Lifestyle changes are frequently insufficient to reduce cardiovascular (CV) risk in patients with dyslipidemia. This study aims to characterize the long-term evolution of lipid profile and CV risk of patients under primary prevention.
MethodsA retrospective study was performed of outpatients at a Portuguese cardiovascular risk clinic with ≥2 CV risk factors, followed for ≥2 years between 1995 and 2015. Statin therapy had been initiated early, in accordance with the clinic's practice. After written informed consent was obtained, sociodemographic and clinical characteristics were collected from medical charts, at baseline and last visit. Changes in lipid profile and CV risk scores were estimated. Associations between HDL-C or LDL-C changes and gender, age, observation time and treatments were assessed through bivariate analysis and multiple linear regression models.
ResultsOut of 516 participants with mean follow-up of 11.4±4.3 years, 56.6% were female and 91.5% received statins. Lipid profile showed statistically significant improvement, including median changes in LDL-C and HDL-C of -77.0 mg/dl and +19 mg/dl, respectively. CV risk also showed statistically significant improvements according to all scores. Statin therapy resulted in a mean HDL-C increase of 7.4 mg/dl (independently of gender and other treatments) and a mean LDL-C reduction of 51.8 mg/dl (irrespective of age and other treatments).
ConclusionResults from this long-term real-life study indicate that primary prevention, specifically early and continuous therapy with intermediate-intensity statins as an add-on to lifestyle interventions, was important in obtaining consistent and adequate metabolic correction in patients with additional risk factors.
Mudar o estilo de vida é frequentemente insuficiente para reduzir o risco cardiovascular (RCV) em doentes com dislipidemia. Pretendeu-se caracterizar a evolução do perfil lipídico e do RCV de doentes em prevenção primária.
MétodosEstudo retrospetivo com doentes com ≥2 fatores de RCV, seguidos por ≥2 anos numa consulta de metabolismo e risco vascular em Portugal, entre 1995-2015. Neste período, as estatinas foram usadas em dosagem intermédia, conforme a prática do centro. Após consentimento informado, recolheram-se dados dos processos clínicos, na consulta inicial e na última visita. Determinaram-se as variações do perfil lipídico e do RCV e foi avaliada a associação com as variáveis sexo, idade, tempo de observação e tratamentos, por análise bivariada e modelos de regressão linear múltipla.
ResultadosDe 516 participantes seguidos, em média, por 11,4±4,3 anos, 56,6% eram mulheres e 91,5% fizeram estatinas. O perfil lipídico apresentou melhorias estatisticamente significativas (variação mediana, LDLc: -77,0 mg/dL; HDLc: +19 mg/dL). O RCV mostrou melhorias estatisticamente significativas em todos os algoritmos. Em média, as estatinas foram responsáveis pelo aumento de 7,4 mg/dL no HDLc (independentemente do sexo e outros tratamentos) e pela redução de 51,8 mg/dL no LDLc (independentemente da idade e outros tratamentos).
ConclusãoOs resultados deste estudo observacional com um longo seguimento indicam que a prevenção primária, nomeadamente a terapia precoce e prolongada com estatinas de dosagem intermédia em combinação com intervenções no estilo de vida, foi relevante para uma compensação metabólica adequada em doentes com RCV adicional.
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in industrialized countries, and is responsible for 45% of all deaths in Europe.1,2 Coronary atherothrombosis and atherosclerosis are important underlying causes of CVD, for which endothelial dysfunction and dyslipidemia are major risk factors, influencing plaque development and rupture.3
Remarkable advances have been seen in both technical and therapeutic innovations for coronary artery disease and acute myocardial infarction, as well as in legislation to reduce tobacco, alcohol and salt consumption.1 However, the pathophysiology of atherosclerosis remains unchanged and endothelial performance continues to be seriously affected by modern lifestyles, with high levels of stress, sedentarism and obesity.4,5 A healthy diet and physical exercise are the core principles for a healthy lifestyle,2 but are frequently insufficient to reduce cardiovascular risk among patients with dyslipidemia or other risk factors. Hence, early treatment with statins for primary prevention of CVD is now recommended for patients with associated risk factors,3,6–9 as well as for secondary prevention.8
Currently, all clinical guidelines recommend primary prevention of CVD, although their approaches, such as intervention models, differ.10,11 Statins are considered the first-line therapy due to their inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase and individual pleiotropic effects.12 Several therapeutic regimens have been proposed, especially in primary prevention,13–15 although there is a lack of consensus regarding their impact on atherosclerotic CVD.9,16 Furthermore, the pharmacological tolerability and long-term consequences of short- and intermediate-term statin therapy have not been assessed.17
The Intensive-intermediate Statin Therapy Observational (ISTO) study was a real-world, retrospective study at a metabolism and cardiovascular risk clinic in a Portuguese public tertiary hospital that aimed to characterize the evolution of lipid profile and cardiovascular risk of outpatients under primary prevention of atherosclerosis, the vast majority with statins at continuous intermediate-intensity doses. The study also explored the effects of this treatment on the most frequent lipid targets – low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C).
MethodsStudy design and populationA retrospective study was performed with medical chart review of outpatients followed at a metabolism and vascular risk clinic at Centro Hospitalar Universitário de São João, in Porto, Portugal. Eligible participants had at least two personal and/or family-related cardiovascular risk factors; attended a quarterly appointment at the clinic, for a minimum of two years between January 1995 and May 2015; initiated primary prevention measures after baseline; had recorded annual biochemical examinations and cardiovascular complementary exams, according to clinical practice; and provided written informed consent. Participants who fulfilled the first eligibility criteria were contacted by the center, between September 2010 and May 2016, to provide informed consent. No study-based intervention was administered but, in the context of real-world clinical practice, participants were mostly treated with statins, which were initiated early for metabolic correction of dyslipidemia. The study was approved by the Ethics Committee of Hospital de São João. All study procedures were compliant with the Declaration of Helsinki and the Guidelines for Good Pharmacoepidemiological Practice.
Study outcomesAfter patients’ written informed consent was obtained, study data were collected from medical records on the first and the last medical appointments (i.e., baseline and final visit, respectively), between January 1995 and May 2015. Patient charts were reviewed to collect baseline sociodemographic characteristics, medical history and lifestyle factors (alcohol consumption, tobacco use and aerobic exercise). Alcohol consumption was classified as never, moderate (one or two drinks daily), excessive (three drinks daily), alcoholism, or abstinence, if a year had passed since the last drink. Patients were classified as non-smokers, ex-smokers or smokers. Regular exercise was defined as at least 150 min per week of moderate-intensity, or at least 75 min per week of vigorous-intensity aerobic activity.18
After baseline assessment, pharmacological treatment was initiated early for metabolic correction of dyslipidemia, as an add-on to lifestyle changes. Thus, according to the standard of care at the clinic, patients without contraindication were treated with intermediate-intensity statin therapy, namely pitavastatin 2 mg/day, simvastatin 20 mg/day, atorvastatin 10 mg/day, rosuvastatin 10 mg/day, fluvastatin 80 mg/day, or pravastatin 20 mg/day. The use of cardiovascular and metabolic comedications that could affect lipid profile (such as corticosteroids, diuretics, or antihypertensives) was collected.
All study participants had annual biochemical monitoring, as part of the clinic's practice. To describe patients’ metabolic and cardiovascular outcomes, lipid profile – LDL-C, HDL-C, total cholesterol, triglycerides and lipoprotein(a) [Lp(a)] – and the inflammatory markers C-reactive protein (CRP) and fibrinogen were compared between baseline and the final visit. Their 10-year vascular risk was estimated using the Framingham risk score, the European Society of Cardiology's Systematic COronary Risk Evaluation (SCORE) for low risk countries, and the atherosclerotic cardiovascular disease (ASCVD) risk estimator.
Data from cardiovascular complementary exams, including ambulatory blood pressure monitoring, electrocardiogram (ECG), two-dimensional echocardiogram and Doppler ultrasonography of the supra-aortic trunks for measurement of carotid intima-media thickness (IMT) (linear probe 7.5 mHz, Sonos 1000, Hewlett Packard, Andover, MA), were also retrieved from medical records as described elsewhere.19,20
Statistical analysisSummary statistics were calculated for all variables and normality of data distribution was assessed with the Kolmogorov-Smirnov and Shapiro-Wilk tests. Nonparametric tests (Wilcoxon signed-rank test and sign test for paired samples) were used to determine the statistical significance of changes in lipid profile, inflammatory markers and cardiovascular risk.
Since LDL-C and HDL-C are the usual treatment targets, we assessed which treatments could explain changes between baseline and final measurements of HDL-C and LDL-C in patients who began treatment at baseline and who did not die during follow-up. First, bivariate analysis was performed to identify variables associated with changes in these parameters, using the Mann-Whitney nonparametric test when comparing two independent groups, and Spearman's correlation coefficient (rs) when considering follow-up duration. Multiple linear regression was then performed to assess the effect of treatment after adjusting for other variables that showed statistically significant differences in the bivariate analysis (p<0.05), or were considered clinically relevant for changes in these lipid parameters. A stepwise method was used to select variables with significance for the optimized regression model.
This was an exploratory study with no formal calculation of sample size. Even so, assuming a conservative approach, a sample size of 516 patients allowed a margin of error of 5% for a confidence interval of 95%. All statistical tests were two-tailed, considering a significance level of 5%. The statistical analysis was conducted using IBM SPSS® Statistics 19.
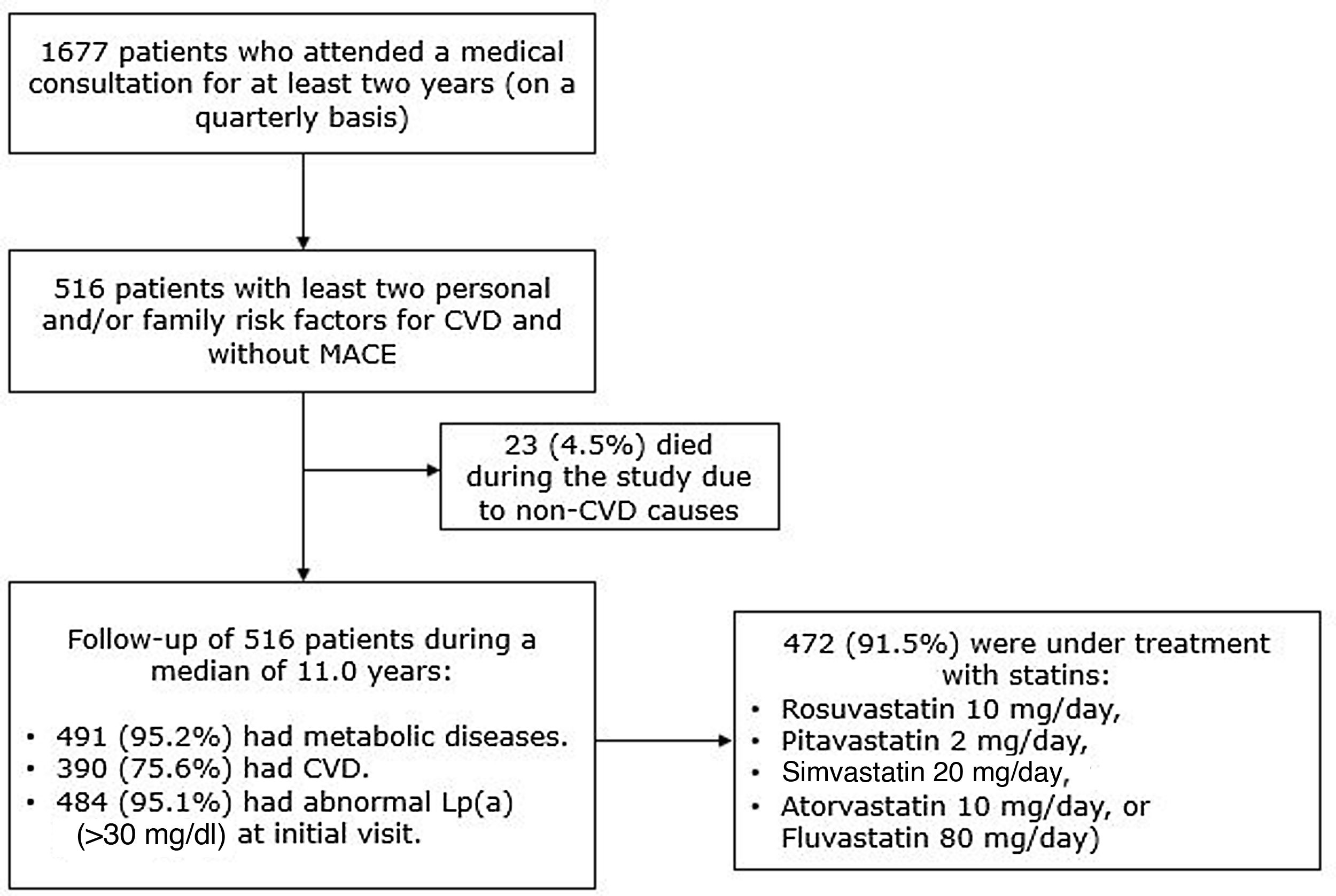
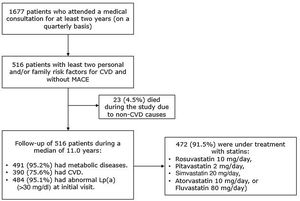
ResultsPatient characteristicsOut of a total of 1677 potentially eligible patients, 516 provided written informed consent and were included in the study (Figure 1). The follow-up duration (mean ± standard deviation) was 11.4±4.3 years (median 11.0). Baseline characteristics of the study participants are summarized in Table 1 and have been described previously.19,20 Briefly, patients’ median age was 46 years at baseline, 56.6% were female and 98.6% were Caucasian. Reductions in tobacco use (13.0% vs. 4.7%) and high alcohol consumption (22.5% vs. 4.9%) and an increase in regular aerobic exercise (28.1% vs. 72.0%) were observed.
Sociodemographic characteristics of study participants at baseline and final visit.
| Baseline | Final | |
|---|---|---|
| Age group (years) | ||
| <20 | 14 (2.7) | 1 (0.2) |
| 20-34 | 104 (20.2) | 36 (7.0) |
| 35-49 | 177 (34.3) | 118 (22.9) |
| 50-65 | 144 (27.9) | 183 (35.5) |
| ≥65 | 77 (14.9) | 178 (34.5) |
| Female gender | 292 (56.6) | |
| Caucasian | 509 (98.6) | |
| Married/living with a partner | 385 (74.6) | 391 (75.8) |
| Educational levela | ||
| Illiterate | 13 (2.5) | 10 (1.9) |
| Elementary school | 312 (60.5) | 293 (56.8) |
| Middle school | 132 (25.6) | 137 (26.6) |
| Secondary school | 33 (6.4) | 38 (7.4) |
| Higher education | 26 (5.0) | 38 (7.4) |
| Employment status | ||
| Active | 374 (72.5) | 275 (53.3) |
| Retired but active | 68 (13.2) | 95 (18.4) |
| Retired | 17 (3.3) | 38 (7.4) |
| Student | 13 (2.5) | 2 8 (0.4) |
| Disabled | 18 (3.5) | 40 (7.8) |
| Not active | 7 (1.4) | 9 (1.7) |
| Housewife/house husband | 19 (3.7) | 57 (11.0) |
| Current smoker | 67 (13.0) | 24 (4.7) |
| Regular exercise | 145 (28.1) | 371 (72.0) |
| High alcohol consumptionb | 116 (22.5) | 25 (4.9) |
| Overweight (BMI ≥25 and <30 kg/m2) | 186 (36.0) | 194 (37.6) |
| Obesity (BMI≥30 kg/m2) | 201 (39.0) | 182 (35.3) |
| Hypertension | 329 (63.8) | 343 (66.5) |
| Diabetes | 158 (30.6) | 158 (30.6) |
All values are number (percentage).
BMI: body mass index.
The majority of the participants (91.5%; n=472/516) were under treatment with intermediate-intensity statins at baseline. Patients not receiving statins (n=44) were younger (mean age: 28.6±14.8 vs. 48.4±13.8 years), had a liver comorbidity or drug interactions, and were treated mainly with beta-blockers (18.2%; 8/44), fibrates (13.6%; 6/44), and diuretics (13.4%; 6/44).
Changes in lipid metabolism, inflammatory markers and cardiovascular riskStatistically significant changes (p<0.001) were found for triglycerides, total cholesterol and respective fractions (Table 2). From baseline to the final visit, median LDL-C values decreased from 172.0 mg/dl to 92.0 mg/dl and median HDL-C increased from 29.0 mg/dl to 49.0 mg/dl. Median Lp(a) (59.0 mg/dl vs. 30.0 mg/dl) and homocysteine (21.0 mg/dl vs. 10.1 mg/dl) levels also presented a statistically significant reduction. A significant reduction of median IMT was also observed (2.90 mm vs. 1.40 mm).
Changes in lipid parameters, inflammatory markers and cardiovascular risk.
| Baseline | Final | Change | p | |
|---|---|---|---|---|
| Total cholesterol, mg/dl | 287.0 [98;457] | 175.5 [74;281] | -101.0 [-308;88] | <0.001 |
| HDL-C, mg/dl | 29.0 [11.0;134] | 48.0 [23;109] | 19.0 [-74;60] | <0.001 |
| LDL-C, mg/dl | 172.0 [39;299] | 92.0 [37;211] | -77.0 [-224;59] | <0.001 |
| VLDL-C, mg/dl | 24.0 [7;294] | 16.0 [6;155] | -6.0 [-139;45] | <0.001 |
| Triglycerides, mg/dl | 147.0 [18;1506] | 110.0 [21;538] | -42.5 [-968;144] | <0.001 |
| Lp(a), mg/dl | 59.0 [9.9;110.0] | 30.0 [1.0;84.0] | -30.0 [-76.0;4.1] | <0.001 |
| Homocysteine, mmol/l | 21.0 [7.9;44.0] | 10.1 [4.0;39.0] | -9.0 [-35.0;23.0] | <0.001 |
| CRP, mg/dl | 0.80 [0.5;9.7] | 0.50 [0.1;9.5] | -0.10 [-7.8;7.9] | <0.001 |
| Fibrinogen, mg/dl | 369.0 [89;651] | 299.0 [129;811] | -59.0 [-412;292] | <0.001 |
| Carotid IMT, mm | 2.9 [0.0;7.2] | 1.4 [0.1;6.6] | -1.3 [-4.4;1.7] | <0.001 |
| Framingham risk score, % | 32.6 [1.1;99.5] | 10.1 [0.3;84.6] | -12.0 [-79.2;28.6] | <0.001* |
| ASCVD score, % | 20.6 [0.9;95.7] | 7.3 [0.2;74.5] | -3.5 [-87.7;40.1] | <0.001* |
| SCORE, low-risk, countries, % | 2.2 [0.1;27.6] | 0.8 [0.1;8.3] | -0.2 [-14.5;2.8] | 0.001* |
Values are median [range].
ASCVD: atherosclerotic cardiovascular disease risk estimator; CRP: C-reactive protein; HDL-C: high-density lipoprotein cholesterol; IMT: intima-media thickness; LDL-C: low-density lipoprotein cholesterol; Lp(a): lipoprotein(a); SCORE: European Society of Cardiology's Systematic COronary Risk Evaluation; VLDL-C: very-low-density lipoprotein cholesterol.
All p-values from Wilcoxon signed-rank test, except * from sign test.
Regarding vascular risk scores, a significant reduction was observed during follow-up from the median baseline values of the Framingham score (32.6% high risk), ASCVD score (20.6%; high risk) and SCORE (2.2%; moderate risk), with all final scores within the categories of low vascular risk.
Treatment associated with changes in low- and high-density lipoprotein cholesterolNo statistically significant association was observed between follow-up duration and changes in HDL-C (rs=0.02; p=0.723) or in LDL-C (rs=-0.03; p=0.456). Age at baseline was not associated with HDL-C changes (rs=0.05; p>0.05) but a statistically significant association was found with LDL-C changes (rs=-0.24; p<0.0001). In accordance with the bivariate analysis and clinical relevance for HDL-C change, gender (female as reference) and use of statins, angiotensin receptor blockers (ARBs), antiplatelets and serotonin reuptake inhibitors, were selected as the variables entering the initial model (Table 3). The optimized final model revealed that HDL-C increased in patients taking statins by an average of 7.4 mg/dl more than in those who did not, after adjustment for gender (Supplementary Table S1). In addition, female gender had more favorable changes in HDL-C than male gender, after adjustment for statins: on average, the HDL-C increase in men was 5.1 mg/dl less than in women.
Changes in high- and low-density lipoprotein cholesterol between baseline and final visits, by gender and treatment (bivariate analysis).
| HDL-C change | LDL-C change | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Median [range] | p | n | Mean ± SD | Median [range] | p | |
| Gender | ||||||||
| Female | 286 | 21.34±11.71 | 20.00 [-22;60] | <0.001 | 284 | -73.25±39.61 | -75.50 [-222;59] | 0.213 |
| Male | 207 | 16.79±12.00 | 17.00 [-74;46] | 202 | -79.77±42.47 | -80.00 [-224;42] | ||
| Statins | ||||||||
| Yes | 451 | 19.96±10.99 | 19.00 [-22;60] | 0.013 | 446 | -81.08±37.16 | -81.00 [-224;40] | <0.001 |
| No | 42 | 13.67±19.34 | 13.50 [-74;51] | 40 | -18.90±37.59 | -11.50 [-145;59] | ||
| ACEIs | ||||||||
| Yes | 264 | 19.99±10.98 | 20.00 [-22;60] | 0.265 | 261 | -87.23±34.64 | -87.00 [-224;20] | <0.001 |
| No | 229 | 18.78±13.13 | 18.00 [-74;57] | 225 | -62.88±43.70 | -67.00 [-203;59] | ||
| ARBs | ||||||||
| Yes | 211 | 20.31±13.44 | 20.00 [-74;60] | 0.053 | 208 | -87.10±38.57 | -87.00 [-224;20] | <0.001 |
| No | 282 | 18.77±10.84 | 18.00 [-16;57] | 278 | -67.62±40.68 | -71.00 [-186;59] | ||
| Oral antidiabetic drugs | ||||||||
| Yes | 144 | 17.99±12.67 | 18.50 [-74;52] | 0.282 | 140 | -86.31±31.52 | -86.50 [-194;-2] | <0.001 |
| No | 348 | 19.98±11.72 | 19.00 [-16;60] | 345 | -71.71±43.53 | -74.00 [-224;59] | ||
| Antiplatelets | ||||||||
| Yes | 266 | 20.62±11.03 | 20.00 [-22;57] | 0.017 | 264 | -86.56±35.87 | -86.00 [-224;24] | <0.001 |
| No | 226 | 18.06±13.01 | 17.00 [-74;60] | 221 | -63.60±42.92 | -67.00 [-222;59] | ||
| CCBs | ||||||||
| Yes | 193 | 20.02±11.48 | 19.00 [-22;60] | 0.518 | 191 | -88.89±33.89 | -88.00 [-224;20] | <0.001 |
| No | 300 | 19.05±12.38 | 19.00 [-74;57] | 295 | -67.59±42.88 | -69.00 [-203;59] | ||
| Allopurinol | ||||||||
| Yes | 242 | 19.42±12.53 | 19.00 [-74;56] | 0.871 | 238 | -85.58±35.65 | -84.00 [-224;20] | <0.001 |
| No | 251 | 19.43±11.56 | 19.00 [-22;60] | 248 | -66.73±43.49 | -68.00 [-222;59] | ||
| SRIs | ||||||||
| Yes | 386 | 20.33±12.28 | 20.00 [-74;60] | <0.001 | 379 | -74.98±40.62 | -77.00 [-224;59] | 0.295 |
| No | 107 | 16.18±10.54 | 15.00 [-13;51] | 107 | -79.42±41.89 | -80.00 [-203;42] | ||
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; CCBs: calcium channel blockers; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol: SD: standard deviation; SRI: serotonin reuptake inhibitors.
All p-values from nonparametric Mann-Whitney (MW) test: MW(df)=value: p<0.001
Age, statins, angiotensin-converting enzyme inhibitors (ACEIs), ARBs, oral antidiabetics, antiplatelets, calcium channel blockers and allopurinol were selected for the initial regression model, due to their clinical influence on LDL-C variation (Table 3). Results from the optimized final model (Supplementary Table S2) revealed that LDL-C decreased in patients taking statins by 51.8 mg/dl more than in those who did not, after adjustment for ACEIs and ARBs. LDL-C decreased in patients taking ACEIs by 12.6 mg/dl more than in those who did not, after adjustment for statins and ARBs. Moreover, LDL-C decreased in patients taking ARBs by 8.2 mg/dl more than in those who did not, after adjustment for statins and ACEIs.
DiscussionResults from this real-life study with long-term follow-up revealed that primary prevention pharmacological treatment, especially early and continuous therapy with intermediate-intensity statins, was important in obtaining consistent and adequate metabolic correction as an add-on to lifestyle interventions.
Among our treated patients (>90% under statin therapy), we observed statistically significant improvements in lipid profile, including marked increases in HDL-C and reductions in LDL-C. Although the final HDL-C level (48 mg/dl) was less than the proposed therapeutic target of 60 mg/dl,21 this improvement was clinically relevant when framed within the significant reductions in all lipid parameters, especially the 45% reduction in LDL-C to below 2.6 mmol/dl (100 mg/dl),10 and the reduction of Lp(a) by 50%. Previously, our group showed that higher Lp(a) values were strongly associated with all markers of cardiovascular risk, such as carotid IMT, LDL-C and homocysteine, as well as with non-alcoholic hepatic steatosis.19,20 A large prospective population-based study conducted in Europe also showed that Lp(a) and LDL-C were independently associated with CVD risk and that low levels of LDL-C (<2.5 mmol/l) reduced the risk caused by increased Lp(a) levels.21,22 Despite the evidence that Lp(a) is an independent risk factor for CVD, specific therapeutic interventions to lower Lp(a) concentrations are still lacking.23
The results from our real-world study are consistent with those from randomized clinical trials that show clinical benefits from intensive statin therapy, although these were conducted mostly in secondary prevention.24 In secondary prevention, studies on high-dose short- and intermediate-duration statins should be interpreted with caution, since there is little follow-up information. In our study, patients treated with statins showed greater improvement in HDL-C and LDL-C levels and, as reported elsewhere, increased Lp(a) levels,20 even after adjusting for gender and other treatments affecting lipid metabolism.25 Indeed, we observed that in patients under statin therapy, HDL-C increased by 7.4 mg/dl more (independently of gender) and LDL-C reduced by 51.8 mg/dl more than in those not taking statins (independently of ACEI or ARB use). As reported by others, LDL-C levels are directly correlated with carotid IMT progression and the effect of statins on lipid profile results in carotid IMT reduction,26,27 and reductions in atherosclerotic plaque after long-term treatment (around 19.7 months) have been reported.28 In our study, carotid IMT was assessed as a marker of early atherosclerosis,29–31 regularly and by the same physician and device (to reduce measurement variability). We observed a significant reduction in median carotid IMT values, which confirms the efficacy of intermediate-intensity statins.32 We also observed a significant improvement in inflammatory markers (CRP and fibrinogen), similar to the findings of others when analyzing patients under early intermediate-intensive statin therapy.33 The pleiotropic effects of statins include attenuation of the inflammatory process, including lowering CRP and raising nitric oxide levels and thus improving endothelial performance.12 Thus, prolonged statin therapy at intermediate-intensity dosages can be effective in reducing carotid IMT in patients as part of the primary prevention of CVD.
Previously, we showed that Lp(a) decreased by 10.6 mg/dl more in patients taking statins than in those who did not, after adjustment for antiplatelets, allopurinol and antidepressants.19,20 We also reported significant reductions in parameters related to type 2 diabetes, such as hemoglobin A1c and fructosamine, as well as stable insulin secretion supported by improvement in C-peptide profile.20 Recent studies have demonstrated that, despite the overall favorable results of this therapeutic approach, there are some disadvantages, such as the occurrence of type 2 diabetes, mainly due to a dose-effect relation.17,34 However, our observations suggest that statins – when used early and continuously at intermediate doses – have positive effects on glucose metabolism and can be useful in patients with high Lp(a) levels for primary prevention of cardiovascular events.35
Regarding overall cardiovascular risk, statistically and clinically significant reductions were observed in the three most common risk scores. These results are consistent with the effect of statins on lipid and inflammatory biomarkers and are in line with other studies.36,37 However, the use of vascular risk scores in primary prevention is not consensual, as it requires transposition of readings and interpretation of results obtained in secondary prevention.2,38 They should therefore be part of a comprehensive patient approach that includes physical, psychological and behavioral factors,39 as well as family history,40 and consideration of other vascular biomarkers such as Lp(a).41
The ISTO study presents some limitations that should be taken into consideration. First, its observational and retrospective design limited assessment of confounding factors and indication bias regarding the effects of statin therapy on lipid profile changes, despite adjustment for all treatments that potentially affected metabolism. Information missing from patients’ medical charts limited possible analyses, such as those related to treatment regimens and patient adherence to statins and lifestyle strategies. Despite the long observation period (1995 to 2015), the duration of patient follow-up was not associated with changes in either HDL-C or LDL-C, and our clinical practice remained stable during this period, irrespective of guideline updates, with all patients undergoing primary prevention with intermediate-intensity statins. A survival bias was introduced in our study, due to the ethical requirement for written informed consent. Finally, caution should be exercised when generalizing our single-center results, since our setting differs from the primary care in which most patients begin primary prevention of CVD.
One of the major strengths of the ISTO study is that it provides real-world evidence of the effect of statin therapy over a long follow-up period. Our results are in line with those of the WOSCOPS study and its post-hoc analysis, which demonstrated the significant benefits of early and continuous use of intermediate-intensity statins in primary prevention.37,42,43 Nevertheless, there are other relevant factors associated with improving patients’ lifestyles that might have influenced this outcome.44 Indeed, we observed significant lifestyle changes, including reductions in weight and smoking, as well as increased exercise. Moreover, a reliable physician-patient interaction is crucial to implementing early-onset therapy and keeping a patient without cardiovascular events motivated to follow clinical guidance, for long-term results.24,45
ConclusionsThis real-world study with a long follow-up suggests that primary prevention strategies, specifically early and continuous therapy with intermediate-intensity statins combined with lifestyle measures, promote consistent and adequate metabolic correction and long-term cardiovascular benefits among patients with additional risk factors. Further studies will be conducted to corroborate the improvement in Lp(a) for therapeutic intervention in primary prevention.
FundingThe authors have no funding to declare.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank CTI, Clinical Trial & Consulting Services Portugal, for the statistical analysis and editorial support provided for this manuscript.