Cardiovascular disease is one of the main causes of morbidity and mortality worldwide. Control of its risk factors, particularly diabetes and dyslipidemia, through reduction of LDL cholesterol, is crucial to reduce cardiovascular risk. This work aims to assess and improve the medical approach to dyslipidemia in diabetic patients.
MethodsThis is a quality improvement study aimed at family doctors. It included patients with diabetics and dyslipidemia enrolled in the primary health care units of Além D’Ouro, S. Miguel and Oceanos. A quality standard was defined for each of the criteria assessed, and the results were compared using the chi-square test with p-value<0.05. Data analysis was performed using Microsoft Excel 2010® and IBM SPSS®.
ResultsComparing the first and second assessments, 14.6% vs. 22.2% (p=0.016) of the patients, respectively, achieved the LDL cholesterol target level of <70 mg/dl. Of those who did not meet the target level, 11.0% vs. 13.6% (p=0.395) had their pharmacological therapy changed and 4.6% vs. 3.3% (p=0.448) had their lipid profile reassessed within three months.
ConclusionsControl of dyslipidemia in patients with diabetes continues to be a major factor in the health of these patients, but it is carried out in an unsatisfactory way in the three health units studied. It is essential to increase the literacy of family doctors and to encourage the search for the best possible lipid control, in order to reduce cardiovascular risk, as well as to raise awareness among patients to increase adherence to therapy.
A doença cardiovascular é uma das principais causas de morbimortalidade no mundo. O controlo dos seus fatores de risco, nomeadamente da diabetes mellitus e da dislipidemia, através da redução de C-LDL, é essencial para reduzir o risco cardiovascular. Este trabalho pretende avaliar e melhorar as práticas médicas relativas à abordagem da dislipidemia em pacientes diabéticos.
MétodosTrata-se de um estudo de qualidade integrando um circuito de avaliação e melhoria aplicado aos médicos. A população incluiu os diabéticos com dislipidemia inscritos nas Unidades de Saúde Familiar de Além D’Ouro, S. Miguel e Oceanos. Definiram-se padrões de qualidade para cada critério avaliado e aplicou-se um teste qui-quadrado para comparação dos resultados, considerando p < 0,05. A análise de dados foi efetuada recorrendo ao Microsoft Excel® e SPSS®.
ResultadosComparando a 1.ª e a 2.ª avaliação, 14,6 versus 22,2% (p=0,016) utentes cumpriam o valor-alvo de C-LDL. Entre os não cumpridores, em 11,0 versus 13,6% (p=0,395) foi alterada a terapêutica antidislipidémica e 4,6 versus 3,3% (p=0,448) realizaram consulta de reavaliação dos parâmetros lipídicos em três meses.
ConclusãoO controlo da dislipidemia em doentes diabéticos continua a ser um fator preponderante na sua saúde, mas realizado de forma muito insatisfatória nas unidades estudadas. É fundamental aumentar a literacia dos médicos e incentivar a procura do melhor controlo lipídico possível com o objetivo de reduzir o risco cardiovascular, assim como sensibilizar os utentes sobre a dislipidemia e o risco cardiovascular para aumentar a adesão à terapêutica.
Cardiovascular (CV) disease remains one of the main causes of morbidity and mortality worldwide, being responsible for over four million deaths annually in Europe. It has a higher incidence in women (2.2 million compared to 1.8 million men).1 In Portugal, CV and cerebrovascular diseases are the leading cause of death (29.7% of deaths in mainland Portugal in 2015), disability, suffering and use of economic resources.2,3 In this context, the United Nations, together with medical societies including the World Heart Federation, American Heart Association, American College of Cardiology Foundation, European Heart Network and European Society of Cardiology, set the goal of a 25% reduction in CV mortality and associated risk factors by 2025.2
Against this epidemiological background, control of CV risk factors takes on paramount importance. Calculation of overall CV risk using the Systematic Coronary Risk Evaluation (SCORE) is an essential tool for assessment of patients, providing an indication of the level of intervention and intensity of clinical care required in the prevention and treatment of CV disease.4,5
Diabetes is among the most important of all CV risk factors, as is dyslipidemia; the prevalence of hypercholesterolemia in Portugal is around 56% according to the HIPOCRATES study.6
The guidelines of both the European Atherosclerosis Society/European Society of Cardiology and the American Heart Association/American College of Cardiology stress the importance of reducing low-density lipoprotein cholesterol (LDL-C) levels in order to reduce CV risk.4,7 The clinical guideline published by the Portuguese Directorate-General for Health (DGS) on the management and treatment of dyslipidemias takes a similar approach, emphasizing monitoring and management of patients according to their LDL-C level.8 According to these guidelines, patients with type 2 diabetes and dyslipidemia are classified as being at very high CV risk, in whom the target LDL-C level is <70 mg/dl (1.8 mmol/l) or a reduction of at least 50% from baseline.4,7,8
In the 2019 update to the European guidelines, diabetic patients with dyslipidemia are classified as being at high or very high CV risk, depending on the duration of diabetes and other history and CV risk factors. The target LDL-C level for patients at very high CV risk is <55 mg/dl (1.4 mmol/l) and a reduction of ≥50% from baseline.1 Comparison of the DGS guidelines published in 2011 and still in force with the 2019 ESC/EAS guidelines shows that the latter are more ambitious and thorough, which makes it important to understand the current management of these patients in the real-world context of primary health care.
In terms of pharmacological therapy, statins are the first-line treatment for dyslipidemias.4,8 If LDL-C goals are not achieved with maximum tolerated doses of this drug class, associating them with other drugs may confer additional reductions, but there is limited evidence from clinical trials for this effect.
Finally, as stated by the DGS guideline, these patients should undergo laboratory testing every four months until the target LDL-C level is reached, and annually thereafter.8
ObjectivesThe main objective of this quality improvement cycle is to assess and foster improvement in the approach to dyslipidemias in patients at high CV risk, specifically those with diabetes, with regard to:
- –
Prescription of laboratory testing of patients with dyslipidemia;
- –
Therapeutic approach in light of the results of laboratory testing;
- –
Monitoring and management of patients with dyslipidemia;
- –
Monitoring of the goals and indicators specified in the DGS guideline.
The specific aims are as follows:
- –
To determine whether target LDL-C levels have been achieved in high CV risk patients;
- –
To analyze changes in therapeutic management of dyslipidemias when target LDL-C levels were not met;
- –
To determine whether lipid parameters were reassessed within three months;
- –
To propose measures to be taken that will lead to improvements.
This study took the form of a two-stage quality improvement cycle addressing the problem of the management of dyslipidemia in patients with very high CV risk, specifically those with diabetes. We set out to assess and implement an intervention aimed at the technical and scientific quality of physicians responsible for managing these patients.
In the first stage, the situation was analyzed and the resulting diagnosis was presented to and discussed with the health professionals being assessed. Measures intended to lead to improvements were proposed and implemented and the results were evaluated in the second stage.
This quality improvement cycle aimed to improve the performance of 27 physicians.
The study population included all patients who were enrolled at the beginning of the study period in the primary health care units (Family Health Units [FHUs]) of Além D’Ouro, S. Miguel and Oceanos diagnosed as having insulin-dependent diabetes (T89 in the International Classification of Primary Care classification) or non-insulin-dependent diabetes (T90) and lipid disorder (T93) as coded in the SClínico® clinical database.
A randomized sample of the patient population in December 2017 and December 2018 was selected from listings obtained using MIM@UF software, with an expected prevalence of 50%, precision of 0.05 and 95% confidence interval. The number of patients from each FHU included in the sample was weighted according to the total number of patients per unit who fulfilled the inclusion criteria.
The data collected were anonymized by randomizing the eligible population from each FHU based on each patient's record number, to which an identifying code was attributed in a Microsoft Office Excel® spreadsheet, protected by a password known only to the study authors.
The following patients were excluded: those who did not complete the diabetes monitoring program in the two preceding six-month periods; those who died or were transferred to another unit between the time of sample selection and data collection; those attending hospital consultations or under private medical care; those whose LDL-C levels could not be measured; and those resident in care homes or followed in home visits only. During the data collection process, any patient who had been excluded was replaced by the following patient in the randomized listing in order to preserve the total number of eligible patients calculated for each FHU.
The first assessment was at the time of diabetes monitoring consultations between July 1, 2017 and December 31, 2017, while the second assessment took place at consultations between January 1, 2019 and June 30, 2019.
After selection of the study participants, information on the continuous and categorical variables presented in Table 1 was extracted for each patient from the SClínico® database records.
Variables analyzed in all patients in the study sample.
| Variable type | Variable | Description |
|---|---|---|
| Continuous | Age | Patient's age at the time of data collection |
| HbA1c | Last HbA1C level recorded | |
| LDL-C | Last LDL-C level recorded | |
| Categorical | Gender | Male or female |
| Statin therapy | Whether the patient was taking statins at the time of the consultation | |
| Type of statin | Specific statin being taken | |
| Other lipid-lowering therapy | Whether the patient was taking any other lipid-lowering therapy | |
| Target LDL-C level | Whether the patient's LDL-C was <70 mg/dl |
HbA1c: glycated hemoglobin; LDL-C: low-density lipoprotein cholesterol.
In cases in which LDL-C measurements were above the target level of <70 mg/dl (1.8 mmol/l), the variables presented in Table 2 were analyzed.
Categorical variables analyzed in patients who did not meet the target LDL-C level.
| Variable | Description |
|---|---|
| Current change in therapy | Change in lipid-lowering therapy at the time of consultation when LDL-C above the target level was determined |
| Previous reduction in LDL-C | Previous ≥50% reduction in LDL-C |
| Previous change in therapy | Previous change of the statin prescribed |
| Repeat lipid profile determination | Whether a consultation was scheduled to reassess lipid parameters |
| Time before reassessment | Whether lipid parameters were reassessed within three months |
LDL-C: low-density lipoprotein cholesterol.
The information collected was entered into a Microsoft Excel® database and the statistical analysis was performed using IBM SPSS® version 25.0. Categorical variables were expressed as absolute (n) and relative (%) frequencies. The Kolmogorov-Smirnov or Shapiro-Wilk tests and skewness and kurtosis tests were used to determine whether the continuous variables under study followed a normal distribution. The chi-square test was used to compare categorical variables that constituted quality criteria between the two assessments, with a p-value of <0.05.
The quality criteria adopted are shown in Table 3.
Quality criteria assessed in the present study.
| Criterion | Quality standards | |
|---|---|---|
| Criterion 1: target LDL-C level achieved | ≥75% | Good |
| ≥50 and <75% | Adequate | |
| ≥25 and <50% | Inadequate | |
| <25% | Very inadequate | |
| Criterion 2: change in lipid-lowering therapy at the time of consultation when LDL-C above the target level was determined | ≥75% | Good |
| ≥50 and <75% | Adequate | |
| ≥25 and <50% | Inadequate | |
| <25% | Very inadequate | |
| Criterion 3: scheduling of a reassessment consultation within three months and monitoring of lipid parameters if target LDL-C level was not achieved | ≥50% | Good |
| ≥25 and <50% | Adequate | |
| <25% | Inadequate | |
LDL-C: low-density lipoprotein cholesterol.
Confidentiality of patient data was ensured and such data were used only for statistical purposes. The study did not violate any ethical principles and was approved by the heads of the FHUs involved, the ethics committee of the Regional Health Authority of the North region, the Research Working Group of the Espinho/Gaia Health Center Group (ACeS), and the Clinical Health Committee of ACeS Matosinhos.
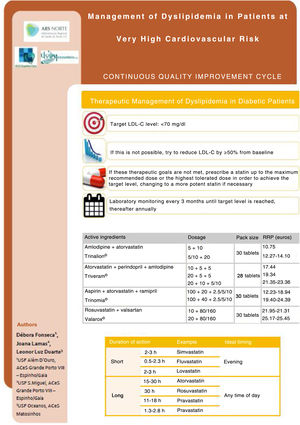
Corrective measuresAs well as presenting the DGS guideline on therapeutic management of dyslipidemia in adults8 to the multidisciplinary team, the authors also led discussions with the physicians being assessed concerning the results of the first stage of the study, any uncertainties or resistance to compliance with the criteria used, and suggestions for improvement. Suggestions were recorded and applied from January 2019 onward and a memorandum (Figure 1) was delivered in paper form to all physicians involved in January and again in March 2019. The existence of the present quality improvement study was communicated to those involved.
ResultsThe sample for the first assessment included 329 patients and the second included 333. Table 4 presents the distribution of patients excluded at the two assessments according to the exclusion criteria.
Reasons for exclusion of patients from the study samples.
| Exclusion criterion | First assessment | Second assessment |
|---|---|---|
| Failure to complete the diabetes monitoring program | 20 | 31 |
| Followed in hospital consultations | 1 | 43 |
| Care home or home visits | 0 | 8 |
| Died | 12 | 6 |
| Transferred to another unit | 1 | 3 |
| LDL-C level unmeasurable | 4 | 1 |
| Total | 37 | 92 |
| LDL-C: low-density lipoprotein cholesterol. | ||
Table 5 presents the characteristics of the study samples at both assessments in terms of gender, age, glycated hemoglobin (HbA1c) levels, and pharmacological therapy with statins and/or other lipid-lowering agents. The table also shows LDL-C levels in both samples. The first assessment analyzed the laboratory tests from the second half of 2017 and before, while the second assessment recorded the results of tests in 2018 and later. This explains the lack of an LDL-C measurement in 58 patients in the second assessment, which led to a total of 275 patients in whom it was possible to determine whether they had achieved the target LDL-C level.
Characteristics of the study samples.
| Variable | First assessment | Second assessment |
|---|---|---|
| Female, n (%) | 170 (51.70%) | 168 (50.5%) |
| Mean age, years (± D) | 67.86±10.179 | 67.58±10.520 |
| Median HbA1c, % (IQR) | 6.6 (1.3) | 6.7 (1.2) |
| Statin therapy, n (%) | 265 (80.5) | 273 (82) |
| Other lipid-lowering therapy, n (%) | 33 (10.4) | 56 (16.8) |
| Mean LDL-C level, mg/dl (±SD) | 104.18±34.76 | |
| Median LDL-C level, mg/dl (IQR) | 92.4 (37.6) | |
| Target LDL-C level achieved, n (%) | 48 (14.6) | 61 (22.2) |
| HbA1c: glycated hemoglobin; IQR: interquartile range; LDL-C: low-density lipopoprotein cholesterol; SD: standard deviation. | ||
Table 6 presents the distribution of patients under statin therapy according to type of statin.
Distribution of patients under statin therapy according to type of statin.
| Intensity | Statin | First assessment, n (%) | Second assessment, n (%) |
|---|---|---|---|
| Low | Simvastatin 10 mg | 6 (2.3) | 3 (1.1) |
| Pravastatin 10-20 mg | 5 (1.9) | 4 (1.5) | |
| Medium | Lovastatin 20 mg | 3 (1.1) | 1 (0.4) |
| Atorvastatin 10-20 mg | 63 (23.8) | 77 (28.2) | |
| Rosuvastatin 5-10 mg | 21 (7.9) | 14 (5.1) | |
| Simvastatin 20-40 mg | 133 (50.2) | 119 (43.6) | |
| High | Pravastatin 20-80 mg | 18 (6.8) | 21 (7.7) |
| Fluvastatin 40 mg twice daily | 1 (0.4) | 0 (0) | |
| Pitavastatin 2-4 mg | 3 (1.1) | 3 (1.1) | |
| Atorvastatin 40-80 mg | 11 (4.2) | 23 (8.4) | |
| Rosuvastatin 20-40 mg | 1 (0.4) | 8 (2.9) |
For patients who had not achieved the target LDL-C level by the time of their consultation, the following criteria were assessed, as presented in Table 7: previous ≥50% reduction in LDL-C; previous change in lipid-lowering therapy; change in therapy at the time of the consultation when LDL-C above the target level was determined; whether a consultation was scheduled to reassess lipid parameters; and whether reassessment was performed within three months.
Treatment of patients who did not achieve the target LDL-C level.
| Variable | First assessment | Second assessment |
|---|---|---|
| Current change in therapy, n (%) | 31 (11.0) | 29 (13.6) |
| Previous reduction in LDL-C, n (%) | 17 (6.0) | 2 (0.9) |
| Previous change in therapy, n (%) | 41 (14.6) | 21 (9.8) |
| Repeat lipid profile determination, n (%) | 133 (47.3) | 86 (40.2) |
| Reassessment within three months, n (%) | 13 (4.6) | 7 (3.3) |
LDL-C: low-density lipoprotein cholesterol.
Table 8 presents the results according to the established quality standards.
Quality standards applied in the study.
| Criterion | First assessment | Second assessment | p | Standard |
|---|---|---|---|---|
| Criterion 1: target LDL-C level achieved | 14.6% | 22.2% | 0.016 | Very inadequate |
| Criterion 2: change in lipid-lowering therapy at the time of consultation when LDL-C above the target level was determined | 11.0% | 13.6% | 0.395 | Very inadequate |
| Criterion 3: scheduling of a reassessment consultation within three months and monitoring of lipid parameters if target LDL-C level was not achieved | 4.6% | 3.3% | 0.448 | Inadequate |
LDL-C: low-density lipoprotein cholesterol.
The results of this study demonstrate that although there was a slight improvement in all of the parameters studied between the first and second stages of the assessment, the management of dyslipidemia in diabetic patients still falls short of the goals set out in the DGS guideline.
The current DGS guideline on therapeutic management of dyslipidemia in adults8 sets a target LDL-C level for diabetic patients with dyslipidemia of <70 mg/dl, and if this is not possible, a reduction of ≥50% from baseline. In our study, there was a statistically significant increase in the number of patients who achieved this target level (14.6% vs. 22.2%, p=0.016), following the implementation of corrective measures among the physicians under study. Given that this guideline was updated in 2017 and that the new version included a change in target LDL-C levels, these results may reflect a lack of awareness on the part of physicians of this change at the time of the first assessment. Another factor that could explain this low percentage is patients’ low level of adherence to drug therapy prescribed by their family physician. The side effects of statins, particularly muscle pain, the usual dosage of one tablet after dinner, the publicity in the media concerning the harm done by these drugs, and campaigns encouraging patients to stop taking them, are some of the factors that contribute to this low adherence.
To overcome these obstacles, physicians must address adherence to treatment at the time of their consultations with diabetic patients with dyslipidemia, in order to dispel myths and misinformation, as well as adopting strategies such as changing the time to take the statin from dinner to breakfast or to use drug combinations that contain statins. All statins apart from simvastatin, fluvastatin and lovastatin have a long-lasting effect and can be taken at any time of day,9,10 and so the time can be changed according to the patient's daily routines in order to minimize the chances of forgetting to take the drug. Furthermore, there are drug combinations on the market that include statins, such as atorvastatin and dopamine; atorvastatin, perindopril and amlodipine; atorvastatin, aspirin and ramipril; and rosuvastatin and valsartan. These combinations are ideal for patients who are prescribed these drugs individually.
The first assessment showed that lipid-lowering therapy was changed at the time of consultation when LDL-C above the target level was determined (criterion 2) in 11% of patients with LDL-C above the target level. At the second assessment, this figure had risen to 13.6%. However, this increase did not reach statistical significance (p=0.395). The authors believe that these results may be due to a failure on the part of the physician to consider dyslipidemia and CV risk as priorities, unlike control of HbA1c, as well as difficulties in managing the time available for the consultation hindering active promotion of better lipid control in these patients. Furthermore, physicians may think that the target LDL-C level of 70 mg/dl is too ambitious to achieve.
Concerning criterion 3 (scheduling of a reassessment consultation within three months), although a repeat lipid profile was requested in 47.3% of the sample at the first assessment and in 40.2% at the second, only 3-5% achieved the reassessment and scheduling within three months, as stipulated in the updated DGS guideline, with a reduction (not statistically significant) from 4.6% to 3.3% (p=0.448) between the two assessments. In our opinion, this may be for two reasons: the inability to schedule a reassessment for these patients at short notice due to overburdened health care services, and the fact that the Clinical Pathway for diabetic patients published by the DGS specifies annual lipid profile assessments.
The main aim of the PATER study, performed in Portugal in 2006, was to characterize standard therapeutic practices in diabetic patients, and it is the most similar to the present study. It found that 37% of diabetic patients with dyslipidemia were under lipid-lowering medication,11 a much lower percentage than in our study (80.5% in the first assessment and 82% in the second), which may be the result of heightened awareness in recent years among physicians of the need to treat this CV risk factor.
With regard to the type of statin prescribed, medium-intensity simvastatin was the most common lipid-lowering therapy (50.2%). This is in line with data from the PRECISE study, in which 52% of hypertensive patients followed in primary health care were medicated with simvastatin,12 and with the DGS guideline, which recommends initial treatment with 40 mg simvastatin in patients at high or very high CV risk indicated for medical therapy.
LDL-C levels fell between the first and the second assessment in our study, from 104.18±34.76 mg/dl to 92.4 (interquartile range 37.6) mg/dl. Levels in the PATER study were higher, falling from 147±41 mg/dl at the first assessment to 125±39 mg/dl after the intervention.11 It should be borne in mind that the PATER study was carried out in 2006, at a time when the recommended target LDL-C level for these patients was 100 mg/dl.
The strengths of the current study include the large number of patients enrolled, representing different geographical areas, as well as its subject, which has an increasingly important impact on CV risk in the population. Another positive feature is the study design of a continuous quality improvement cycle, improving the quality of health care provided, flagging less positive aspects, comparing results, and updating the knowledge of the physicians involved.
A less positive aspect of the study was the lack of any qualification or quantification of the difficulties perceived by physicians in applying the DGS guideline. These difficulties were in fact discussed in meetings between the participants following the first assessment, but the authors considered that a survey of the participants during the first stage (see below) would help prioritize the proposed corrective measures and hence improve the results of the second stage.
A further limitation acknowledged by the authors is the existence of factors influencing the control of LDL-C besides statin therapy that may have affected the results. These include non-adherence to treatment and intolerance of or contraindication to statins. In addition, patients attending consultations in May and June 2019 (at the end of the second assessment) may have gone on to have appropriate management in terms of treatment and follow-up.
The authors propose, as a possible way to improve performance, that a questionnaire should be administered to physicians in order to assess the main difficulties they experience in evaluating CV risk and deciding on the therapeutic approach to dyslipidemia, as well as a survey aiming to identify the main factors influencing patients’ adherence to statin therapy. We also propose that all patients with dyslipidemias, not just those with diabetes, should be referred for a dyslipidemia consultation, given the important contribution of this risk factor to CV risk and the mortality associated with CV disease in Portugal.
ConclusionsOn the basis of the results of this study, we can conclude that the therapeutic approach to dyslipidemia in diabetic patients was not satisfactory in these three FHUs. Following the intervention aimed at the physicians working in each unit, a significant improvement between the two assessments was only seen in achievement of the target LDL-C level, and changes in the other criteria did not reach statistical significance. Furthermore, the 2019 updated European guidelines on the management of dyslipidemias1 specify target LDL-C levels as low as <55 mg/dl. Some of the patients included in our study samples would certainly be within the range of this even more ambitious target, which makes it essential to improve the literacy of health professionals, especially physicians. Peer-to-peer training, continuing medical education and frequent evaluation of interventions are among the ways that the quality of physicians’ performance can be improved. Family doctors are in a unique position regarding disease prevention and health management due to their ability to inform, advise and empower their patients. It is thus crucial for these health professionals to strive for the best possible lipid control and to encourage their patients to adhere to pharmacological therapy and to modify their lifestyles, with a view to reducing their CV risk.
Conflicts of interestThe authors have no conflicts of interest to declare.