The benefit of complete revascularization (CR) on long-term total event reduction in patients with ST-segment elevation myocardial infarction (STEMI) and multivessel disease (MVD), still remains unclear. We assessed the efficacy of three different revascularization strategies on long-term total recurrent events.
MethodsWe retrospectively analyzed 414 consecutive patients admitted with STEMI and MVD who were categorized according to the revascularization strategy used: culprit-vessel-only percutaneous coronary intervention (PCI) (n=163); in-hospital CR (n=136); and delayed CR (n=115). The combined endpoint assessed was all-cause mortality, the total number of myocardial infarctions, ischemia-driven revascularizations or strokes. Negative binomial regression was used to assess the association between the revascularization strategy and total events; risk estimates were expressed as an incidence rates ratio (IRR).
ResultsAt a median follow-up of four years (1.2–6), rates of the combined endpoint per 10 patient-years were 18, 0.8, and 0.6 in culprit-vessel-only PCI, in-hospital CR, and delayed CR strategies, respectively (p<0.001). After multivariable adjustment and when compared with culprit-vessel-only PCI, both in-hospital and delayed CR strategies were significantly associated with a reduction in the combined endpoint (IRR=0.40: 95% confidence interval (CI), 0.25–0.64; p<0.001; and IRR 0.40: 95% CI, 0.24–0.62; p<0.001, respectively). No differences were observed across in-hospital and delayed CR strategies.
ConclusionsComplete revascularization of non-culprit lesions in patients with STEMI and MVD reduces the risk of total recurrent events during long-term follow-up. No differences between in-hospital and delayed CR strategies were found.
O benefício de uma revascularização completa (RC) em termos de redução total de eventos a longo prazo em doentes com enfarte agudo do miocárdio com elevação do segmento ST (EAMCST) e doença coronária multivaso (DCM) ainda não está claro. Avaliamos a eficácia de três estratégias diferentes de revascularização em eventos recorrentes totais a longo prazo.
MétodosAnalisámos retrospetivamente 414 doentes consecutivos admitidos com EAMCST e DCM classificados de acordo com a estratégia de revascularização utilizada: intervenção coronária percutânea (ICP) só de vaso culpado (n=163); RC intra-hospitalar (n=136); e RC retardada (n=115). O parâmetro combinado avaliado foi a mortalidade por todas as causas, o número total de enfarte do miocárdio, revascularizações por isquemia ou acidentes vasculares cerebrais. A regressão binomial negativa foi utilizada para avaliar a associação entre a estratégia de revascularização e os eventos totais e as estimativas de risco foram expressas como taxa de incidência (TI).
ResultadosCom um seguimento mediano de quatro anos (1,2-6), as taxas do parâmetro combinado por 10 pacientes por ano foram de 18, 0,8, e 0,6 em ICP só para o vaso culpado, CR intra-hospitalar, e estratégia de CR retardada, respetivamente (p<0,001). Após ajustamento multivariável e quando comparadas com a ICP apenas em vasos culpados, tanto as estratégias de RC intra-hospitalar como a RC retardada foram significativamente associadas a uma redução do parâmetro combinado (TI=0,40: intervalo de confiança de 95% [IC], 0,25-0,64; p<0,001; e TI=0,40: 95% IC, 0,24-0,62; p<0,001, respetivamente). Não se observaram diferenças entre as estratégias de CR intra-hospitalar e de CR retardada.
ConclusõesA RC de lesões não culpadas em doentes com EAMCST e MVD reduz o risco de eventos totais recorrentes durante o seguimento a longo prazo. Não foram observadas diferenças entre as estratégias de RC intra-hospitalar e de RC retardada.
Primary percutaneous coronary intervention (PCI) is the treatment of choice in patients with ST-segment elevation myocardial infarction (STEMI) since it has been shown to reduce the risk of cardiovascular death and myocardial infarction (MI), when compared with thrombolysis.1 In about 40–50% of the cases, STEMI is associated with multivessel coronary artery disease (MVD), conferring a worse prognosis.2,3 Most randomized clinical trials (RCT) support the concept that complete revascularization (CR), either during primary PCI or as a staged procedure, reduces adverse clinical events.4–8
Although the optimal timing of CR is still a matter of debate, the latest 2017 European Society of Cardiology (ESC) STEMI Guidelines recommend a CR strategy preferably during index admission before discharge.9
Traditionally, the time-to-first event approach has been the classical method for evaluating the risk of adverse events in ischemic cardiomyopathy, including the risk of readmission.10 However, in recent years, some experts have argued in favor of replacing classical time-to-first analyses by evaluating all (first and recurrent) events that occurred during follow-up. This longitudinal approach would hypothetically improve the evaluation of morbidity burden across different treatment strategies. Unfortunately, in this regard, there is no information about the efficacy of different revascularization strategies in STEMI patients on the risk of total long-term adverse clinical events.
In this study, we sought to evaluate the risk of total cardiovascular events among three different revascularization strategies in patients with STEMI and MVD using recurrent event analysis.
MethodsStudy populationThis is a retrospective observational study from a prospective registry carried in a third-level university hospital. We analyzed 688 consecutive patients with STEMI who underwent successful primary PCI and showed MVD in the coronary angiography between February 2010 and May 2019.
Multivessel coronary artery disease was defined as the presence of at least one significant lesion in a non-infarct related epicardial coronary artery (N-IRA), which was amenable to successful treatment with PCI and located in a vessel with a diameter of at least 2.5 mm. Angiographically, significant lesions were deemed when the vessel diameters presented at least a 70% stenosis on visual estimation. The N-IRA lesion was never stented as part of the index culprit lesion PCI.
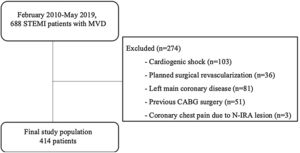
Patients with cardiogenic shock at admission (n=103), those with planned surgical revascularization (n=36), left main coronary disease (≥50% diameter stenosis) (n=81), previous coronary artery bypass grafting (CABG) surgery (n=51), and those who presented a new coronary chest pain during index admission due to N-IRA lesion (n=3) were excluded (Figure 1).
We classified our study population (n=414) in three different groups according to the type of revascularization strategy undertaken: (1) culprit-vessel-only revascularization during primary PCI (n=163), leaving untreated the N-IRA lesions; (2) CR during hospital admission (in-hospital CR) (n=136), where the N-IRA lesions were treated before hospital discharge; and (3) CR after hospital discharge (delayed CR) (n=115), where the N-IRA lesions were treated in a staged procedure time after discharge.
The decision of which type of revascularization strategy should be performed on the N-IRA lesions was based on consensus between the attending clinician and the interventional cardiologist.
Clinical and laboratory data were registered from medical records. The study complies with the Declaration of Helsinki and was approved by institutional ethics committee of the Consorcio Hospital General Universitario de Valencia. Individual consent for this retrospective analysis was waived.
Follow-up and endpointsIn this study, we evaluated the efficacy of each type of revascularization strategy in terms of:
- a)
Endpoint 1: Composite of all-cause mortality, the total number of MI, ischemia-driven revascularizations or strokes.
- b)
Endpoint 2: Composite of the total number of MI, admissions for heart failure (HF), ischemia-driven revascularizations, strokes, or visits to the emergency department with acute chest pain.
- c)
Length of hospital stay.
Myocardial infarction was defined according to the third universal definition and was subclassified according to type.11 For defining ischemia-driven revascularization, we included all PCI or CABG occurring after the baseline procedure and justified by recurrent symptoms or objective evidence of significant ischemia on provocative testing. Stroke was defined as the presence of a new focal neurologic deficit suspected to have a vascular origin, with signs or symptoms lasting more than 24 hours. It was classified as ischemic, hemorrhagic, or type uncertain. HF will be defined as a hospital admission >24 hours with any of the following signs and symptoms: worsening breathlessness, fatigue, fluid overload, pulmonary edema, elevated venous pressure and requirement of iv diuretics or inotropes. Confirmation of HF according to local expert judgment and evidence of impaired left ventricular function was required for the event to be classified as an HF admission. We considered visits to the emergency department with acute chest pain as those in which there was a suspected coronary origin.
All events were identified and quantified by consensus of two cardiologists from the patients health records, including hospital ward admission and emergency room visits.
Statistical analysisContinuous variables are presented as mean±standard deviation or median (inter-quartile range), where appropriate. Categorical variables are expressed as absolute and relative frequencies. Baseline continuous variables were compared across revascularization strategies with ANOVA, adopting Kruskal–Wallis test for non-parametric variables. Discrete variables were compared using the chi-square test.
A descriptive analysis of recurrent events was performed by counting the number of events during the entire follow-up. Crude incidence rates (expressed as the number of events per 10 person-year) were calculated for each endpoint across revascularization strategies. Negative binomial regression evaluated the association among the revascularization strategies and the number of total events during the entire follow-up. Because an increase in adverse events is supposed to be associated with an increased risk of subsequent death, it has been suggested that any analysis of recurrent intermediate endpoints should also account for death as a terminal event. We used the STATA (StataCorp. 2015. Stata Statistical Software: Release 14.1. College Station, TX: StataCorp LP) command bivcnto, a novel method which enables risk estimates to be obtained for total readmission and death accounting for the positive correlation among them.12 Thus, estimates of risk are more adjusted. Covariate selection was performed based on previous medical knowledge. The covariates included in the time-to-first and recurrent events final models were: age, gender, hypertension, diabetes mellitus, smoker, dyslipidemia, Killip class, anterior STEMI, number of vessels pending revascularization, Syntax score, and left ventricular ejection fraction.
Multivariable linear regression analyses were used to evaluate the association between different revascularization strategies and length of hospital stay. Estimates of risks in this latter model included the following covariates: age, gender, hypertension, diabetes mellitus, smoker, dyslipidemia, Killip class, anterior STEMI, number of vessels pending revascularization, Syntax score, and left ventricle ejection fraction.
A two-tailed p value of <0.05 was considered statistically significant for all analyses. All survival analyses were performed using STATA 15.1 (StataCorp. 2015. Stata Statistical Software: Release 14.1. College Station, TX: StataCorp LP). The ‘Bivcnto’ Stata module was used in the multivariable regression models for bivariate count outcomes.
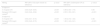
ResultsBaseline characteristicsThe mean age of the patients was 65±3 years, 15.7% were female, and 43% had an anterior STEMI. Most of the patients (88.6%) presented Killip class I at admission, and 39% presented left ventricle ejection fraction of less than 50%. N-IRA lesions were more frequently located in the circumflex artery (47%), and 30.2% had two or more vessels pending revascularization.
The distribution of the cohort across revascularization strategies was as follows: culprit- vessel-only PCI, n=163 (39.3%); in-hospital CR, n=136 (33%); and delayed CR, n=115 (27.7%).
Baseline characteristics among revascularization strategies are presented in Table 1. Patients in the culprit-vessel-only PCI strategy were significantly older and showed more prevalence of diabetes. The proportion of patients who presented two or more vessel diseases and the residual Syntax score were similar across the three groups. Median time to CR was two days (1–3) and 28 days (21–32), for in-hospital and delayed CR strategies, respectively.
Baseline and procedural characteristics.
| Culprit vessel(n=163) | In-hospital CR(n=136) | Delayed CR(n=115) | p value | |
|---|---|---|---|---|
| Demographics and medical history | ||||
| Age, (years) | 68.3±14 | 64±12 | 63±11 | <0.001 |
| Male, n (%) | 130 (79.8) | 114 (84.1) | 105 (91.3) | 0.03 |
| Hypertension, n (%) | 120 (73.6) | 87 (64.1) | 73 (63.5) | 0.10 |
| Diabetes, n (%) | 72 (44.2) | 47 (34.5) | 30 (26.1) | <0.001 |
| Dyslipidemia, n (%) | 89 (54.6) | 86 (63.2) | 61 (53.1) | 0.2 |
| Previous IHD, n (%) | 20 (12.3) | 15 (11) | 7 (6.1) | 0.2 |
| Current smoker, n (%) | 62 (38) | 68 (50) | 50 (43.5) | 0.1 |
| Chronic kidney diseasea, n (%) | 18 (11) | 9 (6.6) | 5 (4.3) | 0.1 |
| LVEF <50%, n (%) | 71 (43.5) | 47 (34.5) | 43 (37.4) | 0.2 |
| Anterior STEMI, n (%) | 77 (47.2) | 52 (38.2) | 49 (42.6) | 0.3 |
| Killip class I, n (%) | 132 (81) | 124 (91.2) | 111 (96.5) | <0.001 |
| Coronary angiography | ||||
| Residual Syntax score | 5 (2.8) | 4 (2.9) | 4 (2.8) | 0.7 |
| Residual diseased vessel ≥2, n (%) | 51(31.3) | 39 (28.7) | 34 (29.6) | 0.4 |
| Location of N-IRA lesions, n (%) | ||||
| Proximal LAD | 14 (8.6) | 19 (14.1) | 13 (11.3) | 0.35 |
| Middle LAD | 41(25.1) | 49 (36) | 42 (36.5) | 0.08 |
| Circumflex artery | 84 (51.5) | 57 (42.1) | 54 (47) | 0.41 |
| Right coronary artery | 63 (38.6) | 44 (32.3) | 46 (40) | 0.6 |
| Length of hospital stay | ||||
| Total hospital stay, (days) | 6 (4.8) | 7 (5.9) | 5 (4.6) | <0.001 |
Data are given as n (%), or median (interquartile range). CR: complete revascularization; IHD: ischemic heart disease; LAD: left anterior descending artery; LVEF: left ventricle ejection fraction; STEMI: ST elevation myocardial infarction.
Continuous values are expressed as mean±SD or median (interquartile range).
No patients in the delayed CR strategy experienced an event until CR was performed.
Revascularization strategies and risk of recurrent cardiovascular eventsDuring a median follow-up of four years (1.2–6), 95 (23%) deaths were registered, 43 recurrent MI in 35 (8.4%) patients, 38 ischemia-driven revascularizations in 35 (8.4%) patients, 22 recurrent strokes in 21 (5%) patients, 28 admissions for HF in 23 (5.5%) patients, and 195 visits to the emergency department for acute chest pain in 109 (26.3%) patients. The total number of events for endpoints one and two were 81 (19.5%) and 153 (37%), respectively.
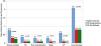
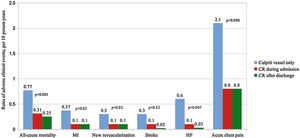
Figure 2 presents the incidence rates of events across revascularization strategies. Culprit-vessel-only PCI strategy showed the highest rates of recurrent MI, ischemia-driven revascularization, admissions for HF, and visits to the emergency department for acute chest pain. No significant differences in the incidence rates of stroke were observed.
Regarding ischemia-driven revascularization, in the culprit-vessel-only PCI strategy, the vessel which underwent ischemia-driven revascularization occurred principally due to a known lesion in the culprit vessel or in the N-IRA, in eight (4.9%) patients and 15 (9.2%) respectively; while revascularization was due to a de novo lesion in only one (0.6%) patient. On the other hand, in the in-hospital and delayed CR strategy groups, the most frequent ischemia-driven revascularization was due to a de novo lesion – in four patients (2.9%) and 4 (3.5%), respectively – and due to a known lesion in the culprit vessel – in three patients and three patients, respectively; while no patient experienced revascularization in an N-IRA.
We also assessed the number of subsequent events after a first non-fatal event. Of the 414 patients, 43 (10.4%) patients died during the first 12 months of follow-up. Therefore, of the 371 patients who were still alive, 34 (9.2%) patients presented a subsequent event (all-cause mortality, MI, ischemia-driven revascularization or stroke) after a first non-fatal event during the entire follow-up: 22 (64.7%) patients in the culprit-vessel-only PCI group, 6 (17.6%) in the in-hospital CR group and 6 (17.6%) in the delayed CR group; (p<0.001).
Risks of all-cause mortality, the total number of myocardial infarctions, ischemia-driven revascularization or stroke (endpoint 1)During follow-up, 81 (19.5%) patients experienced this combined endpoint. The crude incidence rates were 18 per 10 patients-year; 0.8 per 10 patients-year and 0.6 per 10 patients-year; (p<0.001), for culprit-vessel-only PCI, in-hospital CR, and delayed CR strategies, respectively.
After multivariate adjustment, and when compared with culprit-vessel-only PCI group, either in-hospital CR and delayed CR were significantly associated with a reduction in this composite endpoint (IRR=0.40: 95% (CI), 0.25–0.64; p<0.001; and IRR=0.40: 95% CI, 0.24–0.62; p<0.001, respectively). Likewise, when compared with delayed CR, culprit-vessel-only PCI strategy was significantly associated with an increased risk (IRR=2.55: 95% CI, 1.55–3.91; p<0.001). No differences were observed when comparing delayed CR vs. in-hospital CR strategy (IRR=0.96: 95% CI, 0.53–1.74; p=0.90) as is shown in Table 2.
Risk of endpoints 1 and 2 in patients with in-hospital complete revascularization when compared with those with culprit vessel only revascularization and delayed complete revascularization in the multivariate models.
| NBreg | IRR (95% CI)Culprit vessel as reference | p value | IRR (95% CI)Delayed CR as reference | p value |
|---|---|---|---|---|
| Endpoint 1 | ||||
| In-hospital CR | 0.40 (0.25–0.64) | <0.001 | 0.96 (0.53–1.74) | 0.90 |
| Delayed CR/Culprit vessela | 0.40 (0.24–0.62) | <0.001 | 2.55 (1.55–3.91) | <0.001 |
| Endpoint 2 | ||||
| In-hospital CR | 0.53 (0.41–0.70) | <0.001 | 1.00 (0.72–1.40) | 0.90 |
| Delayed CR/Culprit vessela | 0.53 (0.40–0.70) | <0.001 | 1.90 (1.43–2.41) | <0.001 |
CI: confidence interval; CR: complete revascularization; IRR: incidence rate ratio; NBreg: negative binomial regression.
The second combined endpoint occurred in 153 (37%) patients. The crude incidence rates were 23 per 10 patients-year; 2.5 per 10 patients-year and 1.8 per 10 patients-year; (p<0.001), for culprit-vessel-only PCI, in-hospital CR, and delayed CR groups, respectively.
After multivariate analysis, and when compared with the culprit-vessel-only PCI group, both in-hospital CR and delayed CR strategies were significantly associated with a reduction in the risk of presenting the second combined endpoint (IRR=0.53: 95% CI, 0.41–0.70; p<0.001; and IRR=0.53: 95% CI, 0.40–0.70; p<0.001, respectively). Likewise, when compared with delayed CR, culprit-vessel-only PCI strategy was also significantly associated with an increased risk (IRR=1.90: 95% CI, 1.43–2.41; p<0.001). No differences were found when comparing delayed CR vs. in-hospital CR (IRR=1: 95% CI, 0.72–1.40; p=0.90) (Table 2).
Length of hospital stay according to revascularization strategiesThe median length of hospital stay was 6 days (4–8). Patients who underwent in-hospital CR exhibited the most prolonged hospital stay, seven days (5–9) when compared with culprit-vessel-only PCI and delayed CR, which showed 6 (4–8) and 5 (4–6) days, respectively (p<0.001).
After multivariate adjustment and when compared with culprit-vessel-only PCI strategy, in-hospital CR was an independent predictor of longer hospital stay, (β-coefficient 1.34: 95% CI, 0.51–2.17; p=0.002). When compared with in-hospital CR, delayed CR was associated with a significant reduction in days of admission (β-coefficient −2.28: 95% CI, −3.17 to −1.40; p<0.001).
DiscussionThe main finding observed in the current study is that a staged CR approach, either during admission or after discharge, is associated with a significant reduction in the risk of presenting long-term recurrent cardiovascular events compared to the culprit-vessel-only PCI strategy.
Revascularization strategies and impact on clinical outcomesRandomized clinical trials have shown meaningful reductions in the risk of composite endpoints when CR is attempted, and lesions in a N-IRA are treated by PCI. However, the beneficial clinical effect was essentially driven by the reduction in time-to-first composite outcomes (MACE) or repeated revascularizations.7–10 Along this line, the long-term follow-up of the CULPRIT trial has also shown that the reduction in the composite endpoint (all-cause death, IM, HF, ischemia-driven revascularization) of the CR group was maintained at long-term follow-up (median of 5.6 years).13 However, no data on the impact of revascularization strategies on total long-term events were available in the literature.
The current work evaluated the efficacy of three different revascularization strategies using a recurrent event methodology, which enabled us to better quantify the burden of morbidity. We found an increased risk of both combined events and an increase in individual components in those patients who received a culprit-vessel-only PCI strategy.
To the best of our knowledge, this is the first work that compared in-hospital CR and delayed CR strategies in terms of long-term recurrent events. In our study, no significant differences were observed in the incidence of recurrent cardiovascular events between them.
Similar results were found in a subgroup analysis of the COMPLETE trial, where no differences were reported in the combined outcome of cardiac death or new MI between an early and a late CR strategy.8 The aim, therefore, of the most recent meta-analysis by Elgendy et al. was to evaluate the effectiveness of four different strategies in STEMI patients with MVD: culprit-vessel-only PCI, CR during primary PCI, in-hospital CR and delayed CR; the reduction in the risk of MACE observed, was irrespective of the timing of the N-IRA revascularization.14 Recently, Gershlick et al., in the long-term follow-up of the CULPRIT trial, assessed the rate of a subsequent event in those patients with a prior nonfatal event. Their research revealed that the culprit-vessel-only PCI group presented the highest rate (32%) when compared with a CR strategy (23.1%). Similarly, in our cohort, we also found a larger rate of recurrent events in those patients with culprit-vessel-only PCI (64.7%), when compared with patients with in-hospital and delayed CR (17.6% vs. 17.6%, respectively). Confirming again the excessive high-risk conferred by the culprit-vessel only strategy when compared with the other two CR strategies.
Timing of complete revascularization and hospital stayThe benefits of CR in total events reduction were consistently observed in our work regardless of whether the N-IRA PCI was performed during hospital admission or after discharge. Even more, no differences were observed between both strategies when analyzing each individual event. Nevertheless, we observed a reduction of up to two days in-hospital stay in the delayed CR group when compared with the in-hospital CR group, being in-hospital CR an independent predictor of longer hospital stay. To date, no study has addressed the potential benefit in terms of hospital length of an N-IRA PCI performed in a post-discharge staged procedure.
In summary, our findings confirm the prohibitive burden of total cardiovascular events in STEMI patients undergoing a culprit-vessel-only PCI strategy. Both, an in-hospital CR or delayed CR strategy, were independently associated with a reduction in recurrent events during a long-term follow-up, observing no differences between them. Thus, a delayed CR may allow us an early and safe discharge in STEMI patients with MVD, probably reducing the costs associated to their prolonged stays.
Study limitationsSome limitations need to be acknowledged. This work is an observational study in which several unmeasured confounders might be playing a meaningful role. First, this is a single-center observational study in which hidden bias might be operating. Second, the choice of which revascularization strategy was left to the discretion of the clinician and interventional cardiologist, an issue that may be a source of selection bias. Third, an immediate revascularization strategy during the primary PCI of the N-IRA lesion was not evaluated.
ConclusionsCompared to culprit-vessel-only PCI strategy, a staged CR approach, either during admission or after discharge, has shown to be associated with a reduction in the risk of recurrent cardiovascular events during a long-term follow-up. Moreover, a delayed CR approach was additionally associated with a reduction in hospital stay, and therefore could allow us an early and safe discharge.
FundingThis work was supported by grants from Centro de Investigación Biomédica en Red Cardiovascular (16/11/00420).
Conflicts of interestThe authors have no conflicts of interest to declare.