Patients with advanced heart failure (HF) have high morbidity and mortality, with only a small proportion being eligible for advanced therapies. Intermittent outpatient levosimendan infusion has been shown to provide symptomatic relief and reduce the rate of HF events. Our aim was to assess the safety and efficacy of outpatient levosimendan administration in an advanced HF population.
MethodsThis is a report of a single-center experience of consecutive advanced HF patients referred for intermittent intravenous outpatient administration of levosimendan, between January 2018 and March 2021. Baseline and follow-up evaluation included clinical assessment, laboratory tests, transthoracic echocardiography and cardiopulmonary exercise testing. Baseline and clinical follow-up data were compared using the Wilcoxon signed-rank test.
ResultsA total of 24 patients (60.8 years, 83% male, mean left ventricular ejection fraction [LVEF] 24%), with a median of 1.5 HF hospitalizations in the previous six months, were referred for outpatient levosimendan pulses, the majority as a bridge to transplantation or due to clinical deterioration. At six-month follow-up there was a significant reduction in HF hospitalizations to 0.4±0.7 (p<0.001). NYHA class IV (52.2% to 12.5%, p=0.025) and NT-proBNP (8812.5 to 3807.4 pg/ml, p=0.038) were also significantly reduced. Exercise capacity was significantly improved, including peak oxygen uptake (p=0.043) and VE/VCO2 slope (p=0.040). LVEF improved from 24.0% to 29.7% (p=0.008). No serious adverse events were reported.
ConclusionRepeated levosimendan administration in advanced HF patients is a safe procedure and was associated with a reduction in HF hospitalizations, functional and LVEF improvement, and reduction in NT-proBNP levels during follow-up.
Doentes com insuficiência cardíaca avançada (ICA) apresentam uma elevada morbimortalidade, sendo apenas uma pequena proporção elegível para terapêuticas avançadas. A administração intermitente de levosimendan em hospital de dia demonstrou proporcionar alívio sintomático e reduzir a taxa de eventos de IC. O nosso objetivo foi avaliar a segurança e eficácia da administração intermitente de levosimendan em contexto de ambulatório.
MétodosTrata-se do relato de uma experiência unicêntrica de doentes com ICA consecutivamente referenciados para administração intermitente de levosimendan em ambulatório, entre janeiro de 2018 e março de 2021. A avaliação inicial e de follow-up incluiu uma avaliação clínica, laboratorial e ecocardiográfica, bem como a realização, de prova de esforço cardiorrespiratória. Os dados iniciais e de follow-up foram comparados com recurso ao Wilcoxon signed-rank test.
ResultadosForam referenciados 24 doentes (60,8 anos, 83% do sexo masculino, fração de ejeção ventricular esquerda média [FEVE] de 24%), com uma média de 1,7 hospitalizações por IC nos seis meses anteriores, para pulsos de levosimendan em ambulatório, a maioria como ponte para transplante ou devido a agravamento clínico. Aos seis meses de follow-up, verificou-se uma redução significativa de hospitalizações por IC para 0,4±0,7, p<0,001. Verificou-se igualmente uma redução significativa de doentes em classe NYHA IV (52,2% para 12,5%, p=0,025) e dos níveis de NT-proBNP (de 8.812,5 para 3.807,4 pg/mL, p=0,038). A capacidade funcional melhorou de forma significativa, nomeadamente o consumo máximo de oxigénio (p=0,043) e o declive VE/VCO2 (p=0,040). Houve uma melhoria da FEVE de 24,0% para 29,7%, p=0,008. Não se registaram eventos adversos significativos.
ConclusãoA administração de levosimendan em contexto de ambulatório em pacientes com ICA é um procedimento seguro e conduziu a uma redução das hospitalizações por IC, melhoria da classe funcional e da FEVE e redução dos níveis de NT-proBNP durante o follow-up.
Heart failure (HF) is a progressive condition that affects around 26 million people worldwide and 1–2% of the European population.1,2 It is associated with high symptomatic burden and mortality, as well as a significant impact on health care systems, accounting for around 2% of health expenditure in Western countries,3,4 with 40–60% of this cost being attributable to hospital admissions.5
It is estimated that the prevalence of HF in Portugal will double, affecting 500000 individuals by 2035, with an expected increase in the rate of HF hospitalizations and HF-associated mortality, leading to a significant economic burden.6,7
In recent decades, evidence-based pharmacological and device therapies have led to significant reductions in event rates in this population. However, it is estimated that 1–10% of the overall HF population will progress to an advanced stage of the disease.8,9 Advanced HF is characterized by persistent symptoms despite maximal therapy, extremely poor quality of life (QoL) and a high event rate, specifically recurrent episodes of systemic and/or pulmonary congestion or arrhythmic events that result in recurrent hospitalizations and poor prognosis, with one-year mortality ranging between 25% and 75%.10,11
In advanced HF, heart transplantation (HT) or mechanical circulatory support (MCS) are the treatments of choice, not only improving QoL but also conferring a survival benefit compared to conventional management. However, only a small proportion of advanced HF patients are eligible for HT or MCS.10,11
To fulfill this unmet need in the management of advanced HF patient, infusion of inotropes has been proposed as a bridge to HT or MCS, leading to improvement in symptoms. However, a meta-analysis of randomized trials suggested increased mortality with long-term inotropic support, and this strategy is not currently supported by HF guidelines.11
The inodilator levosimendan has emerged as an interesting therapeutic option due to its unique pharmacological, hemodynamic and cardioprotective properties: its inotropic effect may last for approximately two weeks, despite being less pro-arrhythmic than traditional inotropes.12–14
Three randomized, placebo-controlled, double-blind clinical trials (Levo-Rep, LION-HEART, and LAICA) examined the application of repeated cycles of levosimendan therapy in outpatients with advanced HF, and demonstrated that it reduces N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels and admissions for acute decompensated HF, with a trend towards reduced mortality.15–17 Furthermore, this approach has been shown to be cost-effective, generating savings for health care systems compared to the no-treatment option, mainly driven by a significant reduction in HF hospitalizations.18
Our aim was to assess the safety and efficacy of intermittent outpatient administration of levosimendan in a cohort of advanced HF patients at our center.
MethodsPatient population and study designThis is a single-center prospective observational registry including consecutive patients with a clinical diagnosis of advanced HF referred for intermittent intravenous outpatient administration of levosimendan, between January 2018 and March 2021. Patient enrollment, as well as outpatient levosimendan administration, was interrupted between March and June 2020 due to the covid-19 pandemic.
Patients who met all of the following criteria were considered eligible for the procedure: age >18 years, left ventricular ejection fraction (LVEF) ≤35%, severe and persistent symptoms of heart failure (New York Heart Association [NYHA] functional class III or IV), persistently high NT-proBNP values, at least one HF hospitalization in the previous six months or severe impairment of exercise capacity (peak oxygen uptake ≤12–14 ml/kg/min if the patient was under beta-blocker therapy or a 6-min walk test distance ≤300 m). Exclusion criteria included hemodynamic instability, documented hypersensitivity to levosimendan or any of its metabolites, cardiac conditions associated with impaired cardiac filling or output obstruction (hypertrophic/restrictive cardiomyopathy), and inability to give informed consent.
The investigation conforms to the principles outlined in the Declaration of Helsinki and the institutional ethics committee approved the study protocol. All patients provided written informed consent. Herein, we report results of cases between January 2018 and March 2021.
InterventionLevosimendan was administered every two weeks via a six-hour intravenous infusion (target dose 0.2 μg/kg/min, without bolus) for 12 weeks (six cycles) in an outpatient administration setting with non-invasive monitoring of vital signs. Patients on the active HT list continued treatment after six cycles until a donor was available or significant clinical improvement made the treatment unnecessary.
In the first treatment cycle, a dose of 0.05 μg/kg/min was started, titrated to 0.1 μg/kg/min if well tolerated in the first two hours and to 0.2 μg/kg/min after another two hours of treatment. In patients referred for HT, treatment was maintained after six cycles, in order to prevent further end-organ deterioration that could preclude their eligibility for advanced therapies.
Patient observation during infusion was entrusted to the nursing team, with the following tasks being an integral part of the activity: (1) obtain vital signs, weigh and monitor the patient, collect peripheral venous access and peripheral venous blood gas (to assess potassium level) and a routine blood sample; (2) start therapy according to medical prescription; (3) review the patient's vital signs at 30-min intervals or sooner if required by the patient's clinical condition; (4) monitor the patient's urine output; (5) reinforce patient education to improve self-monitoring of HF, with special emphasis on the importance of therapeutic adherence, triggering factors for HF decompensation, dietary measures and knowledge of the comorbidities of each patient; (6) replace potassium during treatment, if considered necessary; (7) at the end of the treatment, collect peripheral venous blood gas to assess potassium levels, remove the intravenous access, weigh the patient again and collect vital signs. The patient's attending physician was contacted if the patient developed hypotension or electrical instability during treatment. In the event of symptomatic hypotension or systolic blood pressure <75 mmHg without symptoms, the dose was reduced by half (to 0.1 μg/kg/min), and tolerability to this dose assessed later, with a subsequent reduction to 0.05 μg/kg/min or discontinuation of treatment if symptoms persisted. HF therapy was maintained during the day of the procedure and the diuretic dose on the treatment day was halved or maintained according to clinical decision. If venous blood gas analysis documented a potassium level <3.5 mmol/l, potassium supplements were given until a level of 3.5 mmol/l was obtained, to start the treatment with the goal of a potassium level of 4–5 mmol/l. The same goal was used for venous blood gas analysis at the end of the treatment.
Baseline and follow-up assessmentBefore inclusion in the outpatient program, patients underwent a clinical assessment by the attending physician at the outpatient clinic, including laboratory, electrocardiographic, echocardiographic and cardiopulmonary exercise testing (CPET) data. A follow-up visit was scheduled 24 weeks after the outpatient levosimendan administration program was concluded, during which the baseline assessment was repeated, as well as the transthoracic echocardiogram and CPET.
Guideline-directed medical therapy management was at the discretion of the attending cardiologist.
Statistical analysisBaseline characteristics and follow-up workup results were summarized as frequencies (percentages) for categorical variables, as means and standard deviations for continuous variables when normality of distribution was confirmed, and as medians and interquartile range when normality was not confirmed by the Kolmogorov-Smirnov test. Baseline and clinical follow-up data were compared using the Wilcoxon signed-rank test (patients who underwent elective or urgent HT while on outpatient levosimendan infusions or during the first six-month follow-up were excluded from this analysis). A two-tailed p-value of <0.05 was considered statistically significant. All data were analyzed using IBM SPSS 21.0.
ResultsBaseline characteristicsDetailed baseline population characteristics are described in Table 1. Mean age was 61±13 years and 20 patients (83%) were male. Half of the cohort had ischemic etiology, mean LVEF was 24% and mean baseline NT-proBNP was 8813 pg/ml. A high proportion of patients were under HF foundational therapy, with 100% receiving beta-blockers and angiotensin-converting enzyme inhibitors/angiotensin receptor-neprilysin inhibitors; 92% were on mineralocorticoid receptor antagonists (MRA) and 46% on sodium-glucose cotransporter 2 inhibitors. All patients had an implantable cardioverter-defibrillator.
Clinical profile of patients on outpatient levosimendan infusion program (n=24).
| Age, years | 60.8±13.01 |
| Male gender | 20 (83.3%) |
| Ischemic etiology | 12 (50.0%) |
| HF hospitalizations in the previous 6 months | 1.7±0.9 |
| Heart failure medication | |
| Beta-blocker | 12 (100%) |
| ACEi/ARB | 11 (45.8%) |
| ARNI | 13 (54.2%) |
| MRA | 22 (91.7%) |
| SGLT2i | 11 (45.8%) |
| Ivabradine | 4 (16.7%) |
| Digitalis | 2 (8.3%) |
| Oral anticoagulation | 15 (62.5%) |
| Furosemide dose, mg | 84.2±38.7 |
| Ferric carboxymaltose | 14 (58.3%) |
| Cardiac rehabilitation | 6 (25.0%) |
| Hypertension | 13 (54.2%) |
| Dyslipidemia | 14 (58.3%) |
| Diabetes | 10 (41.7%) |
| Current or former smoker | 13 (54.2%) |
| Atrial fibrillation | 11 (45.8%) |
| Previous sustained VT or VF | 9 (37.5%) |
| ICD | 24 (100%) |
| CRT device | 6 (25.0%) |
| CKD | 12 (50.0%) |
| PAD | 4 (16.7%) |
| COPD | 10 (41.7%) |
| Active transplantation list | 9 (37.5%) |
| NYHA class IV, n %) | 13 (54.2) |
| Hemoglobin, g/dl | 13.2±1.7 |
| Creatinine, mg/dl | 1.5±0.5 |
| hs-cTnI, ng/ml | 30.2±20.3 |
| NT-proBNP, pg/ml | 8812.5±6100.9 |
| LVEDD, mm | 70.2±10.0 |
| LVESD, mm | 59.5±12.3 |
| LVEF, % | 24.0±6.2 |
| GLS, % | −4.2±−3.3 |
| TAPSE, mm | 15.7±4.4 |
| LA indexed volume, ml/m2 | 68.9±22.3 |
| PASP, mmHg | 45.5 ±12.9 |
| CPET duration, min | 6.5±3.0 |
| pVO2, ml/kg/min | 11.6±2.8 |
| VE/VCO2slope | 44.9±10.1 |
Values are mean±SD, n (%), or median (interquartile range).
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CPET: cardiopulmonary exercise testing; CRT: cardiac resynchronization therapy; GLS: global longitudinal strain; hs-cTnI: high-sensitivity cardiac troponin I; ICD: implantable cardioverter-defibrillator; LA: left atrial; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; NT-proBNP: N-terminal pro-B-type brain natriuretic peptide; NYHA: New York Heart Association; PAD: peripheral artery disease; PASP: pulmonary artery systolic pressure; pVO2: peak oxygen uptake; SGLT2i: sodium/glucose cotransporter 2 inhibitor; TAPSE: tricuspid annular plane systolic excursion; VE/VCO2: ventilatory efficiency; VF/VT: ventricular fibrillation/ventricular tachycardia.
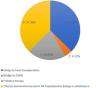
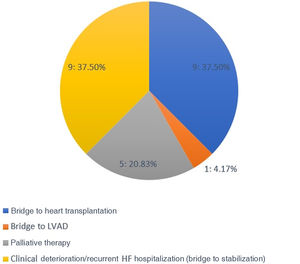
This population was highly symptomatic, with 54% in NYHA class IV, a mean baseline peak oxygen uptake of 11.6 ml/kg/min, and a mean of 1.7 HF hospitalizations in the previous six months. These patients presented a high burden of cardiovascular risk factors and comorbidities, which not only made their management very difficult but also precluded their referral for advanced HF therapies. The majority were referred for outpatient levosimendan infusions due to clinical deterioration with recurrent HF hospitalizations either as a bridge to HT or left ventricular assist device (LVAD), (42%) or bridge to clinical stabilization (37%), and the remainder (21%) were referred as palliative therapy (Figure 1).
There was a significant increase in MRA dosage, from 25.1±17.2 to 31.7±19.3 mg (p=0.026) (Table 2). There were numerically higher dosages of the other HF drugs, although this difference did not reach statistical significance. At six-month follow-up there was a non-significant reduction in loop diuretic dosage (p=0.331).
Changes in heart failure medication dosages.
| Baseline | 6-Month follow-up | p | |
|---|---|---|---|
| Beta-blocker (bisoprolol equivalent dose), mg | 5.5±2.8 | 6.0±2.9 | 0.344 |
| ACEi (ramipril equivalent dose), mg | 2.2±2.2 | 2.4±4.3 | 1.000 |
| ARNI, mg | 37.5±58.3 | 42.8±53.8 | 0.655 |
| MRA (spironolactone equivalent dose), mg | 25.1±17.2 | 31.7±19.3 | 0.026 |
| Loop diuretic (furosemide equivalent dose), mg | 116.3±96.7 | 84.2±38.7 | 0.331 |
Values are mean±SD, n (%), or median (interquartile range).
ACEi: angiotensin-converting enzyme inhibitor; ARNI: angiotensin receptor-neprilysin inhibitor; MRA: mineralocorticoid receptor antagonist.
Each patient underwent a mean of 8.3±5.2 cycles of outpatient levosimendan infusion, with a mean dose per session of 35.6±7.5 μg/min. By the second scheduled infusion, all patients had achieved the target dose of 0.2 μg/kg/min. There were no major safety events and infusions were hemodynamically well tolerated. Intravenous potassium chloride replacement was frequently required, correlated with the diuretic response.
The mean follow-up was 10.2±4.3 months, during which five patients underwent elective HT, all of whom were transplanted while on the outpatient levosimendan infusion program. One patient improved while on the program, and was taken off the HT list.
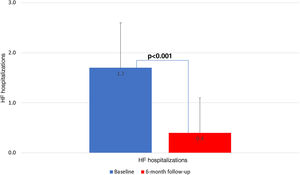
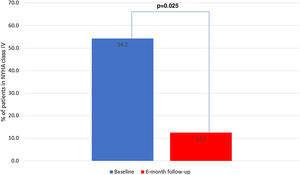
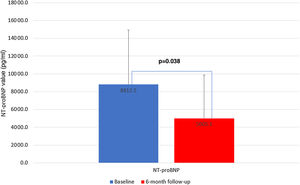
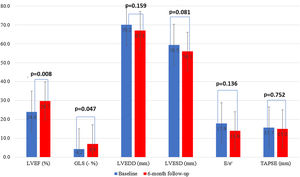
There were five deaths, of which two (40%) were due to cardiovascular causes (end-stage HF and refractory ventricular tachycardia) and occurred during the six-month follow-up period (Table 3). There were seven unplanned HF hospitalizations, four during levosimendan infusion and three during the six-month follow-up. In the first six months of follow-up, there was a significant reduction in HF hospitalizations in comparison with the six months previous to enrollment in the program: 0.4±0.7 vs. 1.7±0.9 (p<0.001) (Figure 2). There was also a significant reduction in the proportion of patients in NYHA functional class IV, from 54.2% to 12.5% (p=0.025) (Figure 3). This was accompanied by a statistically significant reduction in NT-proBNP, from 8812.5±6100.9 to 5005.1±4885.5 pg/ml (p=0.038) (Figure 4). Regarding echocardiographic variables, there was a significant improvement in left ventricular (LV) systolic function at six-month follow-up, with a mean improvement in LVEF of 5.7% (from 24.0±6.2% to 29.7±9.1%, p=0.008) and global longitudinal strain (GLS) of 2.7% (from −4.2±−3.3% to −6.7±−2.6%, p=0.047) (Figure 5). There was no positive effect on either diastolic function or LV filling pressures, assessed by E/e′ (p=0.136) and pulmonary artery systolic pressure (p=0.260), or on right ventricular systolic function, as assessed by tricuspid annular plane systolic excursion (p=0.752).
Changes in echocardiographic parameters between baseline and six-month follow-up. GLS: global longitudinal strain; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; TAPSE: tricuspid annular plane systolic excursion.
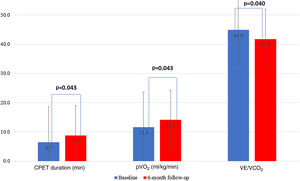
The outpatient levosimendan infusions led to improvement in cardiopulmonary fitness at six months, as shown by an increase in CPET duration (mean improvement of 2.3 min, from 6.5±3.0 to 8.8±4.0 min, p=0.043) and peak oxygen uptake (from 11.6±2.8 to 14.1±4.8 ml/kg/min, p=0.043) and improvement in ventilatory efficiency (VE/VCO2 slope) (from 44.9 to 41.8, p=0.040) (Figure 6).
DiscussionAdvanced HF is associated with marked reductions in QoL, volume overload and/or hypoperfusion, end-organ dysfunction, diuretic resistance and inability to tolerate standard HF therapies, and survival without heart transplantation or LVAD therapy remains extremely poor.10,11 Furthermore, there are few data on the effectiveness of medical therapy for patients with advanced HF and a lack of trials addressing this population. For this reason, management in these patients is less evidence-based than in the earlier stages of the disease. It is thus of paramount importance to integrate palliative care into evidence-based heart failure management in order to improve QoL in this setting.19
A recent trial (LIFE) showed that sacubitril/valsartan on top of optimal medical therapy did not reduce NT-proBNP or improve clinical outcomes among patients with advanced HF and reduced ejection fraction (HFrEF) and comorbidities during a mean follow-up of 24 weeks.20 However, GALACTIC-HF revealed that in patients with HFrEF, those who received omecamtiv mecarbil had a lower risk of a composite of HF events (hospitalization or urgent visit for HF) and cardiovascular death than those who received placebo; this benefit was consistent across most prespecified subgroups, including LVEF, systolic blood pressure and renal function, patients with LVEF ≤28% having a greater benefit.21 This was the first trial showing that an oral inotrope could improve the prognosis of patients with advanced HF. Likewise, in the VICTORIA trial, high-risk HF patients with a recent HF hospitalization or intravenous diuretic use (mean LVEF 29%, 40% in NYHA class III and median NT-proBNP of 2816 pg/ml) receiving vericiguat had a lower rate of cardiovascular death or hospitalization for HF.22
Despite the survival improvement associated with advanced HF therapies, patients on the waiting list for HT or LVAD have a poor QoL and are still at a high risk for HF events, which can lead to functional deterioration and end-organ damage that may render them ineligible for these therapies.10,11 Historically, there has been an interest in drugs that can increase cardiac contractility and cardiac output, however, traditional beta-agonist inotropes are associated with adverse outcomes related to an increase in cyclic adenosine monophosphate (cAMP), which predisposes to life-threatening arrhythmias, cell death and development of tolerance.23 In this context, levosimendan, a calcium sensitizer, has emerged as an interesting therapeutic option, as it does not lead to an increase in intracellular calcium, and is thus less pro-arrhythmic, consumes less energy and does not induce tolerance. Furthermore, the long half-life of its active metabolite confers a long-lasting clinical effect.
Several clinical studies have addressed the use of intermittent outpatient levosimendan infusions in advanced HF patients. Levo-Rep, a prospective, randomized, double-blind, placebo-controlled, multicenter trial that was the largest study on this strategy, revealed that the administration of four cycles of levosimendan therapy did not significantly improve functional capacity or QoL as compared with placebo, despite a numerical trend in favor of levosimendan.15 The LION-HEART trial revealed that biweekly infusions of levosimendan for 12 weeks reduced NT-proBNP, improved QoL and reduced hospitalizations without adverse effects in patients with advanced HF, increasing the probability of survival in Kaplan-Meier curve analysis at 180 days.16 LAICA, which investigated monthly 24-hour infusions of levosimendan for a year in addition to optimal medical therapy, did not demonstrate statistical significance for the primary endpoint, but the results favored levosimendan in terms of both fewer admissions for acute decompensated HF and lower mortality.17 Despite a trend in favor of the repeated use of levosimendan in the majority of studies,14 more robust data on associated hospitalization and mortality rates are needed, like the ongoing LeoDOR trial.24
Our analysis aimed to determine the real-life safety and beneficial effect of six-hour outpatient levosimendan infusions in patients with advanced HF, aiming to fulfill an unmet clinical need in contemporary cardiology and HF therapy. In this regard, our study's protocol was very similar to that of the above-mentioned trials, as we included only highly symptomatic patients with evidence of poor functional status and a recent HF hospitalization, despite optimal medical therapy, in a total of more than 200 outpatient sessions. There were no major safety events and the infusions were well tolerated.
In line with the available literature, our population experienced a significant symptomatic improvement with intermittent outpatient pulses of levosimendan, as there was a 41.7% reduction in patients in NYHA functional class IV at six-month follow-up.15–17 Furthermore, CPET parameters also improved, as assessed by mean improvements in CPET duration, peak oxygen uptake and VE/VCO2 slope. Mushtaq et al. showed that a 24-h cycle of levosimendan significantly improved peak oxygen uptake and reduced VE/VCO2 slope as assessed by CPET performed 24 hours after treatment.25 However, data are scarce regarding the effect of repeated levosimendan cycles on CPET variables and exercise performance, and our work may lay the foundations for further studies in this area.
In the LION-HEART study, outpatient levosimendan treatment led to a significant reduction in NT-proBNP levels, a finding which was replicated in our analysis, with a mean reduction of 3807.4 pg/ml at six months (p=0.038).16 Natriuretic peptide concentrations after treatment have prognostic significance as treatments leading to a greater relative reduction are associated with a better prognosis, and it is well established that discharge natriuretic peptide concentrations are an excellent predictor of one-year death or rehospitalization among patients with acute HF.26 Thus, the reduction of NT-proBNP levels and the improvement in CPET parameters after six months are reflected in a reduction in HF events, as assessed by a significant reduction in HF hospitalizations during follow-up.
Several small studies have assessed the effect of different regimens of levosimendan infusion on various echocardiographic parameters, including LVEF and end-diastolic and end-systolic volumes and dimensions, showing that it is associated with improvements in LV systolic function and some degree of reverse remodeling.27 Similarly, in our analysis treatment was associated with significant improvements in LV systolic function in terms of both LVEF and GLS, with mean improvements of almost 6% and 5%, respectively. Although there was a trend toward reverse remodeling after six months, with reductions in end-diastolic and end-systolic diameters, these differences did not attain statistical significance.
At baseline, all patients were receiving optimal HF medical and device therapy, however, there was a significant increase in MRA dosage at six-month follow-up, as well as numerically higher dosages of the other HF drugs, including beta-blockers and ARNI. This may be either due to frequent patient follow-up that enables uptitration of HF medication or a consequence of levosimendan therapy, as its beneficial effect on cardiac contractility could make uptitration more easily tolerated, without causing symptomatic hypotension. The optimization of HF medical therapy enabled by the levosimendan outpatient program may thus result from the beneficial effects of calcium sensitization.
Our work establishes outpatient levosimendan infusion as a promising approach not only for patients referred for advanced HF therapies (as a bridge to HT or LVAD), reducing morbidity events and preventing end-organ damage that could render these patients ineligible for such therapies, but also to reduce the symptomatic burden of end-stage patients (as palliative therapy). Furthermore, it can also be assumed that it could be useful as a bridge to recovery during the vulnerable post-discharge period after a prolonged hospitalization for acute coronary syndrome or HF, enabling stabilization of LV function and uptitration of neurohormonal blockade medication in patients with a hypotensive profile. This is an important point to address in future trials.
Study limitationsThere are limitations in our study that should be mentioned, including its single-center design and the lack of a control group. Furthermore, some of the endpoints assessed, such as NYHA functional class, are subjective, and it would have been valuable to assess the effect on both symptomatic status and QoL using a more refined tool, such as the Kansas City Cardiomyopathy Questionnaire. Not only may some echocardiographic parameters present significant inter- and intra-observer variability, but they may also be subject to bias, if the operators are not blinded to the intervention. Moreover, the study population was very heterogeneous, including advanced HF patients on the waiting list for advanced therapies as well as patients who underwent the program as part of a palliative approach.
ConclusionIn this single-center experience, outpatient levosimendan administration proved to be a safe strategy and led to significant reductions in natriuretic peptides and HF events at six months, as well as improvement in exercise performance and LV systolic function of patients suffering from advanced HF. It is important to raise awareness of this therapeutic weapon, as it can both help stabilize HF patients eligible for HT or LVAD, preventing further end-organ damage that could render them unsuitable for advanced therapies, and improve QoL and reduce the symptomatic burden of patients referred for palliative care.
Conflicts of interestThe authors have no conflicts of interest to declare.