52 Here, the primary endpoint was the change in LV mass index (LVMI) from baseline to week 30, with multiple exploratory endpoints including changes in cellular hypertrophy, left atrial volume index (LAVI), LV function, myocardial fibrosis measured by extracellular volume fraction (ECVF) and late gadolinium enhancement (LGE), NT-proBNP and hs-cTnI.
Thirty-five patients with a mean age of 60.3 years were randomized (1:1) into a mavacamten group (n=17) and a placebo group (n=18). This substudy analysis provided interesting results, with the mavacamten group showing a greater reduction in: LVMI (mean difference: -15.8 g/m2 [95% CI, -22.6 to -9.0]; p<0.0001), LV mass (difference: -30.0 g [95% CI, -43.3 to -16.7]; p<0.0001]), absolute intracellular myocardial mass index (mean difference: -13.1 g/m2 [95% CI, -18.7 to -7.5]; p=0.0002), maximum LAVI (mean difference: -10.3 mL/m2 [95% CI, -16.0 to -4.6]; p=0.0004), as well as greater reduction in NT-proBNP (80%) and hs-cTnI (50%). Finally, mavacamten was also associated with a greater reduction in maximum LV wall thickness (LVWTmax) (difference: -2.4 mm [95% CI, -3.9 to -0.9]; p=0.0079).
As such, the EXPLORER-HCM CMR substudy showed, for the first time, the full beneficial impact of mavacamten on cardiac remodeling. LAVI, LVMI, and LVWTmax are considered important predictors of poor prognosis in HOCM patients, and all of these were significantly reduced after mavacamten treatment.1,2,53 LAVI is an index that has been shown to be an important predictor of mortality, independent of LV geometry.54 Much like LAVI, LVMI has been shown to be an independent predictor of SCD.55 A decrease in LVMI coupled with a reduction in LVWTmax demonstrates the mavacamten's ability to effectively lower cardiac hypertrophy. Combined with changes in LVOT gradient, fibrosis stabilization, and decreases in serum biomarkers, these new results could translate into a significant impact on the risk of SCD in HCM patients. As such, beyond identifying a short-term favorable effect of mavacamten on cardiac structure, it is possible that this new agent can also work towards the reduction of SCD and improvement of diastolic function in HCM patients with both obstructive and nonobstructive phenotypes, thus broadening its potential indications beyond LVOT gradient reduction and quality of life improvement.
MAVA-LTE & VALOR-HCMMAVA-LTE (NCT03723655) is an ongoing long-term safety extension study of patients that completed the MAVERICK-HCM and EXPLORER-HCM trials. Involving 310 participants, its primary objective is to assess the frequency and severity of adverse effects over a period of 252 weeks. Three-arm groups are selected, treated with mavacamten: 1) a base target concentration, 2) a higher target concentration, and 3) a dose titrated to clinical response. It could be interesting to lower BB dosage in these patients, to further evaluate mavacamten's true effect on HOCM patients.
VALOR-HCM (NCT04349072) is also an ongoing randomized, double-blind, placebo-controlled study counting with 100 participants, which aims to evaluate mavacamten's effect on reducing the number of SRT procedures performed in patients with HOCM with eligibility criteria based on current guidelines. Importantly, this study will not only allow background medication with BB or NDH-CCB, but also disopyramide therapy, thus being the first study to analyze the efficacy and safety profile of this new drug when combined with the full standard therapy for HOCM patients. Furthermore, severely symptomatic patients (NYHA class IV) will also be included. The primary endpoint is a composite of 1) the number of patients who decide to undergo SRT prior to or at week 16, and 2) the number of patients who remain guideline eligible for SRT at week 16 (defined as a (LVOT gradient of ≥50 mmHg and NYHA class III-IV). Multiple secondary outcomes are included to further evaluate mavacamten's effect on NYHA, KCCQ, NT-proBNP, and LVOT gradient.
ConclusionLeft ventricular outflow tract obstruction is an important cardinal feature of HCM, associated with high morbidity and an increased risk of cardiovascular death, and a topic of inevitable interest within the cardiology community. Although major medical advances have been observed, it seems that the treatment options for these patients had stagnated. With no definitive treatment available, clinicians aimed to improve symptoms, functional capacity, diminish disease progression, and prevent the ever so-feared SCD.
In the absence of effective targeted therapies, therapeutic approaches relied on empirical pharmacological treatment, SCD prevention strategies, and invasive interventions such as septal myectomy, SAA and mitral valve manipulation, achieving variable success. As demonstrated in this review of recently published clinical trials with mavacamten, this drug offers a potential new approach, paving the way for targeted therapies that can provide optimized and specialized treatment to HCM patients.
Currently there are, however, important gaps in knowledge that prevent its immediate wide-spread use in clinical practice. It is not yet clear whether NYHA class I and IV patients would benefit from such a therapy, since this population was not included in the EXPLORER-HCM trial. Furthermore, mavacamten's place in the current treatment algorithm remains unclear – should it be used together with BB as second line therapy, or with BB and disopyramide, or even as monotherapy? Additionally, mavacamten was shown to cause a transient decrease in LVEF which could pose an important limitation on its use and may depend on basal LVEF value. Finally, it is as yet unclear whether HCM patients without the classic sarcomeric mutations would benefit equally from mavacamten treatment.
Conflicts of interestThe authors have no conflicts of interest to declare.