Idiopathic ventricular fibrillation (IVF) is diagnosed in patients who survive sudden cardiac arrest (SCA), preferably with documented ventricular fibrillation (VF), without any identifiable structural or electrical abnormality. Current evidence provides limited guidance on the diagnosis and follow-up of these patients. Our aim was to assess the clinical outcomes of survivors of an aborted SCA attributed to IVF.
MethodsWe retrospectively collected clinical data from all patients who survived SCA and implanted a cardiac defibrillator (ICD) between 2005 and 2023.
ResultsA total of 38 patients, 36.8% female, with a mean age of 44±14 years old were included. Median follow-up time was 8.7 years (interquartile range (IQR) 4.7–14.7 years). All patients underwent a comprehensive diagnostic evaluation that excluded structural and coronary disease. During follow-up, underlying diagnoses were established in 34.2% of the whole cohort. Genetic testing, performed in 37.2%, revealed underlying diagnoses in 57.1% of those tested, compared to only 26.3% of patients who did not undergo genetic testing [p=0.035, OR=5.1 (95% confidence interval (CI) 1.2–21.5)]. Mortality was 10.5% (due to non-arrhythmic causes) and 36.8% patients received appropriate therapies with a median time to first ICD therapy of 39 [5.4–47.3] months.
Conclusion(s)Etiological diagnosis and recurrence prediction in patients with IVF remains challenging, even with extensive diagnostic evaluation and long-term follow-up. In our study, genetic testing enhanced diagnostic yield. Consistent with previous findings, our cohort experienced a notable arrhythmic recurrence, with no cardiac deaths, underlining the pivotal role of ICD implantation in these patients.
Fibrilhação Ventricular Idiopática (FVI) refere-se ao diagnóstico atribuído a sobreviventes de paragem cardíaca súbita (PCR) sem cardiopatia estrutural ou causa elétrica identificável após investigação exaustiva. A evidência atual é limitada quanto ao diagnóstico e à história natural destes doentes. O nosso objetivo é avaliar os resultados clínicos de doentes com o diagnóstico de FVI.
MétodosAnálise retrospetiva de dados clínicos de sobreviventes a PCR em ritmo desfibrilhável e que implantaram um cardio-desfibrilhador (CDI) entre 2005 e 2023.
ResultadosForam incluídos 38 doentes, 36,8% mulheres, com uma idade média de 44±14 anos e um tempo de seguimento mediano de 8,7 anos (intervalo inter-quartil: 4,7-14,7 anos). Os doentes foram submetidos a uma avaliação diagnóstica compreensiva, tendo-se excluído doença coronária e doença estrutural em todos. Durante o seguimento, o diagnóstico foi estabelecido em 34,2% de todos os doentes. O teste genético, realizado em 37,2%, levou à identificação da etiologia subjacente em 57,1% dos doentes testados, em comparação com 26,3% de diagnósticos alternativos identificados nos doentes que não realizaram teste [p=0,035, OR=5,1 (95% CI 1,2-21,5)]. A mortalidade foi de 10,5% (devido a causas não-arrítmicas) e 36,8% receberam terapias apropriadas com tempo mediano até primeira terapia de 39 meses [IQR 5,4-47,3 meses].
ConclusãoO diagnóstico etiológico e predição de recorrência de arritmias em doentes com FVI continua a ser um desafio, mesmo após uma avaliação extensiva e um longo seguimento. O teste genético desempenhou um papel crucial na identificação de etiologias subjacentes à FVI. A nossa coorte experienciou uma recorrência arrítmica consistente com achados prévios, mas sem mortes cardíacas, realçando o papel fundamental da implantação de CDI nestes doentes.
Sudden cardiac death (SCD) remains a major, potentially preventable cause of death. It accounts for approximately 50% of all cardiovascular deaths and may present as the first manifestation of cardiac disease in up to 50% of cases.1 In Europe, it is estimated to be responsible for 10–20% of all deaths.1
Incidence increases with age, especially after the fifth decade of life, mostly caused by coronary artery disease or cardiomyopathies.1,2 However, it remains a major cause of death in young individuals, in whom primary arrhythmic syndromes, cardiomyopathies, myocarditis and coronary anomalies are the predominant underlying etiology.1,2
Despite exhaustive clinical assessment with up-to-date diagnostic tools, a significant number of patients with life-threatening ventricular arrhythmias remain without a diagnosis. Idiopathic ventricular fibrillation (IVF) is a diagnosis of exclusion in survivors of sudden cardiac arrest (SCA), preferably with documented VF, when there's no evidence of arrhythmic syndromes, structural, metabolic, or toxicological etiologies.1,2 Its prevalence varies in different studies, ranging from 5% to 37%, mostly due to heterogeneity among centers in the diagnostic evaluation performed in SCA survivors.3,4
Genetic testing could improve diagnostic yield in this heterogenous group of patients, and it has been increasingly performed in recent years.5 However, indiscriminate use of genetic testing might lead to harmful interpretations, assigning significance prematurely to an otherwise ambiguous variant of uncertain significance (VUS). Therefore, current guidelines recommend (class I recommendation) genetic testing when there is a suspected genetic cause (and testing should only include genes with an evident gene disease association) and might be considered in all IVF patients (with a IIB class of recommendation).1
Although IVF prevalence is expected to decline with technical advances of diagnostic tools and the identification of new primary arrhythmic syndromes, current evidence still provides limited guidance on the diagnosis, follow-up, and prognosis of these patients.
ObjectivesThe aim of our study was to assess the clinical outcomes of survivors of an aborted SCA attributed to IVF.
MethodsStudy populationWe screened a total of 2801 patients referred to the São João Local Health Unit, a tertiary center in Porto, Portugal, for ICD implantation between January 2005 and May 2023. All consecutive survivors of SCA with a shockable rhythm were retrospectively screened for IVF.
Demographic characteristics, personal medical history, family medical history and circumstances before SCA were collected. Results from laboratory testing (including toxicological screening), 12-lead electrocardiogram (ECG) (all reviewed by cardiologists), exercise stress test, cardiac imaging [echocardiography, cardiac magnetic resonance (CMR), computed tomography/coronary angiography], provocative pharmacological testing (sodium channel blocker challenge to exclude Brugada syndrome) and endomyocardial biopsy were collected at presentation and patients with abnormalities in these exams were excluded. IVF was diagnosed according to current criteria.1
Genetic testing was not considered as a baseline assessment since the result is only available several weeks after index hospitalization. In fact, the impact of the genetic test result on redefining diagnosis during follow-up was explored in our study. The genetic testing panel was chosen according to initial clinical suspicion (channelopathies and/or cardiomyopathies) or single gene testing was performed if there was a family member with an identified genetic cardiac condition associated with ventricular arrhythmias. Supplementary Table 1 illustrates the panel of genes included in the genetic testing.
The diagnostic investigation was performed at the physicians’ discretion during initial hospitalization, but four investigators reviewed medical records to ensure that appropriate diagnostic criteria were used. All procedures performed during index hospitalization were planned before cases had been labeled as IVF.
Follow-up data, including ICD data, were retrospectively collected, and analyzed from paper and digital records and reviewed by cardiologists. All patients were followed in a specialized cardiac implantable electronic devices outpatient clinic, with regular device evaluations, usually, the day after, one week, three months and then every six months after implantation. This study was approved by the institutional ethics committee.
Statistical analysisCategorical variables are presented as numbers (percentages) and were compared using χ2 or Fisher's exact tests, as appropriate. All continuous variables were tested for normality with one-sample Kolmogorov–Smirnov test. Normally distributed variables were described as mean±standard deviation (SD) and compared using Student's t-test. Non-normally distributed variables were described as median [interquartile range (IQR)] and compared by Mann–Whitney U test. p values <0.05 were considered significant. Statistical analysis was performed in IBM SPSS Statistics version 27.
ResultsThirty-eight patients with IVF met the inclusion criteria and were retrospectively analyzed with a median follow-up time of 8.7 years [IQR 4.7–14.7 years]. Mean age at index event was 36.3±11.7 years old and 63.2% were male. Patient baseline characteristics are summarized in Table 1.
Clinical characteristics of patients with idiopathic ventricular fibrillation.
| Mean age, years ±SD | 36.3±11.7 |
| Median follow-up time, years (IQR) | 8.7 (4.7–14.7) |
| Male (%) | 24 (63.2%) |
| Arterial hypertension | 7 (18.4%) |
| Diabetes mellitus | 2 (5.3%) |
| Dyslipidemia | 6 (15.8%) |
| Current or previous smoking habits | 7 (18.4%) |
| Chronic kidney disease | 2 (5.3%) |
| Atrial fibrillation | 5 (13.2%) |
| Family history of SCD/unexplained sudden death | 6 (15.8%) |
| Previous symptoms | |
| Syncope | 5 (13.2%) |
| Pre-syncope | 2 (5.3%) |
| Palpitations | 1 (2.6%) |
| No symptoms | 30 (78.9%) |
| Baseline electrocardiogram | |
| Normal | 29 (65.8%) |
| Atrial fibrillation | 3 (7.9%) |
| First-degree AVB | 1 (2.6%) |
| Second-degree AVB Mobitz I | 1 (2.6%) |
| Complete RBBB | 2 (5.3%) |
| Incomplete RBBB | 1 (2.6%) |
| Early repolarization pattern | 1 (2.6%) |
AVB: atrioventricular block; IQR: interquartile range; RBBB: right bundle branch block; SD: standard deviation.
All patients received an ICD during the index hospitalization and most patients (86.8%) had a transvenous ICD implanted (65.8% single chamber, 21% dual chamber), while five patients (13.2%) received a subcutaneous ICD.
The initial rhythm was VF in 73.7% and pVT in 26.3% of patients. Cardiac arrest occurred during daily life activities in 52.9%, at rest in 17.6%, during emotional stress in 11.8%, during exercise in 11.8% and while asleep in 5.9% of our cohort. Of note, 13.2% of patients had a previous history of syncope and 15.8% had family history of sudden cardiac death or unexplained sudden death at a young age in a first-degree family member.
Baseline ECG was normal in most patients (65.8%). The most frequent abnormalities found were atrial fibrillation (7.9%), followed by complete right bundle branch block (RBBB, 5.3%) and early repolarization pattern (2.6%).
Diagnostic evaluation included toxicology tests, electrocardiogram, echocardiogram and invasive or non-invasive coronary angiography in all patients, cardiac magnetic resonance (CMR) in 75.7% patients, genetic testing during index hospitalization in 37.2%, electrophysiology study in 21.1%, pharmacological provocative test with flecainide or ajmaline in 13.2%, stress exercise testing in 13.2% and endomyocardial biopsy in 2.6%.
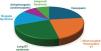
An etiological diagnosis was established in 13 patients (34.2%) during follow-up (Figure 1). In three patients (23%) VF episodes were attributed to vasospasm, three patients (23%) were diagnosed with short-coupled polymorphic ventricular tachycardia (SCPVT), three patients (23%) diagnosed with long QT syndrome (LQTS), two patients (14.4%) with Brugada syndrome, one patient (7.7%) with arrhythmogenic cardiomyopathy and in one patient (7.7%) an Ankyrin-B Syndrome was identified.
Patient in whom genetic testing was performed had an underlying diagnosis identified in 57.1% of cases, compared to only 26.3% of patients who did not have genetic testing performed [p=0.035, OR=5.1 (95% CI 1.2–21.5)]. Besides pathogenic or likely pathogenic variants, two other patients had VUS identified – one patient was found to be heterozygous for RYR2 c.4735G>A (p.Val1579Met) and another patient with VUS in several genes: SCN5A, LAMA4, FLNC and TTN.
During follow-up, 10.5% patients died, all from non-arrhythmic causes, 5.3% developed heart failure and 23.7% developed atrial fibrillation. As for arrhythmic recurrence, 36.4% patients received appropriate anti-tachycardia therapies for VF (in 28.5%) or VT (7.9%), with a median time to first therapy of 39 months [IQR 5.4–47.3 months] (Figure 2). Almost half of the patients (42.8%) with arrhythmic recurrence had an alternative diagnosis established during follow-up. One patient received appropriate therapies for VF in the context of vasospasm, two patients had SCPTV recurrence, two were patients with LQTS and the remaining patient had a diagnosis of Ankyrin-B Syndrome. Most of these patients started or were already on pharmacological treatment – beta-blockers in 42.9%; sodium channel blockers in 14.3%; sotalol in 14,3%; calcium channel blockers 14.3% and amiodarone in 7.1%.
Five patients (13.2%) received inappropriate therapies, due to sinus tachycardia in 60% of patients and other supraventricular tachycardias in the remaining. ICD programming was adjusted in these patients, with no recurrence of inappropriate therapies.
Gender, family history, arrhythmic recurrence during index hospitalization, identification of alternative diagnosis during follow-up and genetic testing results were similar among patients with or without arrhythmic recurrence.
DiscussionDespite technical diagnostic improvement, higher availability, and easier access to diagnostic tests, such as CMR and genetic testing, and a deeper understanding of pathologies and genetic associations, there remains a subset of patients with SCA in whom an underlying cause is not identifiable.
An etiologic diagnosis is of utmost importance as it may guide therapeutic approach and family screening. A recent systematic review showed a comprehensive diagnostic testing yield of 43% in survivors of unexplained cardiac arrest.6 Despite the importance of diagnostic investigation, previous studies showed that diagnostic work-up in IVF is consistently incomplete, leading to underdiagnosis of underlying cardiac pathologies and a worse prognosis.5,7–9
Based on the Dutch national IVF registry, Groeneveld et al.5 demonstrated there is a high testing rate for coronary angiography/coronary computed tomography angiography (96%), CMR (75%), exercise stress testing (70%), sodium channel blocker provocation (SCBP) (63%). Those who had a high number of high-yield tests performed had less chances of being given an alternative diagnosis on follow-up. Compared to these findings, our study also showed a high testing rate regarding coronary angiography (100%) and CMR (73.7%). However, stress testing (13.1%) and SCBP (13.2%) were underused.
Although previous studies underlined the importance of a comprehensive work-up before labeling cases as IVF, there was no standardized set of testing recommended for these patients in previous European guidelines.1 This leads to an underuse of some key investigation tools, as was observed in our cohort.
Consistent with previous reports,2,10 a non-negligible proportion of patients had symptoms prior to cardiac arrest (21.1%), including a preceding syncope in 13.2%. Also, in line with previous findings,3,11 baseline ECG was normal in most of our patients.
Genetic testing showed a high diagnostic yield in our cohort, leading to the identification of the underlying cause of IVF in 57% of patients in whom it was performed. Genetic testing diagnostic yield varies greatly between studies, ranging from 3% to 48%.5,12–17 Differences are explained by heterogeneity in population, testing based on clinical suspicion vs indiscriminate testing, as well as differences in genetic panel selection. The difference between our study results and previous findings might be explained by a longer follow-up and a careful selection of tested genes.
This study includes patients with an index event in the early 2000s, when genetic testing was not as easily available and testing panels were less broad. Since then, our knowledge of different cardiomyopathies and arrhythmic syndromes and their genetic basis has expanded, and novel large gene next-generation sequencing panels might increase the clinical value of genetic testing in patients with IVF. The impact over time of evolving genetic testing panels was not directly assessed in our study. However, 35.7% of genetic tests were performed after 2020 and 85.7% tests were performed after 2010. Only 5.3% of our cohort repeated genetic testing during follow-up and no pathogenic variants were found. Future studies assessing the impact of evolving genetic testing panels and evolving classification of genetic variants are needed and repetition of genetic testing with recent genetic panels might be appropriate in certain cases.
In previous studies,14 up to 30% of patients had one or more VUS identified. The number of VUS increases when larger genetic panels are analyzed, which does not necessarily translate in a significant rise in yield. In a patient group such as IVF patients, with uncertainties regarding missing diagnosis, increasing uncertainty with VUS is unfavorable and appropriate pre- and post-test counseling by a specialized genetic counselor is pivotal. On the one hand, new variants that might be associated with disease might be identified, improving our knowledge. On the other hand, a VUS that might be benign will increase a proband's and relative's anxiety.
In our cohort, the patient with a VUS identified in the RYR gene was assessed in a cardio-genetic specialized consultation. Whole exome sequencing was performed, and information from his parents’ genetic testing was also integrated. The same VUS was identified in his father, who is a healthy subject. Taking all this data into account, it is unlikely that this is a disease-causing variant.
Even though our study included patients labeled as IVF, one-third had an alternative diagnosis identified after extensive work-up and long-term follow-up. One of the most frequent diagnoses at follow-up was short-coupled polymorphism ventricular tachycardia (SCPVT) (23% of all diagnosis). SCPVT is a polymorphic ventricular tachycardia variant initiated by an extremely premature ventricular contraction (<300 ms) arising frequently from peripheral His-Purkinje system.1 Previously considered to be rare, this variant was observed in up to 30% of IVF.3,18,19 Our study emphasizes the hypothesis of this disease as a major trigger in this group of patients.
Interestingly, vasospasm was similarly encountered (23% of all diagnoses) in our cohort during follow-up. This observation supports the potential role of ergonovine, or acetylcholine challenge aimed at provoking coronary spasm during the initial evaluation of an episode of unexplained SCA. However, their use requires careful consideration of safety concerns and patient selection, since these tests have not been evaluated in a trial dedicated do SCA survivors, and results should be interpreted in conjunction with other clinical and diagnostic findings.20–22
Concerning patient clinical outcomes during follow-up, one-third of our patients experienced arrhythmic recurrence after a median time of 39 months [IQR 5.4–47.3 months]. These results are consistent with previous findings,1,8,23,24 highlighting the importance of ICD implantation and maintenance of regular clinical and device assessment.
Contrary to previous findings, we did not find a significant association between arrhythmic recurrence and potential risk factors, such as arrhythmic recurrence during index hospitalization,20 etiological diagnosis achieved during follow-up5 or identification of pathological/probably pathological or VUS variants.16 This might be explained by our small sample size, the heterogeneity in variants found and the small absolute number of patients with an alternative diagnosis identified during follow-up.
LimitationsThe main limitation of our study is its single-center retrospective observational design, which introduces selection bias and concerns regarding generalization and broader applicability. The small sample size does not allow for multivariate analysis to be performed and caution is needed in data generalization. Data should be validated and confirmed in future studies, especially prospective, multi-centric ones.
The broad inclusion period (from 2005 to 2023) might have limited the use of CMR and genetic testing in early recruited patients. Since only a small number of patients repeated CMR and genetic testing during follow-up, that could have led to underdiagnosis of some cardiac pathologies.
ConclusionThis real-life study with one of the longest follow-up times available to date highlights the considerable challenges regarding etiological diagnosis and prediction of recurrence IVF. In our population, genetic testing played a crucial role in enhancing the diagnostic yield, leading to the identification of a distinct clinical entity in a significant proportion of patients. Our cohort experienced arrhythmic recurrence in about one-third of patients, although with an overall good prognosis as shown by the absence of cardiac deaths. These results underline the pivotal role of ICD implantation in these patients.
Learning points/take home messages- 1.
Sudden cardiac death is an uncommon event in the absence of structural heart disease.
- 2.
Etiologic diagnosis in patients with IVF is crucial for patient's management, prognosis definition and family screening.
- 3.
An exhaustive diagnostic evaluation is pivotal to avoid underdiagnosis that might lead to a worse prognosis.
- 4.
Adherence to an adequate diagnostic work-up is variable between centers and in our cohort some of the diagnostic tools were underused.
- 5.
Long-term follow-up and a thorough diagnostic evaluation during follow-up led to identification of an underlying etiology in 34.2% of patients.
- 6.
Genetic testing along with patient's follow-up significantly improved the diagnostic yield.
- 7.
Recurrence of life-threatening arrhythmias occur in a significant number of patients (36.4%), emphasizing the importance of ICD implantation and future studies to define better treatment strategies.
The authors have no conflicts of interest to declare.