Acute blood glucose but not glycated hemoglobin (HbA1c) predicts poor outcome in acute heart failure (HF). The stress hyperglycemia ratio (SHR) has been proposed as a prognostic predictor in various clinical settings.
ObjectivesWe assessed the prognostic implications of the SHR in acute HF patients with and without diabetes.
MethodsWe performed a retrospective analysis of an acute HF registry conducted between 2009 and 2010. Estimated average glucose (eAG) was calculated as (28.7×HbA1c)−46.7 and SHR as acute blood glucose divided by eAG. The primary endpoint was all-cause mortality. Follow-up was three months. Patients were grouped by SHR tertiles (≤0.88, 0.89–1.16, and >1.16). Cox regression analysis was used to test the association of SHR (cut-off 0.88) with all-cause mortality. Analysis was stratified according to the presence of diabetes. Multivariate models were built accounting for acute blood glucose and for eAG (models 1 and 2, respectively).
ResultsWe studied 599 patients, mean age 76±12 years, of whom 62.1% had reduced ejection fraction and 50.9% had diabetes. Median acute blood glucose, eAG and SHR were 136 (107–182) mg/dl, 131 (117–151) mg/dl, and 1.02 (0.20–3.34), respectively. During follow-up 102 (17.0%) died. In patients with diabetes, those in the lowest SHR tertile had a hazard ratio (HR) of 2.24 (95% CI: 1.05–5.22) (model 1) and 2.34 (1.25–4.38) (model 2). In patients without diabetes, the HR of three-month death in the lowest SHR tertile was 0.71 (95% CI: 0.36–1.39) and 1.02 (0.58–1.81). Significant interaction was observed between diabetes and SHR.
ConclusionsIn HF patients with diabetes, a SHR ≤0.88 was associated with a more than twofold higher three-month mortality risk. No such association was found in non-diabetic patients. The presence of diabetes influences the association of the SHR with mortality.
A glicemia aguda (GA), mas não a hemoglobina glicada (HbA1c), prediz pior prognóstico na insuficiência cardíaca aguda (ICA). O ratio da hiperglicemia de stress (SHR) associou-se a pior prognóstico em várias patologias.
ObjetivosAvaliamos as implicações prognósticas do SHR em doentes com ICA com ou sem diabetes mellitus (DM).
MétodosAnálises retrospetiva de um registo de ICA (2009-2010). Glicemia crónica estimada (GCE)=(28,7×HbA1c)−46,7. SHR=GA/GCE. Objetivo-primário: mortalidade por qualquer causa. Seguimento: três meses. Os doentes foram agrupados de acordo com tercis de SHR (≤0,88; 0,89<shr≤1,16;> 1,16). Uma regressão de Cox foi usada para testar a associação entre SHR (ponto-de-corte 0,88) e mortalidade. Análise estratificada de acordo com DM/não DM. Construídos modelos multivariados incluindo a GA (modelo 1) e a GCE (modelos 2).</shr≤1,16;>
ResultadosEstudados 599 doentes. Idade média 76 (±12) anos, 62,1% tinham fração de ejeção reduzida, 50,9% DM; GA, GCE e SHR mediana 136 (107-182) mg/dL, 131 (117-151) mg/dL e 1,02 (0,20-3,34), respetivamente. Morreram 102 doentes (17,0%). Doentes com DM no tercil mais baixo de SHR tinham um HR de morte aos três meses de 2,24 (95% IC: 1,05-5,22) (modelo 1) e de 2,34 (1,25-4,38) (modelo 2); já em doentes sem DM os HR nos doentes com SHR ≤0,88 foram de 0,71 (95% IC: 0,36-1,39) e 1,02 (0,58-1,81). Havia interacção entre DM e SHR.
ConclusõesEm doentes diabéticos com ICA, um SHR ≤0,88 associou-se a mais do dobro do risco de mortalidade. Não se encontrou esta associação nos doentes sem DM. A presença de DM influenciou a associação da SHR com a mortalidade.
Diabetes has long been known to increase the risk of incident heart failure (HF).1 Furthermore, patients with HF and concomitant diabetes have worse prognosis than those with HF and no coexistent diabetes.2–4 The degree of glycemic control seems to play an important role in this association. Higher glycated hemoglobin (HbA1c) is associated with increased HF incidence,5 however, studies show conflicting results concerning its association with mortality in chronic HF patients. The association between HbA1c values and mortality in chronic HF has been reported as inverse,6,7 direct,8,9 and U-shaped.10,11 Only one recently published study assessed the impact of HbA1c on mortality in acute HF patients and no association was reported.12
Blood glucose has also been studied as a prognostic predictor in HF. Acutely elevated glucose levels at admission are a strong predictor of poor outcome in HF patients irrespective of the coexistence of diabetes.13–17 Hyperglycemia in hospitalized patients is likewise associated with higher mortality in patients with acute myocardial infarction, stroke, exacerbation of chronic obstructive airway disease, and in critical illness.18–21 In patients with diabetes, hyperglycemia reflects both the acute exacerbation caused by the intercurrent illness and the degree of chronic glycemic control. Hyperglycemia may also occur de novo in patients without a previous diabetes diagnosis, as a transient response to the acute illness process (stress hyperglycemia). Patients with stress hyperglycemia and no history of diabetes appear to have higher mortality than diabetic patients with stress hyperglycemia, meaning that stress hyperglycemia portends an even worse prognosis in non-diabetic than in diabetic patients.18,19,22
The stress hyperglycemia ratio (SHR) or acute-to-chronic glycemic ratio has been proposed to account for background blood glucose.18 This ratio focuses on the rise in blood glucose above background levels and therefore gives information about the current acute glycemic response to the illness. The SHR is associated with worse prognosis in a number of settings including acute illness18,23 and myocardial infarction,24 and after mechanical thrombectomy for ischemic stroke25 or percutaneous coronary intervention.26 It appears to have different prognostic implications according to the patient's diabetes status.24,26 The association between the SHR and mortality has never been addressed in an acute HF setting.
We aimed to assess the short-term impact on mortality of the SHR in acute HF patients with and without diabetes.
MethodsWe conducted a retrospective analysis in a cohort of hospitalized acute HF patients who were part of an acute HF registry conducted between January 2009 and December 2010 in the internal medicine ward of the Centro Hospitalar Universitário São João, Porto. The registry's main objectives were to characterize a cohort of real-world acute HF patients and to study prognostic predictors in acute HF. The diagnosis of HF was made according to the European Society of Cardiology guidelines.27 Briefly, all patients consecutively admitted with a primary diagnosis of acute HF, both new-onset and decompensated chronic HF, and patients with both reduced and preserved ejection fraction, were eligible for inclusion in the registry. Patients were excluded if symptoms were attributed to causes other than HF, if an acute coronary syndrome was the cause underlying acute HF, if no structural or functional abnormality was detected on the echocardiogram, or if no echocardiogram was performed. As part of the registry's protocol a series of procedures were performed, including a complete physical examination and venous blood sample collection both at admission and on the day of discharge. All patients underwent echocardiography, medications in use on admission and at discharge were recorded, and demographic data as well as data on comorbidities were collected. All echocardiographic images were obtained with a standard ultrasound machine (System 6, GE Vingmed, Horten, Norway) with a 2.5-MHz probe. Left ventricular ejection fraction (LVEF) was calculated by the biplane Simpson's method from apical 4-chamber and 2-chamber views. Patients with LVEF of at least 50% were considered to have preserved ejection fraction (HFpEF), those with LVEF <40% to have HF with reduced ejection fraction (HFrEF), and those with LVEF between 40 and 49% to have HF with mid-range ejection fraction (HFmrEF); severe left ventricular systolic dysfunction was defined as LVEF lower than 30%.
Comorbidities were defined as follows. Coronary heart disease (CHD) was defined as a history of acute myocardial infarction or significant CHD confirmed by imaging studies. Diabetes was defined as either a known previous diagnosis, current prescription of an oral hypoglycemic agent or insulin, or HbA1c ≥6.5%. All patients had type 2 diabetes. Renal function was assessed by calculating the glomerular filtration rate (GFR) following the Modification of Diet in Renal Disease formula. HbA1C was determined by an ion-exchange high-performance liquid chromatography system with a Bio-Rad D-10 analyzer (Bio-Rad, Porto, Portugal). Patients and clinicians treating them were aware of the ongoing registry and treatment was strictly according to the attending physician. The study protocol conformed to the ethical guidelines of the declaration of Helsinki and was approved by the local ethics committee.
The outcome under analysis was all-cause mortality and follow-up was set at three months from hospital admission. A total of 659 patients were included in the registry, of whom 60 with no measurement of venous glucose on admission to the emergency department or no measurement of HbA1c during hospital stay were excluded, resulting in a study population of 599 patients. Acute blood glucose corresponded to the venous glucose in the blood sample collected in the emergency department. The registry's protocol included HbA1c measurement during hospitalization, which was used to estimate average blood glucose concentration using the equation: estimated average glucose (eAG)=(28.7×HbA1c)−46.7, derived by Nathan et al.28 The relative hyperglycemia or stress hyperglycemia ratio (SHR) was defined as acute blood glucose divided by eAG. The relationships of glucose and SHR with short-term mortality in acute HF patients were examined.
Statistical analysisPatients were grouped according to SHR tertiles (≤0.88, 0.89–1.16, and >1.16). The chi-square test was used to compare categorical variables between groups, one-way analysis of variance to compare normally distributed continuous variables, and the Kruskal-Wallis test to compare non-normally distributed variables. Cox regression analysis was used to study the association of SHR with short-term mortality. Patients in the lowest SHR tertile were compared with the remainder. The analysis was stratified according to the presence of diabetes. Two multivariate models were built. In both models, adjustments were made considering a set of variables known to be associated with HF outcome: age, ischemic etiology, left ventricular dysfunction, renal function as assessed by GFR, and evidence-based HF therapies. Model 1 also accounted for acute venous glucose, while model 2 accounted for estimated chronic blood glucose along with the other reported variables. Interaction between diabetes and the SHR was formally tested. Kaplan-Meier cumulative survival curves were used to display mortality according to SHR with a cut-off of 0.88 (corresponding to the 33rd percentile).
The p-value considered to represent statistical significance was 0.05. Data were stored and analyzed using IBM SPSS software, version 20.0 (IBM Corp., Armonk, NY).
ResultsThis was a cohort of 599 elderly acute HF patients, mean age 76 years, mostly women (55.9%). The HF etiology was ischemic in 40.4%, valvular in 20.2%, hypertensive in 18.2%, idiopathic in 8.3%, alcoholic in 4.7%, and other rarer etiologies in 8.2%. Sixty percent of the patients presented in New York Heart Association (NYHA) class IV and the sample showed significant neurohumoral activation, with a median B-type natriuretic peptide (BNP) of 1640.9 pg/ml. Patients presented a high comorbidity burden and half of them had diabetes. Median acute blood glucose was 136 (107–182) mg/dl and median eAG was 131 (117–151) mg/dl. HFrEF patients composed 50.8% of the cohort, HFpEF composed 37.9% and HFmrEF 11.4%. Patient characteristics and comparison between those with and without diabetes are depicted in Table 1. As expected, hypertension was more frequent, HF was more often ischemic and GFR was significantly lower in patients with than without diabetes. Importantly, despite anticipated differences in AG and eAG, the groups were similar with regard to SHR.
Patient characterization and comparison between patients with and without diabetes.
| All (n=599) | Without diabetes (n=294) | With diabetes (n=305) | p | |
|---|---|---|---|---|
| Age, years, mean (SD) | 76 (12) | 76 (14) | 76 (10) | 0.97 |
| Male gender, n (%) | 264 (44.1) | 131 (44.6) | 133 (43.6) | 0.81 |
| Diabetes, n (%) | 305 (50.9) | |||
| Hypertension, n (%) | 441 (76.2) | 187 (65.8) | 254 (86.1) | <0.001 |
| Ischemic HF, n (%) | 242 (40.4) | 105 (35.7) | 137 (44.9) | 0.02 |
| HFpEF, n (%) | 227 (37.9) | 119 (40.5) | 108 (35.4) | |
| HFmrEF, n (%) | 68 (11.4) | 30 (10.2) | 38 (12.5) | |
| HFrEF, n (%) | 304 (50.8) | 145 (49.3) | 159 (52.1) | 0.38 |
| NYHA class I, n (%) | 0 | 0 | 0 | |
| NYHA class II, n (%) | 9 (1.5) | 5 (1.7) | 4 (1.3) | |
| NYHA class III, n (%) | 227 (38.2) | 117 (40.2) | 110 (36.3) | |
| NYHA class IV, n (%) | 358 (60.3) | 169 (58.1) | 189 (62.4) | 0.55 |
| Intermediate/intensive care unit admission, n (%) | 101 (16.9) | 50 (17.0) | 51 (16.7) | 0.93 |
| Inotropic/vasoactive drugs, n (%) | 55 (9.2) | 28 (9.5) | 27 (8.9) | 0.80 |
| Beta-blocker at admission, n (%) | 297 (49.6) | 142 (48.3) | 155 (50.8) | 0.51 |
| ACEi/ARB at admission, n (%) | 372 (62.1) | 160 (54.4) | 212 (69.5) | <0.001 |
| MRA at admission, n (%) | 84 (14.9) | 50 (17.0) | 34 (11.1) | 0.04 |
| ICD and/or CRT, n (%) | 18 (3.0) | 12 (4.1) | 6 (2.0) | 0.15 |
| Admission hemoglobin, g/dl, mean (SD) | 11.8 (2.1) | 11.9 (2.2) | 11.6 (2.0) | 0.07 |
| GFR, ml/min/1.73 m2, median (IQR) | 46 (33–59) | 48 (34–62) | 44 (30–58) | 0.007 |
| Admission BNP, pg/ml, median (IQR) | 1640.9 (960.2–2801.4) | 1859.1 (1011.7–2835.1) | 1554.0 (909.6–2789.1) | 0.13 |
| Acute blood glucose, mg/dl, median (IQR) | 136 (107–182) | 116 (98–144) | 163 (124–220) | <0.001 |
| HbA1c (%), median (IQR) | 6.2 (5.7–6.9) | 5.8 (5.5–6.1) | 6.9 (6.5–7.7) | |
| HbA1c (mmol/mol), median (IQR) | 44 (39–52) | 40 (37–43) | 52 (48–61) | <0.001 |
| Estimated chronic blood glucose, mg/dl | 131 (117–151) | 120 (111–128) | 151 (140–176) | <0.001 |
| SHR, median (IQR) | 1.02 (0.83–1.29) | 1.00 (0.83–1.26) | 1.05 (0.81–1.35) | 0.40 |
| Insulin at admission, n (%) | 56 (18.4) | |||
| Metformin at admission, n (%) | 81 (26.6) | |||
| Sulfonylurea at admission (%) | 84 (27.5) | |||
| DPP-4i at admission, n (%) | 28 (9.2) |
ACEi/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BNP: B-type natriuretic peptide; CRT: cardiac resynchronization therapy; DPP-4i: dipeptidyl peptidase 4 inhibitors; GFR: glomerular filtration rate; HbA1c: glycated hemoglobin; HF: heart failure; HFmrEF: heart failure with mid-range ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; ICD: implantable cardioverter-defibrillator; IQR: interquartile range; MRA: mineralocorticoid receptor antagonist; NYHA: New York Heart Association; SD: standard deviation; SHR: stress hyperglycemia ratio.
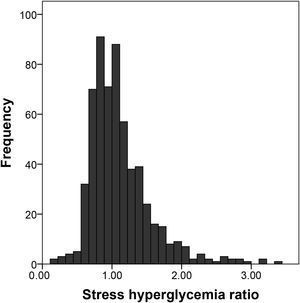
The SHR showed a slightly skewed, right-tailed distribution, with a median value of 1.02 (range 0.20–3.34). Figure 1 shows the histogram of SHR distribution. No significant differences were observed when SHR distribution was compared between patients with and without diabetes: median (IQR) 1.05 (0.81–1.35) in patients with diabetes vs. 1.00 (0.83–1.26) in patients without diabetes (p=0.38, Mann-Whitney U test).
When patients were classified according to SHR tertiles (first tertile ≤0.88, second tertile 0.89–1.16 and third tertile >1.16), patients in the first tertile tended to be younger and patients in the third tertile presented in higher NYHA classes. Apart from the expected differences in glucose levels, they were similar in terms of comorbidities, systolic function, renal function and neurohumoral activation. Table 2 shows the comparison of patients according to SHR tertiles.
Comparison between patients according to stress hyperglycemia ratio tertiles.
| SHR ≤0.88 | SHR 0.89–1.16 | SHR >1.16 | p | |
|---|---|---|---|---|
| Age, years, mean (SD) | 75 (14) | 77 (11) | 76 (12) | 0.06 |
| Male gender, n (%) | 90 (45.9) | 88 (42.3) | 86 (44.1) | 0.77 |
| Diabetes, n (%) | 97 (49.5) | 103 (49.5) | 105 (53.8) | 0.61 |
| Hypertension, n (%) | 144 (73.5) | 145 (69.7) | 152 (77.9) | 0.19 |
| Ischemic HF, n (%) | 87 (44.4) | 73 (35.1) | 82 (42.0) | 0.14 |
| HFpEF, n (%) | 70 (35.7) | 90 (43.3) | 67 (34.4) | |
| HFmrEF, n (%) | 19 (9.7) | 21 (10.2 | 28 (14.4) | |
| HFrEF, n (%) | 107 (54.6) | 97 (46.6) | 100 (51.3) | 0.19 |
| NYHA class I, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| NYHA class II, n (%) | 4 ((2.1) | 3 (1.4) | 2 (1.0) | |
| NYHA class III, n (%) | 83 (43.2) | 86 (41.3) | 58 (29.9) | |
| NYHA class IV, n (%) | 105 (54.7) | 119 (57.2) | 134 (69.1) | 0.04 |
| Intermediate/intensive care unit admission, n (%) | 15 (12.8) | 37 (17.8) | 39 (20.3) | 0.15 |
| Inotropic/vasoactive drugs, n (%) | 16 (8.2) | 24 (11.6) | 15 (7.7) | 0.34 |
| Beta-blocker, n (%) | 94 (47.9) | 102 (49.0) | 101 (51.8) | 0.72 |
| ACEi/ARB, n (%) | 116 (59.2) | 117 (56.3) | 139 (71.3) | 0.004 |
| MRA, n (%) | 32 (16.3) | 32 (15.4) | 20 (10.3) | 0.18 |
| ICD and/or CRT, n (%) | 7 (3.6) | 4 (1.9) | 7 (3.6) | 0.53 |
| Admission hemoglobin, g/dl, mean (SD) | 11.7 (2.1) | 11.8 (2.2) | 11.8 (2.1) | 0.76 |
| Admission creatinine, mg/dl, median (IQR) | 1.40 (1.11–1.90) | 1.37 (1.10–1.72) | 1.37 (1.10–1.80) | 0.37 |
| Admission BNP, pg/ml, median (IQR) | 1755.6 (1012.6–2932.7) | 1629.8 (914.4–2798.5) | 1602.5 (933.4–2670.6) | 0.17 |
| Acute blood glucose, mg/dl, median (IQR) | 98 (86–111) | 134 (116–162) | 194 (160–251) | <0.001 |
| Estimated chronic blood glucose, mg/dl | 131 (123–148) | 131 (117–156) | 128 (114–161) | 0.09 |
| Mortality, n (%) | 38 (19.4) | 35 (16.8) | 29 (14.9) | 0.49 |
ACEi/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BNP: B-type natriuretic peptide; CRT: cardiac resynchronization therapy; HF: heart failure; HFmrEF: heart failure with mid-range ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; ICD: implantable cardioverter-defibrillator; IQR: interquartile range; MRA: mineralocorticoid receptor antagonist; NYHA: New York Heart Association; SD: standard deviation; SHR: stress hyperglycemia ratio.
During the three-month follow-up, 102 (17.0%) patients died (21 of them in hospital). Figure 2 depicts the Kaplan-Meier survival curves in patients with and without diabetes according to SHR. In patients with concomitant diabetes, those presenting with a SHR in the first tertile had worse survival, while no association was seen between SHR and short-term mortality in HF patients without diabetes. After multivariate adjustment for age, ischemic heart disease, left ventricular dysfunction and renal dysfunction, this association of lower SHR with three-month all-cause death remained significant only in HF patients with diabetes. Table 3 shows two multivariate models in HF patients with and without diabetes, one including acute blood glucose and the other including estimated chronic blood glucose. Acute HF patients with diabetes in the lowest SHR tertile had a 2.34 hazard ratio (HR) (95% confidence interval [CI]: 1.05–5.22) of three-month death compared to those with SHR >0.88 when acute blood glucose was included and 2.34 (95% CI: 1.25–4.38) when estimated chronic blood glucose was included. The HR of three-month death in non-diabetes patients in the lowest SHR tertile was 0.71 (95% CI: 0.36–1.39) and 1.02 (0.58–1.81) in models 1 and 2, respectively. The interaction between diabetes and SHR tertiles was statistically significant in both models tested (p=0.05 in model 1 and p=0.03 in model 2).
Kaplan-Meier curves of three-month survival according to stress hyperglycemia ratio (SHR) (cut-off 0.88) in patients with diabetes (right) and without diabetes (left). In patients with diabetes, those with SHR ≤0.88 had a three-month survival of 79.5% (95% confidence interval [CI]: 74.9–84.1%) and in those with SHR >0.88 three-month survival was 84.7% (95% CI: 82.3–87.2%) (p=0.03). In patients with no concomitant diabetes the estimated three-month survival for those with SHR ≤0.88 was 80.3% (95% CI: 75.9–84.7%) and for those with SHR >0.88 it was 78.8% (95% CI: 75.3–82.2%) (p=0.67).
Predictors of three-month all-cause mortality: multivariate models separately in patients without and with diabetes.
| Without diabetes | With diabetes | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | P | |
| Multivariate model including acute blood glucosea | ||||
| SHR ≤0.88 | 0.71 (0.36–1.39) | 0.31 | 2.34 (1.05–5.22) | 0.04 |
| Acute blood glucose (per 10 mg/dl) | 0.95 (0.88–1.03) | 0.20 | 1.00 (0.95–1.05) | 0.99 |
| Age (per year) | 1.06 (1.02–1.09) | <0.001 | 1.07 (1.03–1.12) | 0.001 |
| Ischemic etiology | 0.65 (0.35–1.22) | 0.18 | 0.90 (0.47–1.75) | 0.76 |
| Glomerular filtration rate (per 10 ml/min) | 0.92 (0.79–1.08) | 0.33 | 0.94 (0.78–1.12) | 0.47 |
| HFpEF | 1 | 1 | ||
| HFmrEF | 0.98 (0.40–2.43) | 0.97 | 0.91 (0.29–2.83) | 0.87 |
| HFrEF | 1.28 (0.70–2.34) | 0.43 | 1.38 (0.68–2.81) | 0.38 |
| ACEi or ARB at admission | 1.02 (0.59–1.75) | 0.94 | 1.09 (0.55–2.16) | 0.80 |
| Beta-blocker at admission | 0.89 (0.52–1.54) | 0.68 | 1.29 (0.68–2.46) | 0.43 |
| MRA at admission | 1.55 (0.76–3.19) | 0.23 | 0.86 (0.30–2.49) | 0.79 |
| Multivariate model including chromic estimated glycemiab | ||||
| SHR ≤0.88 | 1.02 (0.58–1.81) | 0.94 | 2.34 (1.25–4.38) | 0.008 |
| Estimated chronic blood glucose (per 10 mg/dl) | 0.85 (0.66–1.09) | 0.19 | 1.01 (0.94–1.09) | 0.75 |
| Age (per year) | 1.06 (1.03–1.09) | <0.001 | 1.07 (1.03–1.12) | 0.001 |
| Ischemic etiology | 0.61 (0.33–1.14) | 0.12 | 0.90 (0.47–1.74) | 0.76 |
| GFR (per 10 ml/min) | 0.93 (0.80–1.09) | 0.38 | 0.94 (0.78–1.12) | 0.48 |
| HFpEF | 1 | 1 | ||
| HFmrEF | 0.93 (0.37–2.31) | 0.87 | 0.91 (0.29–2.83) | 0.87 |
| HFrEF | 1.44 (0.77–2.67) | 0.25 | 1.37 (0.68–2.79) | 0.38 |
| ACEi or ARB at admission | 1.00 (0.58–1.71) | 1.00 | 1.09 (0.55–2.16) | 0.80 |
| Beta-blocker at admission | 0.94 (0.55–1.61) | 0.81 | 1.29 (0.68–2.45) | 0.43 |
| MRA at admission | 1.52 (0.75–3.09) | 0.25 | 0.88 (0.30–2.54) | 0.81 |
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; CI: confidence interval; GFR: glomerular filtration rate; HFpEF: heart failure with preserved ejection fraction; HFmrEF: heart failure with mid-range ejection fraction; HFrEF: heart failure with reduced ejection fraction; HR: hazard ratio; MRA: mineralocorticoid receptor antagonist; SHR: stress hyperglycemia ratio.
We found that a SHR of ≤0.88 was associated with increased three-month mortality in our cohort of acute HF patients. This association was only found in patients with diabetes. Patients with diabetes with a SHR ≤0.88 had a more than two-fold higher risk of short-term death.
Acute hyperglycemia may be seen as the sum of chronic background blood glucose and stress-induced hyperglycemia due to critical illness. The former depends on the patient's beta-cell function, insulin resistance, and ongoing diabetes treatment, while the latter is a consequence of increased catecholamines and cortisol release, increased production of inflammatory cytokines and sympathetic system activation in the acutely ill.29,30 Acute hyperglycemia is associated with increased oxidative stress, endothelial dysfunction, mitochondrial dysfunction, platelet activation and procoagulation, increased proinflammatory cytokines and electrolyte and acid-base disturbances, all of which can contribute to increased mortality.18,29,30 The SHR, the ratio between acute blood glucose and chronic hyperglycemia, may be a better prognostic marker that acute hyperglycemia in patients with and without diabetes.18,24,26
Our findings contrast with those in other cardiovascular and critical care settings that show a positive relationship between the SHR and mortality.18,23–26 Interestingly, in a group of 321 patients with acute ischemic stroke treated with mechanical thrombectomy, both the lowest and highest SHR tertiles were associated with 90-day mortality compared to the second tertile used as reference.25 Patients in the lowest tertile had a median SHR of 0.81 (0.70–0.87), a value somewhat closer to ours. In addition, when only patients without background hyperglycemia were analyzed, the association between the lower and higher SHR tertiles lost statistical significance, although a trend toward higher mortality persisted.
A literature review retrieved only one study in acute HF patients focusing on the impact of the relation of acute hyperglycemia to baseline glycemia, by Liao et al.31 The authors defined the glycemic gap as the numerical difference between acute blood glucose and estimated chronic blood glucose. In a cohort of 425 patients with HF and diabetes, they found that a glycemic gap of over 43 mg/dl was associated with a more than seven-fold higher risk of in-hospital mortality compared to lower values. However, the very small number of events under analysis (only 6% of the patients died in-hospital) precludes the drawing of strong conclusions. The multivariate model built by the authors, with seven variables included for a total of 26 events under analysis, may suffer from overfitting. Besides the difference between the variables under study (glycemic gap in Liao et al. and SHR in ours), our study also differed in the duration of follow-up (three months vs. in-hospital mortality) and in the number of events analyzed, making our study potentially more robust in terms of conclusions drawn.
The SHR associated with worse outcome was clearly less than 1, meaning that those patients presented with a lower acute blood glucose than their background blood glucose. This suggests that relative hypoglycemia may play a role in short-term mortality. Hypoglycemia has been associated with increased in-hospital and short- and medium-term mortality,32,33 and the presence of HF is a risk factor for hypoglycemia.34 Thus, HF patients in the lowest SHR tertile (SHR ≤0.88), with blood glucose at least 12% lower than usual, could be at higher risk of in-hospital hypoglycemia and consequently of short-term mortality. Since only diabetic patients would be expected to take hypoglycemic drugs, this could explain the differences between the two groups. Relative hypoglycemia could also represent diabetic cardiovascular dysautonomia and therefore be a risk marker, particularly in this subset of HF patients.35 Furthermore, patients with poorly-controlled diabetes present symptoms of hypoglycemia and elevations of counter-regulatory hormones at more extreme glycemic thresholds than patients without diabetes or well-controlled diabetes patients.36 Therefore, even patients with higher background blood glucose but lower than usual acute blood glucose (and a low SHR, clearly below 1) could have higher activation of counter-regulatory hormones like cortisol and growth hormone, both described as being associated with worse outcome in HF.37,38 On the other hand, chronic hyperglycemia may have a protective effect against the damage caused by acute hyperglycemia through downregulation of glucose transporters.18,29 This could also be why patients with diabetes with higher SHR did not exhibit worse prognosis. Obviously, this could not explain the absence of an association between SHR and mortality in patients without diabetes.
The lack of association between higher SHR and higher mortality in acute HF is puzzling. Elevated SHR did not predict poor outcome in our acute HF population; this could be interpreted as some kind of metabolic reserve in response to stress. Our study is the first to report an association between SHR and mortality in acute HF patients according to diabetes status. The coexistence of diabetes appears to influence the impact of the SHR on HF prognosis: a low SHR seems to represent a survival disadvantage only in patients with diabetes, possibly because it reflects more severe dysautonomia.
Nevertheless, this study has some important limitations. First, it is a retrospective study, with all the inherent weaknesses. Information is lacking regarding blood glucose monitoring during hospitalization, particularly hypoglycemic events. Another major limitation is the fact that acute blood glucose was defined as venous glucose in the blood sample collected in the emergency department, and therefore previously administered medication was not accounted for. However, patients presenting in the emergency department with dyspnea are usually observed less than an hour after admission, and blood is collected immediately, so the probability that medication influencing blood glucose levels had been administered before blood collection is low. It would have been interesting to additionally study particular subgroups of the patient population, especially patients with pre-diabetes or even according to ejection fraction (subgroups of patients with HFrEF, HFmrEF and HFpEF). However, the small number of events precluded the creation of these additional subgroups. Despite all these limitations, the number of events studied (with a non-combined endpoint) was large enough to detect differences in mortality in terms of SHR, and to detect interactions between diabetes and the SHR.
ConclusionsIn acute HF, patients with diabetes and a SHR in the lowest tertile (≤0.88) presented a two-fold higher three-month mortality risk. No association between SHR and mortality was found in non-diabetic HF patients. The presence of diabetes seems to influence the association of the SHR with mortality. Our results suggest that relative hypoglycemia may play a role in short-term mortality in HF patients with diabetes.
Authors’ contributionsFMC and PL wrote the first draft of the manuscript. FMC and PL designed the study and collected and interpreted the data. PB collected and interpreted the data. MC and IF interpreted the data. PL performed the statistical analysis. All authors read and approved the final version of the manuscript.
FundingNone to report.
Conflicts of interestThe authors have no conflicts of interest to declare.







![Kaplan-Meier curves of three-month survival according to stress hyperglycemia ratio (SHR) (cut-off 0.88) in patients with diabetes (right) and without diabetes (left). In patients with diabetes, those with SHR ≤0.88 had a three-month survival of 79.5% (95% confidence interval [CI]: 74.9–84.1%) and in those with SHR >0.88 three-month survival was 84.7% (95% CI: 82.3–87.2%) (p=0.03). In patients with no concomitant diabetes the estimated three-month survival for those with SHR ≤0.88 was 80.3% (95% CI: 75.9–84.7%) and for those with SHR >0.88 it was 78.8% (95% CI: 75.3–82.2%) (p=0.67). Kaplan-Meier curves of three-month survival according to stress hyperglycemia ratio (SHR) (cut-off 0.88) in patients with diabetes (right) and without diabetes (left). In patients with diabetes, those with SHR ≤0.88 had a three-month survival of 79.5% (95% confidence interval [CI]: 74.9–84.1%) and in those with SHR >0.88 three-month survival was 84.7% (95% CI: 82.3–87.2%) (p=0.03). In patients with no concomitant diabetes the estimated three-month survival for those with SHR ≤0.88 was 80.3% (95% CI: 75.9–84.7%) and for those with SHR >0.88 it was 78.8% (95% CI: 75.3–82.2%) (p=0.67).](https://static.elsevier.es/multimedia/08702551/0000004200000005/v1_202305031201/S0870255123000513/v1_202305031201/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)


