The Ross procedure is an alternative to standard aortic valve (AV) replacement in young and middle-aged patients. However, durability and incidence of reoperation remain a concern for most cardiac surgeons. Our aim was to assess very long-term clinical and echocardiographic outcomes of the Ross procedure.
MethodsWe conducted a single-center retrospective analysis of 56 consecutive adult patients who underwent the Ross procedure. Mean age at surgery was 44±12 years (range, 16-65 years) and 55% were male. Clinical endpoints included overall mortality and the need for valve reoperation due to graft failure. The echocardiographic endpoint was the presence of any graft deterioration. Median clinical follow-up was 20 years (1120 patient/years).
ResultsIndications for surgery were dominant aortic stenosis in 50% and isolated aortic regurgitation in 21%. Concomitant mitral valve repair was performed in 21% and a subcoronary technique was most commonly used (86%). Overall long-term survival was 91%, 80% and 77% at 15, 20 and 24 years, respectively. The survival rate was similar to the age- and gender-matched general population (p=0.44). During the follow-up period, freedom from graft reoperation was 80%. Eleven patients (31%) developed moderate AV regurgitation, three (8.6%) developed moderate pulmonary regurgitation and one (2.9%) presented moderate pulmonary stenosis.
ConclusionThe Ross procedure, mostly using a subcoronary approach, proved to have good clinical and hemodynamic results, with low reoperation rates in long-term follow-up. Moderate autograft regurgitation was a frequent finding but had no significant clinical impact.
A cirurgia de Ross é uma alternativa à utilizada na substituição da válvula aórtica em indivíduos jovens e de meia-idade. Contudo, a durabilidade e a incidência da reoperação permanecem uma preocupação para os cirurgiões cardíacos. O objetivo foi avaliar os resultados clínicos e ecocardiográficos a longo prazo da cirurgia de Ross.
MétodosAnálise retrospetiva de centro-único em que foram analisados 56 doentes adultos consecutivos submetidos à cirurgia de Ross. A idade média na cirurgia foi 44±12 anos (intervalo, 16 a 65 anos) e 58% homens. Os endpoints clínicos incluíram mortalidade global e reintervenção valvular por falência de um dos enxertos. Os endpoints ecocardiográficos incluíram presença de deterioração valvular dos enxertos. A mediana do seguimento clínico foi 20 anos (1.120 doentes/ano).
ResultadosA indicação operatória foi, em 50% dos doentes, predomínio de estenose aórtica e 21% de regurgitação. A cirurgia valvular mitral concomitante foi realizada em 21% e a técnica subcoronária foi a mais utilizada (86%). A sobrevida foi 91%, 80% e 77% aos 15, 20 e 24 anos, respetivamente, sendo sobreponível à da população em geral, ajustada para a idade e sexo (p=0,44). A sobrevida-livre de reoperação foi 80%. Onze doentes (31%) apresentavam regurgitação aórtica moderada; 3 (8,6%) regurgitação pulmonar moderada e 1 doente (2,9%) estenose pulmonar moderada.
ConclusãoA cirurgia de Ross, em que foi usada maioritariamente a abordagem subcoronária, mostrou bons resultados clínicos e hemodinâmicos, com baixas taxas de reoperação no seguimento a longo prazo. A regurgitação moderada do autoenxerto foi uma constatação frequente embora sem impacto clínico considerável.
In the Ross procedure, originally introduced in 1967,1 a diseased aortic valve (AV) is replaced by the native pulmonary valve (autograft) and a homograft is implanted in the pulmonary position. Several advantages have been reported in young and middle-age adults compared to mechanical valves: no need for anticoagulation, low risk of endocarditis and thromboembolism, similar hemodynamic performance to the native valve and good quality of life, without restrictions on physical activity.
Although the procedure was popular in the 1990s, today it is rarely used in adults and the number of surgical centers where it is performed is limited because of its complexity.2 Moreover, it requires detailed surgical knowledge of the anatomy of the aortic root and right ventricular outflow tract.
Graft durability remains a concern, especially considering the reintervention rate due to autograft failure. The international guidelines raise major concerns about the procedure, which is suggested only for patients of childbearing age and for those wishing to avoid anticoagulation.3,4
A small number of long-term studies have demonstrated a similar survival rate compared to the age- and gender-matched general population, as well as freedom of reintervention comparable to mechanical AV replacement (mar).5–11 However, there are few data on late outcomes of this operation. Our aim was to assess very long-term clinical and echocardiographic results of the Ross procedure in adult patients.
MethodsStudy populationWe carried out a retrospective observational single-center analysis that included all consecutive patients (aged ≥16 years) who underwent a Ross procedure in our institution between January 1992 and December 1999. Patients under 16 years old were excluded since other associated congenital defects are often present and the prognosis is different from adults.8
In total, 56 patients were included. Data on patient and surgery characteristics were obtained from hospital medical records and the surgical database.
Surgical procedure and postoperative assessmentThe choice of the Ross procedure as an alternative treatment for aortic valve disease was discussed with all patients with an active lifestyle and a desire to avoid lifelong anticoagulation therapy. Surgery was performed after informed consent was obtained. The surgical technique has been described previously.12 Briefly, all patients were operated under standard cardiopulmonary bypass (CPB) conditions with moderate systemic hypothermia, and cardioplegia was administered by anterograde and retrograde infusion. The pulmonary valve was explanted after careful inspection of its structure, and right ventricle to pulmonary artery continuity was re-established using a cryopreserved pulmonary homograft from a local heart valve bank. Two operative techniques were used to implant the autologous pulmonary valve in the aortic position: subcoronary implantation and aortic root replacement (partial with implantation of one coronary artery or total with implantation of both coronaries).12,13 Although the subcoronary technique was more often used (n=48; 86%), the choice between the two main Ross procedure techniques was dependent on aortic root and pulmonary size, presence of aortic root disease, coronary artery anatomy and the surgeon's preference.14
From August 1996, detailed measurements of the pulmonary and aortic valve leaflets were routinely performed in surgery to choose the correct orientation of the pulmonary autograft in relation to the recipient aortic root. Our group has previously reported a method to determine the best fitting position for the autograft in the aortic root that would cause the least distortion and possibly less regurgitation after surgery.15
Intraoperative transesophageal echocardiography was performed in all patients, before and after CPB, to assess the morphology and function of both autograft and homograft and also possible complications, particularly related to left ventricular systolic function. Before discharge from hospital, all patients underwent transthoracic echocardiography.
Follow-upClinical follow-up was performed by the referring cardiologists. Follow-up data were collected from hospital medical records or, if clinical follow-up was performed in a different hospital, by phone contact with the referring cardiologist, patient or family member. Vital status and date of death were obtained from the national registry. Echocardiographic assessments were scheduled according to the preference of the patient's cardiologist.
Clinical follow-up was complete in all patients. Patients who were reoperated on the AV continued to be monitored and were included in the survival analysis, but were excluded from the echocardiographic analysis. Overall, all patients who were alive and who were not reoperated on the homograft or autograft had echocardiographic follow-up (71% performed in our center).
Clinical and echocardiographic endpointsThe clinical endpoints were overall mortality and valve reoperation due to graft failure (any surgical intervention performed due to autograft or homograft degeneration or endocarditis). The echocardiographic endpoint was defined as graft dysfunction with at least moderate regurgitation or stenosis with a mean gradient of ≥20mmHg, in accordance with the European guidelines on valve disease.16 Quantification of aortic regurgitation as at least moderate was defined by the following criteria: effective regurgitant orifice area ≥10mm2 or regurgitant volume ≥30ml, pressure half-time ≤500ms, and ratio of aortic jet width to left ventricular outflow tract diameter >25%.
The survival rate of the study population was compared with that of the general population. Age, gender and surgery-year survival estimates for the general population were obtained from life tables published online by the Portuguese National Statistics Institute.
Statistical analysisContinuous variables were described as mean ± standard deviation, or median and interquartile range (IQR) for variables with non-normal distribution. Categorical variables were represented as percentages. Normality of distribution was assessed with the Kolmogorov-Smirnov test. Cumulative rates of all-cause death and reoperation were estimated using the Kaplan-Meier method. The one-sample log-rank test was used for comparisons with the age- and gender-adjusted standard population. Two-sided p values <0.05 were considered statistically significant. The statistical analysis was performed with IBM SPSS version 22.0.
ResultsBaseline characteristics of the study populationThe preoperative clinical characteristics of the population and surgical data are shown in Tables 1 and 2, respectively. Ages ranged from 16 to 65 years (mean age 44 years) and 55% were male. The main etiologies of aortic disease were rheumatic (32%), bicuspid valve (29%) and calcified tricuspid (27%). The indication for surgery was stenosis in 50%, pure regurgitation in 21% and mixed aortic disease in 29% of cases. Most patients had preserved ejection fraction and concomitant mitral repair intervention was performed in 21%.
Preoperative characteristics of the study population (n=56).
| Age at surgery, years | 44±12 |
| Age group, years | |
| 16-30 | 7 (13%) |
| 30-49 | 27 (48%) |
| ≥50 | 22 (39%) |
| Male gender, n (%) | 31 (55%) |
| BMI, kg/m2 | 24±4 |
| Hypertension, n (%) | 6 (11%) |
| Diabetes, n (%) | 10 (18%) |
| Smoking, n (%) | 2 (3.6%) |
| Aortic valve disease, n (%) | |
| Rheumatic | 18 (32%) |
| Bicuspid | 16 (29%) |
| Calcified tricuspid | 15 (27%) |
| Myxomatous degeneration and prolapse | 4 (7.1%) |
| Endocarditis | 3 (5.4%) |
| Aortic valve lesion, n (%) | |
| Stenosis | 28 (50%) |
| Regurgitation | 12 (21%) |
| Mixed lesion | 16 (29%) |
| Significant mitral disease, n (%) | 11 (20%) |
| Ejection fraction, n (%) | |
| Normal (>55%) | 48 (86%) |
| Mildly impaired (45-55%) | 2 (3.6%) |
| Moderately impaired (30-45%) | 3 (5.4%) |
| Atrial fibrillation (%) | 8 (14%) |
BMI: body mass index.
Surgical data.
| Mean CPB time, min | 153±23 |
| Concomitant procedure, n (%) | |
| Mitral valve repair | 12 (21%) |
| Ascending aorta replacement | 3 (5.4%) |
| CABG, n (%) | 3 (5.4%) |
| Autograft implantation technique, n (%) | |
| Subcoronary | 48 (86%) |
| Root replacement | 8 (14%) |
| Length of hospital stay, days | 9 (IQR: 7-10) |
CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; IQR: interquartile range.
The subcoronary implantation technique was used in 86% of cases. Mean CPB time was 153±23min and median length of hospital stay was nine days (IQR: 7-10).
Clinical and echocardiographic follow-upThe median clinical follow-up was 20 years (IQR: 19-23; 1120 patients/year) and ranged from one to 24 years in all patients and from 16 to 24 years in living patients.
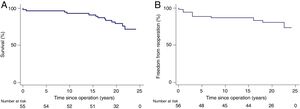
Overall survival, including those who were reoperated for any cause, was 93%, 91%, 80% and 77% at 10, 15, 20 years and the final follow-up, respectively (Figure 1). Overall 30-day mortality was one patient, due to hemorrhagic shock.
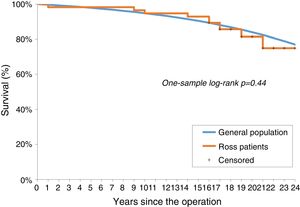
No significant difference was observed between the survival of the Ross population and that of the age- and gender-adjusted standard population (one-sample log-rank test standard mortality ratio 1.24; 95% confidence interval: 0.67-2.28, p=0.44; Figure 2).
Freedom from reintervention due to graft failure was 87.5%, 82.1% and 80.4% at 15, 20 and 24 years, respectively. A total of 11 patients (19.6%) required graft-related reoperation after the initial Ross procedure. Kaplan-Meier curves for reintervention are shown in Figure 1.
The main indication for reoperation was severe autograft regurgitation, except for one patient with homograft stenosis. There was no concomitant replacement of the ascending aorta in patients who underwent reintervention. Eight patients required reoperation due to mitral and/or tricuspid disease.
In the subgroup of patients who underwent the subcoronary technique (n=48), the rate of reoperation was 13% (n=6) and 19% (n=9) at 15 and 24 years, respectively. There was a small difference in the reoperation rate compared with the much smaller group who underwent root replacement technique (19% vs. 25%, p=0.68 respectively), without statistical significance.
After discharge the incidence of stroke at follow-up was 7% (n=4), one of them related to endocarditis, and there was no hemorrhagic stroke. At the latest follow-up, 37 (86%) patients were in New York Heart Association functional class I, five (12%) in class II, and one (2%) in class III.
Median length of echocardiographic follow-up was 19 years (IQR: 17-21 years). Among patients who were not reoperated and who were alive at the end of follow-up (n=35), 11 patients (31%) had moderate and one had severe autograft regurgitation. In the other patients, autograft regurgitation was mild (n=20; 57%) or absent (n=3; 8.6%). No autograft stenosis was observed. Regarding the pulmonary homograft, only three patients had moderate regurgitation and the remainder had mild or no regurgitation. Four patients had mild and one patient had moderate stenosis (Figure 3). At 24 years, freedom from moderate or severe aortic regurgitation was 66%. Except from the patient with severe autograft regurgitation, there was no left ventricular dysfunction or dilatation.
By the end of follow-up, two patients had dilated aortic root combined with moderate secondary autograft regurgitation and five had isolated dilatation of the ascending aorta (45-50mm in three patients and >50mm in two).
Morphological assessment of the pulmonary cusp dimensions was performed in 17 patients (30%). There was no significant difference in reintervention rate (18% [n=3] vs. 21% [n=8], p=0.81) or incidence of moderate or severe aortic regurgitation in this subset of patients compared to the others.
DiscussionIn recent years, there has been growing interest in the Ross procedure, with several registries showing excellent long-term outcomes after the operation.5–11,17 Mortality and valve-related complications after the procedure are very low and quality of life, another important factor, is better compared to patients receiving a mechanical valve.
We report a long-term follow-up of young and middle-aged adults who underwent the Ross procedure at our center, and reveal good results regarding overall mortality and reintervention due to graft failure. Overall survival was 80% at 20 years and 77% at final follow-up. The survival rate was similar to that of the age- and gender-matched general population. Twenty percent of patients underwent reoperation due to graft deterioration, mostly due to severe autograft regurgitation. These findings are evidence that the Ross procedure represents an alternative to mAVR, or even the first choice for individuals with isolated aortic disease and no significant aortic annulus dilatation, in specialized centers.
David et al.5 reported 20-year survival of 94% in a cohort of 212 patients (median age 34 years), similar to that of the age- and gender-matched general population; freedom from valve-related reintervention during the same period was 80%. Their overall survival rate was higher than in our study (94% vs. 80%), although the median follow-up was considerably shorter (14 years) and their patients were much younger. Furthermore, freedom from graft reoperation was similar to that of our study (80% vs. 82%). A study by Charitos et al. in 203 patients revealed freedom from graft reoperation of 87% at 15 years,17 compared to 88% in our group in the same follow-up. Findings from other studies have shown similar positive results regarding this procedure, although outcomes differ between series.6–11
Durability and reoperation rates are the major concerns regarding the Ross procedure. However, these complications are strongly influenced by the volume, experience and expertise of the surgical center, as well as by the surgical technique used. The relatively low rate of graft reoperation in our study may be explained by the significant proportion of patients who underwent a subcoronary technique, as described originally by Ross. The subcoronary approach has been shown to give good clinical results, with lower reoperation rates.18,19
Children were excluded from our cohort, in view of the high prevalence of complex associated defects in children that could alter the prognosis, with more frequent need for reintervention on the pulmonary homograft and right ventricular outflow tract, in comparison with adults.8
Unlike the present study, a recent registry with a long follow-up (median 15 years, maximum 25 years) in 310 patients (mean age 41 years) revealed lower life expectancy after the Ross procedure compared with matched subjects (p<0.0001).20 In our study, the number of patients (n=56) may have been too small to reveal differences between groups. Also, it does not necessarily follow that after more than 20 years of follow-up patients will have a similar survival to the general population during their expected life span. On the other hand, the subcoronary approach was only used in 6% in this registry, and we speculate that this probably contributed to the difference.
In a propensity-matched cohort study in which the Ross procedure was compared to mAVR, survival in the first postoperative decade was similar between the two groups, and to that of the general population.21 Furthermore, Mazine et al. showed in 208 patients after the Ross procedure that freedom from valve-related reintervention and overall survival were comparable to mAVR, but with a significantly lower incidence of major bleeding and stroke in the Ross surgery cohort.6 Also, in a study in a large center, there were no differences in perioperative mortality or neurological complications between these two interventions, despite the greater complexity of the Ross procedure.22
Ross surgery should be considered as a viable alternative to conventional AV replacement in young patients, as they have a greater probability of suffering valve-related complications due to their longer life expectancy. Mechanical valves, by contrast, are associated with various long-term risks, particularly the risk of thrombosis, hence the need for anticoagulation. Additionally, biological prosthetic valves are not a long-term solution due to their limited durability. In a randomized study that compared outcomes of the Ross procedure and aortic homografts, 10-year survival was significantly longer following the former (97% vs. 83%, respectively).23
Even though mostly young patients were selected in this study, 39% of patients were 50 years or older (up to 65 years). We believe that this patient group may also benefit from this procedure.
At least moderate autograft regurgitation was a common finding (34% of patients), which is comparable to previously published studies with shorter follow-up periods, which ranged from 12% at nine years to 15% at 15 years.5,9,11 However, in our study moderate autograft regurgitation had no clinically significant impact in a very long follow-up. Also, pulmonary homograft deterioration was infrequent (three patients with moderate regurgitation and one with moderate stenosis), possibly because of the use of cryopreserved homografts.
Our series, along with numerous previous studies, showed good long-term outcomes following the Ross procedure, which suggests it is time for a hard look at current practices.
LimitationsThis study has some limitations, particularly its retrospective, observational and single-center nature, and its relatively small sample. It is thus more of a descriptive study, and statistical analysis of the small number of events, such as identification of long-term predictors of survival, reoperation or graft dysfunction, was not feasible. However, although the sample was small, the follow-up was very long (median 20 years).
Furthermore, there was no mechanical valve group for comparison, which limited the conclusions that can be drawn. Another limitation is related to the application of two different surgical techniques, although the subcoronary approach was the most used.
ConclusionIn the present study of a single-center cohort of patients who underwent the Ross procedure, mainly using the subcoronary approach, good clinical and hemodynamic results were observed, with low reoperation rates and similar survival to the age- and gender-matched general Portuguese population. These results were similar to previously published series, although these were conducted with a shorter follow-up period. The Ross procedure, using a subcoronary approach, is the surgical technique that has best stood the test of time in terms of durability.
Moderate autograft valve regurgitation was a frequent finding, in agreement with other series, but no significant clinical impact was observed in a median follow-up of 20 years.
Conflicts of interestThe authors have no conflicts of interest to declare.