Breast cancer patients undergoing trastuzumab therapy have greater risk of cardiovascular disease. Risk factors for this effect have been proposed. However, the role of dyslipidemia is not completely understood. This systematic review aimed to explore the role of dyslipidemia in trastuzumab-induced cardiotoxicity.
MethodsThe investigators searched MEDLINE, Scopus, and Web of Science up to October 25, 2020. A random-effects model was used to determine pooled estimates of the results. The primary endpoint was trastuzumab-induced cardiotoxicity in patients with and without dyslipidemia.
ResultsA total of 39 studies were selected for inclusion in our systematic review assessing 21079 patients. One study demonstrated a statistically significant association between dyslipidemia and cardiotoxicity (OR=2.28, 95% CI 1.22–4.26, p=0.01). In all other studies, no such association was observed. Twenty-one studies including 6135 patients were eligible for meta-analysis. In this meta-analysis of unadjusted data, dyslipidemia was significantly associated with cardiotoxicity (OR=1.25, 95% CI 1.01–1.53, p=0.04, I2=0%), however, a subgroup analysis of studies reporting adjusted measures did not demonstrate a significant association (OR=0.89, 95% CI 0.73–1.10, p=0.28, I2=0%).
ConclusionThis systematic review and meta-analysis did not demonstrate a significant association between dyslipidemia alone and the development of cardiotoxicity. In the absence of other relevant cardiovascular risk factors, review of lipid profile may not be obligatory, and management of patients could be performed without referral for cardio-oncology assessment. Further investigation of risk factors for trastuzumab-induced cardiotoxicity is required to confirm these results.
Pacientes com cancro da mama sob tratamento com trastuzumab apresentam maior risco de doença cardiovascular. Fatores de risco têm sido propostos. No entanto, o papel da dislipidemia não é completamente conhecido. Esta revisão sistemática destinou-se a explorar o papel da dislipidemia na cardiotoxicidade induzida por trastuzumab.
MétodosOs investigadores pesquisaram publicações na MEDLINE, Scopus e Web of Science até 25 de outubro de 2020. Um modelo de efeitos aleatórios foi utilizado para determinar as estimativas combinadas dos resultados. A variável de resultado primária foi a cardiotoxicidade induzida por trastuzumab em pacientes com e sem dislipidemia.
ResultadosForam selecionados 39 estudos para inclusão na nossa revisão sistemática, avaliando 21.079 pacientes. Um estudo demonstrou associação significativa entre dislipidemia e cardiotoxicidade (OR=2,28, 95% CI=1,22-4,26, p=0,01). Em todos os restantes não foram observadas semelhantes associações. Foram elegíveis 21 estudos para a meta-análise incluindo 6135 pacientes. Na meta-análise de dados não ajustados a dislipidemia esteve associada significativamente a cardiotoxicidade (OR=1,25, 95% CI=1,01-1,53, p=0,04, I2=0%). No entanto, a análise de subgrupos de estudos que reportaram as medidas ajustadas não demonstrou uma associação significativa (OR=0,89, 95% CI=0,73-1,10, p=0,28, I2=0%).
ConclusãoEsta revisão sistemática e meta-análise não demonstrou uma associação significativa entre dislipidemia isolada e o desenvolvimento de cardiotoxicidade. Na ausência de outros fatores de risco cardiovasculares relevantes, a análise do perfil lipídico nestes pacientes pode não ser obrigatória e a vigilância poderá ser realizada sem referenciação para avaliação por cardio-oncologia. Investigação adicional sobre fatores de risco para cardiotoxicidade induzida por trastuzumab é necessária para confirmar estes resultados.
Treatment of breast cancer has progressed greatly due to advances in systemic therapies, and survival rates have increased.1 Trastuzumab is a monoclonal antibody that targets the human epidermal growth factor receptor 2 (HER-2)/neu oncogene. It is used in combination with chemotherapy in metastatic and (neo)adjuvant settings in breast cancer, demonstrating improvement in survival outcomes and clinical benefit compared to chemotherapy alone.2,3
Trastuzumab therapy causes an increase in lifetime risk of heart failure (HF),4 the incidence of which is higher when combined with anthracyclines.5,6 Although rare (5% in the NSABP B-31 trial and 2.5% in the HERA trial),7 this has important prognostic implications.
In contrast to the well-recognized effect of anthracyclines,8 which have been the focus of research on cancer therapy-related cardiac dysfunction, trastuzumab-induced cardiotoxicity (TIC) is still the subject of debate,9 as it appears not to be dose-dependent, and discontinuation of treatment can often reverse the condition.10,11
Although current guidelines encourage modification of cardiovascular risk factors for patients in this setting,12 the real incidence of TIC, especially in breast cancer patients, is still largely unknown.13
Several potential risk factors for TIC have been proposed. However, their real weight and role as independent predictors are still debated.13
Dyslipidemia is a known risk factor for cardiovascular disease.14 However, the actual susceptibility for TIC of patients with dyslipidemia is not well documented and to our knowledge, there has to date been no systematic review assessing this link.
This systematic review and meta-analysis aimed to explore whether dyslipidemia could be used as a predictor for the development of TIC in breast cancer patients and to quantify its impact.
MethodsThe PRISMA guidelines were followed for the systematic review design.15,16 Patients and the public were not involved in this review.
Search strategyThree electronic databases were searched (MEDLINE, Scopus, and ISI Web of Science) to identify potentially eligible articles using a pre-defined search strategy (Appendix A).
The search encompassed all articles from inception to October 25, 2020.
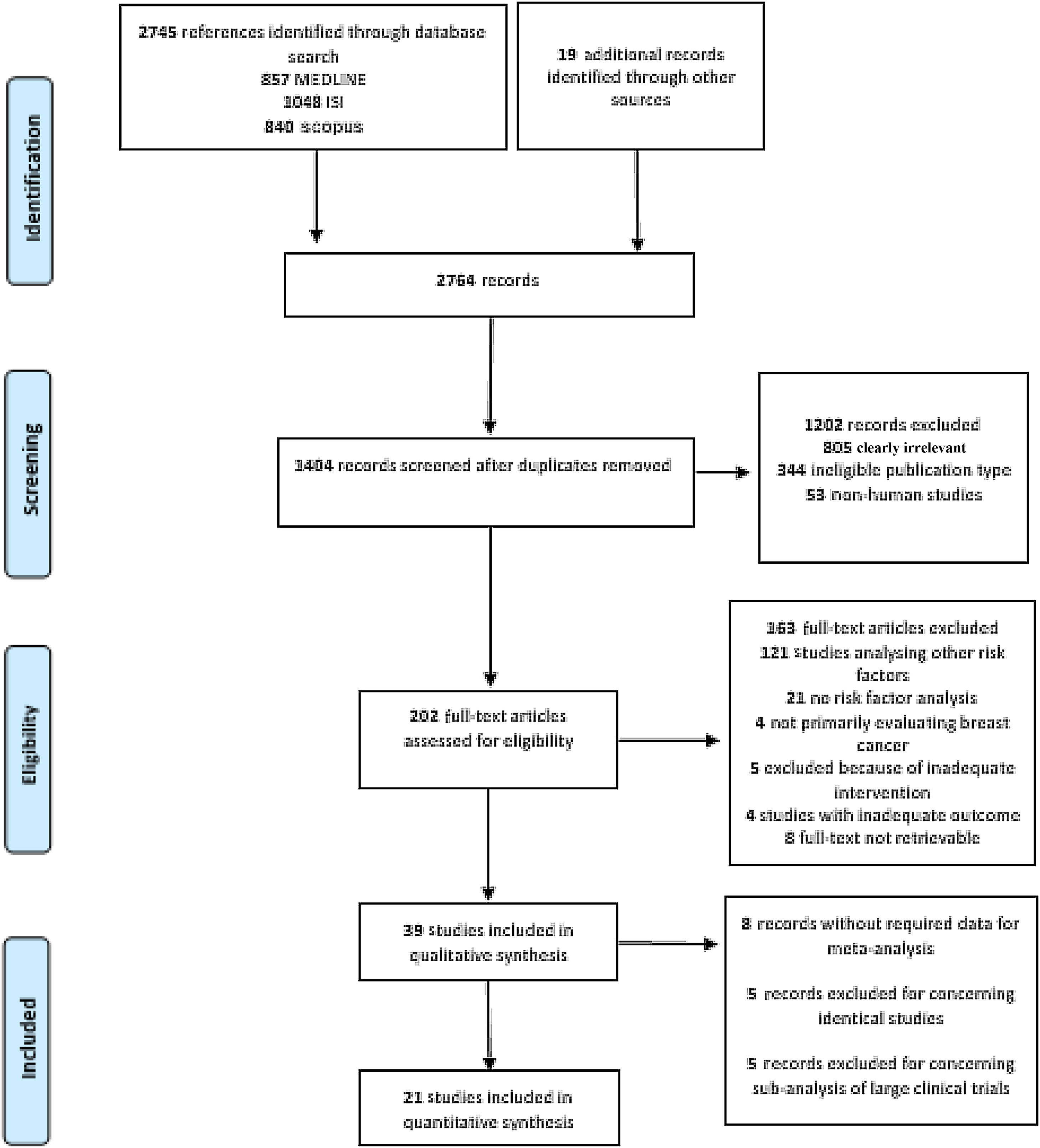
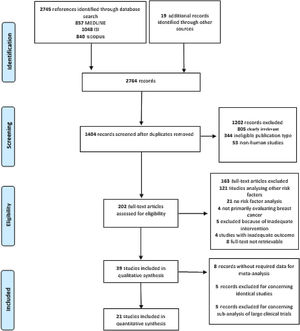
This process resulted in 857 articles in the MEDLINE database, 1048 articles in ISI Web of Science, and 840 in Scopus (Figure 1). A further 19 articles were later identified, mainly through manual searches and citations.
Eligibility criteriaWe considered only human studies assessing the effects of HER-2 directed agents in breast cancer patients undergoing chemotherapy and reporting on independent risk factors. This strategy ensured that articles that did not mention dyslipidemia in their title or abstract due to non-significant results were included. The included studies assessed the impact of risk factors for cardiotoxicity, analyzing at least two study arms, comparing either patients with cardiotoxicity to those without or patients with a given factor to those without. Subsequently, the studies were screened for the role of dyslipidemia in the development of TIC.
Our primary outcome was cardiotoxicity, defined according to the criteria used in the HERA trial17 as symptomatic (e.g. HF and/or dyspnea, and/or referral to a cardiologist) or asymptomatic (e.g. decline in left ventricular ejection fraction [LVEF] >10% from baseline or LVEF <50%). We did not, however, exclude articles that diverged slightly from this definition for the qualitative and quantitative synthesis.
Secondary outcomes consisted of symptomatic cardiotoxicity, discontinuation of trastuzumab due to cardiac causes, recovery of cardiac function after a cardiac event and reintroduction of therapy after discontinuation.
Exclusion criteriaWe excluded (1) studies that mainly focused on anthracycline effects rather than trastuzumab; (2) studies that analyzed outcomes in a pediatric population; (3) non-human studies; (4) studies that followed patients for less than six months; and (5) guidelines, systematic reviews and meta-analyses, case reports, editorials, letters, and/or review articles with no original data.
No articles were excluded based on population size, publication date, or language.
Study selectionAfter removal of duplicates, two reviewers (JFP and MMC) independently screened the articles at title/abstract level according to the predefined inclusion and exclusion criteria. Afterward, the two reviewers (JFP and MMC) independently analyzed the full texts of studies not previously excluded using the same inclusion and exclusion criteria. Disagreements were resolved by consensus with a third reviewer (CDS) serving as final arbitrator. Efforts were made to contact investigators in order to obtain publications not accessible by other means.
Data extractionTwo reviewers (JFP and MMC) independently analyzed the full texts that had met the inclusion criteria and extracted data into a pre-established spreadsheet.
The full text of short-listed articles was systematically appraised for the following items: first author, year of publication, nationality, study setting, study design, number of patients with breast cancer, number of patients treated with anthracyclines and/or trastuzumab, duration of follow-up, patients’ median age and age at diagnosis, method of LVEF assessment, and number of patients developing or not developing cardiotoxicity (or number of patients with and without a given cardiovascular risk factor).
Quality assessment and risk of biasThe risk of bias was assessed at the study level according to the method used by Haffar et al.,18 given that for the most part, the studies included are observational. This method was also used to classify sub-analyses of randomized controlled trials (RCTs) since the primary subject of these studies was not the effect of dyslipidemia. Two reviewers (JFP and MMC) independently analyzed the studies. Any disagreements were resolved by consensus.
Statistical analysisReview Manager® (version 5.4.1)19 was used for statistical analysis and to derive forest plots.
Summary measures and synthesis of resultsThe frequency of a given risk factor was assessed using absolute and relative frequencies. If these were not present, they were calculated.
Odds ratios (ORs) were used as a summary measure. The precision of effect sizes was measured using 95% confidence intervals (CIs) and corresponding p-values for both. We chose the OR since relative estimates are more comparable than absolute effects between studies with different designs, populations, and lengths of follow-up.20
The Cochran Q test (I2)21,22 was used to assess heterogeneity and statistical inconsistency. Small study effects and reporting bias were explored with a visual inspection of asymmetry in funnel plots.23 We classified heterogeneity as 0%, signifying absence of detected heterogeneity; 0–10%, indicating low heterogeneity; 10–50%, indicating moderate heterogeneity; and over 50%, indicating high heterogeneity.
A random-effects model was used to pool data owing to the anticipated heterogeneity in the included trials. In fact, adjusted indirect comparisons that use a fixed-effects model tend to underestimate the standard errors of pooled estimates.24
To better understand the specific effect of dyslipidemia alone as a risk factor for cardiotoxicity, we also performed a pre-planned subgroup analysis involving only studies reporting the most adjusted measures.
ResultsGeneral characteristics of the included studiesThe search returned 2745 records, of which 1407 remained after removal of duplicates. After title and abstract screening, followed by full-text appraisal, 39 articles matched our eligibility criteria and were included in our systematic review.25–63 Afterward, 18 records were excluded from the meta-analysis because of data duplication (n=5), missing data (n=8) or being sub-analyses of large RCTs (n=5), and were thus not suitable for use in pooled estimates in conjunction with observational studies. Hence, 21 studies were further examined by meta-analysis (Figure 1). Table 1 summarizes the characteristics of the included studies.
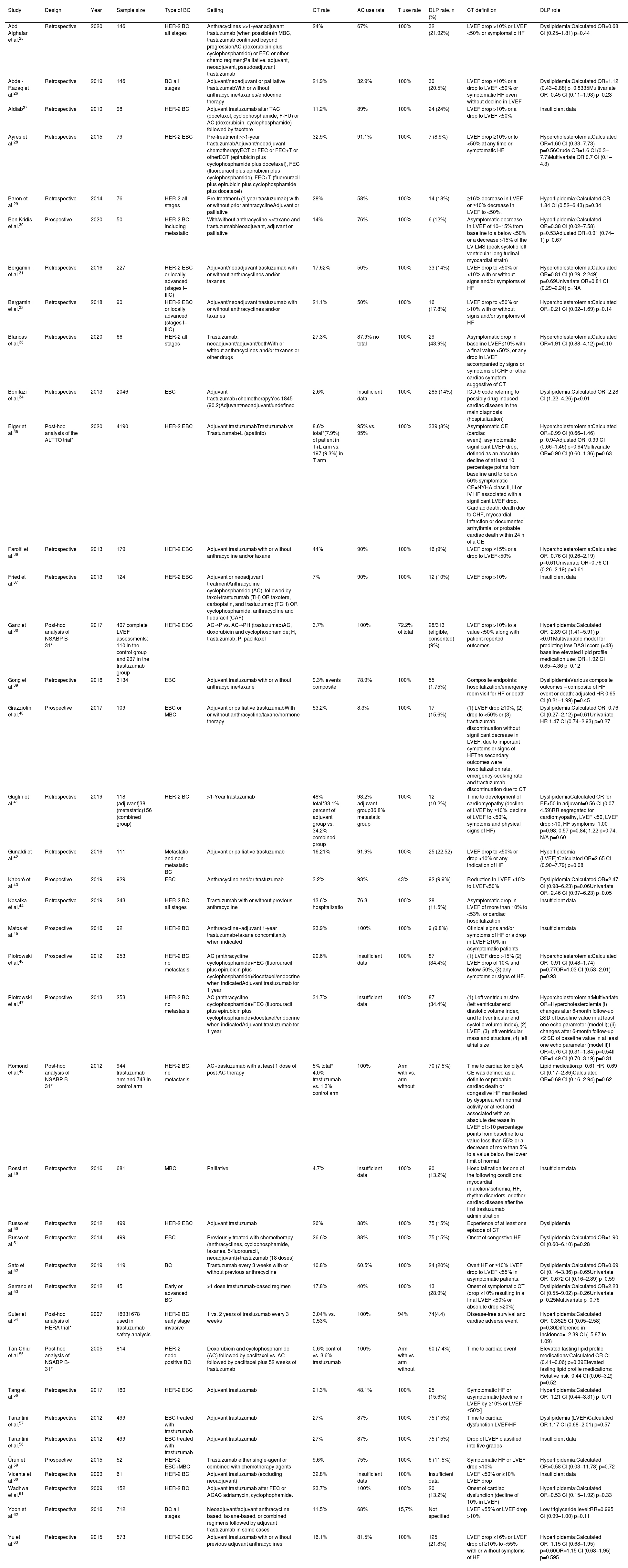
Main features of the included studies.
| Study | Design | Year | Sample size | Type of BC | Setting | CT rate | AC use rate | T use rate | DLP rate, n (%) | CT definition | DLP role |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abd Alghafar et al.25 | Retrospective | 2020 | 146 | HER-2 BC all stages | Anthracyclines >>1-year adjuvant trastuzumab (when possible)In MBC, trastuzumab continued beyond progressionAC (doxorubicin plus cyclophosphamide) or FEC or other chemo regimen;Palliative, adjuvant, neoadjuvant, pseudoadjuvant trastuzumab | 24% | 67% | 100% | 32 (21.92%) | LVEF drop >10% or LVEF <50% or symptomatic HF | Dyslipidemia:Calculated OR=0.68 CI (0.25–1.81) p=0.44 |
| Abdel-Razaq et al.26 | Retrospective | 2019 | 146 | BC all stages | Adjuvant/neoadjuvant or palliative trastuzumabWith or without anthracycline/taxanes/endocrine therapy | 21.9% | 32.9% | 100% | 30 (20.5%) | LVEF drop ≥10% or a drop to LVEF <50% or symptomatic HF even without decline in LVEF | Dyslipidemia:Calculated OR=1.12 (0.43–2.88) p=0.8335Multivariate OR=0.45 CI (0.11–1.93) p=0.23 |
| Aldiab27 | Retrospective | 2010 | 98 | HER-2 BC | Adjuvant trastuzumab after TAC (docetaxol, cyclophosphamide, F-FU) or AC (doxorubicin, cyclophosphamide) followed by taxotere | 11.2% | 89% | 100% | 24 (24%) | LVEF drop >10% or a drop to LVEF <50% | Insufficient data |
| Ayres et al.28 | Retrospective | 2015 | 79 | HER-2 EBC | Pre-treatment >>1-year trastuzumabAdjuvant/neoadjuvant chemotherapyECT or FEC or FEC+T or otherECT (epirubicin plus cyclophosphamide plus docetaxel), FEC (fluorouracil plus epirubicin plus cyclophosphamide), FEC+T (fluorouracil plus epirubicin plus cyclophosphamide plus docetaxel) | 32.9% | 91.1% | 100% | 7 (8.9%) | LVEF drop ≥10% or to <50% at any time or symptomatic HF | Hypercholesterolemia:Calculated OR=1.60 CI (0.33–7.73) p=0.56Crude OR=1.6 CI (0.3–7.7)Multivariate OR 0.7 CI (0.1–4.3) |
| Baron et al.29 | Retrospective | 2014 | 76 | HER-2 all stages | Pre-treatment+(1-year trastuzumab) with or without prior anthracyclineAdjuvant or palliative | 28% | 58% | 100% | 14 (18%) | ≥16% decrease in LVEF or ≥10% decrease in LVEF to <50%. | Hyperlipidemia:Calculated OR 1.84 CI (0.52–6.43) p=0.34 |
| Ben Kridis et al.30 | Prospective | 2020 | 50 | HER-2 BC including metastatic | With/without anthracycline >>taxane and trastuzumabNeoadjuvant, adjuvant or palliative | 14% | 76% | 100% | 6 (12%) | Asymptomatic decrease in LVEF of 10–15% from baseline to a below <50% or a decrease >15% of the LV LMS (peak systolic left ventricular longitudinal myocardial strain) | Hyperlipidemia:Calculated OR=0.38 CI (0.02–7.58) p=0.53Adjusted OR=0.91 (0.74–1) p=0.67 |
| Bergamini et al.31 | Retrospective | 2016 | 227 | HER-2 EBC or locally advanced (stages I–IIIC) | Adjuvant/neoadjuvant trastuzumab with or without anthracyclines and/or taxanes | 17.62% | 50% | 100% | 33 (14%) | LVEF drop to <50% or >10% with or without signs and/or symptoms of HF | Hypercholesterolemia:Calculated OR=0.81 CI (0.29–2.249) p=0.69Univariate OR=0.81 CI (0.29–2.24) p=NA |
| Bergamini et al.32 | Retrospective | 2018 | 90 | HER-2 EBC or locally advanced (stages I–IIIC) | Adjuvant/neoadjuvant trastuzumab with or without anthracyclines and/or taxanes | 21.1% | 50% | 100% | 16 (17.8%) | LVEF drop to <50% or >10% with or without signs and/or symptoms of HF | Hypercholesterolemia:Calculated OR=0.21 CI (0.02–1.69) p=0.14 |
| Blancas et al.33 | Retrospective | 2020 | 66 | HER-2 all stages | Trastuzumab: neoadjuvant/adjuvant/bothWith or without anthracyclines and/or taxanes or other drugs | 27.3% | 87.9% no total | 100% | 29 (43.9%) | Asymptomatic drop in baseline LVEF≤10% with a final value <50%, or any drop in LVEF accompanied by signs or symptoms of CHF or other cardiac symptom suggestive of CT | Hypercholesterolemia:Calculated OR=1.91 CI (0.88–4.12) p=0.10 |
| Bonifazi et al.34 | Retrospective | 2013 | 2046 | EBC | Adjuvant trastuzumab+chemotherapyYes 1845 (90.2)Adjuvant/neoadjuvant/undefined | 2.6% | Insufficient data | 100% | 285 (14%) | ICD-9 code referring to possibly drug-induced cardiac disease in the main diagnosis (hospitalization) | Dyslipidemia:Calculated OR=2.28 CI (1.22–4.26) p<0.01 |
| Eiger et al.35 | Post-hoc analysis of the ALTTO trial* | 2020 | 4190 | HER-2 EBC | Adjuvant trastuzumabTrastuzumab vs. Trastuzumab+L (apatinib) | 8.6% total*(7.9%) of patient in T+L arm vs. 197 (9.3%) in T arm | 95% vs. 95% | 100% | 339 (8%) | Asymptomatic CE (cardiac event)=asymptomatic significant LVEF drop, defined as an absolute decline of at least 10 percentage points from baseline and to below 50% symptomatic CE=NYHA class II, III or IV HF associated with a significant LVEF drop. Cardiac death: death due to CHF, myocardial infarction or documented arrhythmia, or probable cardiac death within 24 h of a CE | Hypercholesterolemia:Calculated OR=0.99 CI (0.66–1.46) p=0.94Adjusted OR=0.99 CI (0.66–1.46) p=0.94Multivariate OR=0.90 CI (0.60–1.36) p=0.63 |
| Farolfi et al.36 | Retrospective | 2013 | 179 | HER-2 EBC | Adjuvant trastuzumab with or without anthracycline and/or taxane | 44% | 90% | 100% | 16 (9%) | LVEF drop ≥15% or a drop to LVEF<50% | Hypercholesterolemia:Calculated OR=0.76 CI (0.26–2.19) p=0.61Univariate OR=0.76 CI (0.26–2.19) p=0.61 |
| Fried et al.37 | Retrospective | 2013 | 124 | HER-2 EBC | Adjuvant or neoadjuvant treatmentAnthracycline cyclophosphamide (AC), followed by taxol+trastuzumab (TH) OR taxotere, carboplatin, and trastuzumab (TCH) OR cyclophosphamide, anthracycline and fluouracil (CAF) | 7% | 90% | 100% | 12 (10%) | LVEF drop >10% | Insufficient data |
| Ganz et al.38 | Post-hoc analysis of NSABP B-31* | 2017 | 407 complete LVEF assessments: 110 in the control group and 297 in the trastuzumab group | HER-2 EBC | AC→P vs. AC→PH (trastuzumab)AC, doxorubicin and cyclophosphamide; H, trastuzumab; P, paclitaxel | 3.7% | 100% | 72.2% of total | 28/313 (eligible, consented) (9%) | LVEF drop >10% to a value <50% along with patient-reported outcomes | Hyperlipidemia:Calculated OR=2.89 CI (1.41–5.91) p=<0.01Multivariable model for predicting low DASI score (<43) – baseline elevated lipid profile medication use: OR=1.92 CI 0.85–4.36 p=0.12 |
| Gong et al.39 | Retrospective | 2016 | 3134 | EBC | Adjuvant trastuzumab with or without anthracycline/taxane | 9.3% events composite | 78.9% | 100% | 55 (1.75%) | Composite endpoints: hospitalization/emergency room visit for HF or death | DyslipidemiaVarious composite outcomes – composite of HF event or death: adjusted HR 0.65 CI (0.21–1.99) p=0.45 |
| Grazziotin et al.40 | Prospective | 2017 | 109 | EBC or MBC | Adjuvant or palliative trastuzumabWith or without anthracycline/taxane/hormone therapy | 53.2% | 8.3% | 100% | 17 (15.6%) | (1) LVEF drop ≥10%, (2) drop to <50% or (3) trastuzumab discontinuation without significant decrease in LVEF, due to important symptoms or signs of HFThe secondary outcomes were hospitalization rate, emergency-seeking rate and trastuzumab discontinuation due to CT | Dyslipidemia:Calculated OR=0.76 CI (0.27–2.12) p=0.61Univariate HR 1.47 CI (0.74–2.93) p=0.27 |
| Guglin et al.41 | Retrospective | 2019 | 118 (adjuvant)38 (metastatic)156 (combined group) | HER-2 BC | >1-Year trastuzumab | 48% total*33.1% percent of adjuvant group vs. 34.2% combined group | 93.2% adjuvant group36.8% metastatic group | 100% | 12 (10.2%) | Time to development of cardiomyopathy (decline of LVEF by ≥10%, decline of LVEF to <50%, symptoms and physical signs of HF) | DyslipidemiaCalculated OR for EF<50 in adjuvant=0.56 CI (0.07–4.59)RR segregated for cardiomyopathy, LVEF <50, LVEF drop >10, HF symptoms=1.00 p=0.98; 0.57 p=0.84; 1.22 p=0.74, N/A p=0.60 |
| Gunaldi et al.42 | Retrospective | 2016 | 111 | Metastatic and non-metastatic BC | Adjuvant or palliative trastuzumab | 16.21% | 91.9% | 100% | 25 (22.52) | LVEF drop to <50% or drop >10% or any indication of HF | Hyperlipidemia (LVEF):Calculated OR=2.65 CI (0.90–7.79) p=0.08 |
| Kaboré et al.43 | Prospective | 2019 | 929 | EBC | Anthracycline and/or trastuzumab | 3.2% | 93% | 43% | 92 (9.9%) | Reduction in LVEF >10% to LVEF<50% | Dyslipidemia:Calculated OR=2.47 CI (0.98–6.23) p=0.06Univariate OR=2.46 CI (0.97–6.23) p=0.05 |
| Kosalka et al.44 | Retrospective | 2019 | 243 | HER-2 BC all stages | Trastuzumab with or without previous anthracycline | 13.6% hospitalizatio | 76.3 | 100% | 28 (11.5%) | Asymptomatic drop in LVEF of more than 10% to <53%, or cardiac hospitalization | Insufficient data |
| Matos et al.45 | Prospective | 2016 | 92 | HER-2 BC | Anthracycline+adjuvant 1-year trastuzumab+taxane concomitantly when indicated | 23.9% | 100% | 100% | 9 (9.8%) | Clinical signs and/or symptoms of HF or a drop in LVEF ≥10% in asymptomatic patients | Insufficient data |
| Piotrowski et al.46 | Prospective | 2012 | 253 | HER-2 BC, no metastasis | AC (anthracycline cyclophosphamide)/FEC (fluorouracil plus epirubicin plus cyclophosphamide)/docetaxel/endocrine when indicatedAdjuvant trastuzumab for 1 year | 20.6% | Insufficient data | 100% | 87 (34.4%) | (1) LVEF drop >15% (2) LVEF drop of 10% and below 50%, (3) any symptoms or signs of HF. | Hypercholesterolemia:Calculated OR=0.91 CI (0.48–1.74) p=0.77OR=1.03 CI (0.53–2.01) p=0.93 |
| Piotrowski et al.47 | Prospective | 2013 | 253 | HER-2 BC, no metastasis | AC (anthracycline cyclophosphamide)/FEC (fluorouracil plus epirubicin plus cyclophosphamide)/docetaxel/endocrine when indicatedAdjuvant trastuzumab for 1 year | 31.7% | Insufficient data | 100% | 87 (34.4%) | (1) Left ventricular size (left ventricular end diastolic volume index, and left ventricular end systolic volume index), (2) LVEF, (3) left ventricular mass and structure, (4) left atrial size | Hypercholesterolemia:Multivariate OR=Hypercholesterolemia (i) changes after 6-month follow-up ≥SD of baseline value in at least one echo parameter (model I); (ii) changes after 6-month follow-up ≥2 SD of baseline value in at least one echo parameter (model II)I OR=0.76 CI (0.31–1.84) p=0.54II OR=1.49 CI (0.70–3.19) p=0.31 |
| Romond et al.48 | Post-hoc analysis of NSABP B-31* | 2012 | 944 trastuzumab arm and 743 in control arm | HER-2 BC, no metastasis | AC+trastuzumab with at least 1 dose of post-AC therapy | 5% total* 4.0% trastuzumab vs. 1.3% control arm | 100% | Arm with vs. arm without | 70 (7.5%) | Time to cardiac toxicityA CE was defined as a definite or probable cardiac death or congestive HF manifested by dyspnea with normal activity or at rest and associated with an absolute decrease in LVEF of >10 percentage points from baseline to a value less than 55% or a decrease of more than 5% to a value below the lower limit of normal | Lipid medication:p=0.61 HR=0.69 CI (0.17–2.86)Calculated OR=0.69 CI (0.16–2.94) p=0.62 |
| Rossi et al.49 | Retrospective | 2016 | 681 | MBC | Palliative | 4.7% | Insufficient data | 100% | 90 (13.2%) | Hospitalization for one of the following conditions: myocardial infarction/ischemia, HF, rhythm disorders, or other cardiac disease after the first trastuzumab administration | Insufficient data |
| Russo et al.50 | Retrospective | 2012 | 499 | HER-2 EBC | Adjuvant trastuzumab | 26% | 88% | 100% | 75 (15%) | Experience of at least one episode of CT | Dyslipidemia |
| Russo et al.51 | Retrospective | 2014 | 499 | EBC | Previously treated with chemotherapy (anthracyclines, cyclophosphamide, taxanes, 5-fluorouracil, neoadjuvant)+trastuzumab (18 doses) | 26.6% | 88% | 100% | 75 (15%) | Onset of congestive HF | Dyslipidemia:Calculated OR=1.90 CI (0.60–6.10) p=0.28 |
| Sato et al.52 | Retrospective | 2019 | 119 | BC | Trastuzumab every 3 weeks with or without previous anthracycline | 10.8% | 60.5% | 100% | 24 (20%) | Overt HF or ≥10% LVEF drop to LVEF <55% in asymptomatic patients. | Dyslipidemia:Calculated OR=0.69 CI (0.14–3.36) p=0.65Univariate OR=0.672 CI (0.16–2.89) p=0.59 |
| Serrano et al.53 | Retrospective | 2012 | 45 | Early or advanced BC | >1 dose trastuzumab-based regimen | 17.8% | 40% | 100% | 13 (28.9%) | Onset of symptomatic CT (drop ≥10% resulting in a final LVEF <50% or absolute drop >20%) | Dyslipidemia:Calculated OR=2.23 CI (0.55–9.02) p=0.26Univariate p=0.25Multivariate p=0.76 |
| Suter et al.54 | Post-hoc analysis of HERA trial* | 2007 | 16931678 used in trastuzumab safety analysis | HER-2 BC early stage invasive | 1 vs. 2 years of trastuzumab every 3 weeks | 3.04% vs. 0.53% | 100% | 94% | 74(4.4) | Disease-free survival and cardiac adverse event | Hyperlipidemia:Calculated OR=0.3525 CI (0.05–2.58) p=0.30Difference in incidence=−2.39 CI (−5.87 to 1.09) |
| Tan-Chiu et al.55 | Post-hoc analysis of NSABP B-31* | 2005 | 814 | HER-2 node-positive BC | Doxorubicin and cyclophosphamide (AC) followed by paclitaxel vs. AC followed by paclitaxel plus 52 weeks of trastuzumab | 0.6% control vs. 3.6% trastuzumab | 100% | Arm with vs. arm without | 60 (7.4%) | Time to cardiac event | Elevated fasting lipid profile medications:Calculated OR CI (0.41–0.06) p=0.39Elevated fasting lipid profile medications: Relative risk=0.44 CI (0.06–3.2) p=0.52 |
| Tang et al.56 | Retrospective | 2017 | 160 | HER-2 EBC | Adjuvant trastuzumab | 21.3% | 48.1% | 100% | 25 (15.6%) | Symptomatic HF or asymptomatic [decline in LVEF by ≥10% or LVEF ≤50%] | Hyperlipidemia:Calculated OR=1.21 CI (0.44–3.31) p=0.71 |
| Tarantini et al.57 | Retrospective | 2012 | 499 | EBC treated with trastuzumab | Adjuvant trastuzumab | 27% | 87% | 100% | 75 (15%) | Time to cardiac dysfunction LVEF/HF | Dyslipidemia (LVEF)Calculated OR 1.17 CI (0.68–2.01) p=0.57 |
| Tarantini et al.58 | Retrospective | 2012 | 499 | EBC treated with trastuzumab | Adjuvant trastuzumab | 27% | 87% | 100% | 75 (15%) | Drop of LVEF classified into five grades | Insufficient data |
| Ürun et al.59 | Prospective | 2015 | 52 | HER-2 EBC+MBC | Trastuzumab either single-agent or combined with chemotherapy agents | 9.6% | 75% | 100% | 6 (11.5%) | Symptomatic HF or LVEF drop >10% | Hyperlipidemia:Calculated OR=0.58 CI (0.03–11.78) p=0.72 |
| Vicente et al.60 | Retrospective | 2009 | 61 | HER-2 BC | Adjuvant trastuzumab (excluding neoadjuvant) | 32.8% | Insufficient data | 100% | Insufficient data | LVEF <50% or ≥10% LVEF drop | Insufficient data |
| Wadhwa et al.61 | Retrospective | 2009 | 152 | HER-2 BC | Adjuvant trastuzumab after FEC or ACAC adriamycin, cyclophophamide. | 23.7% | 100% | 100% | 20 (13.2%) | Onset of cardiac dysfunction (decline of 10% in LVEF) | Hyperlipidemia:Calculated OR=0.53 CI (0.15–1.92) p=0.33 |
| Yoon et al.62 | Retrospective | 2016 | 712 | BC all stages | Neoadjuvant/adjuvant anthracycline based, taxane-based, or combined regimens followed by adjuvant trastuzumab in some cases | 11.5% | 68% | 15,7% | Not specified | LVEF <55% or LVEF drop >10% | Low triglyceride level:RR=0.995 CI (0.99–1.00) p=0.11 |
| Yu et al.63 | Retrospective | 2015 | 573 | HER-2 EBC | Adjuvant trastuzumab with or without previous adjuvant anthracyclines | 16.1% | 81.5% | 100% | 125 (21.8%) | LVEF drop ≥16% or LVEF drop of ≥10% to <55% with or without symptoms of HF | Hyperlipidemia:Calculated OR=1.15 CI (0.68–1.95) p=0.60OR=1.15 CI (0.68–1.95) p=0.595 |
>>: followed by; AC: doxorubicin plus cyclophosphamide; AC rate: anthracycline use rate; BC: breast cancer; CT: cardiotoxicity; CE: cardiac event; CI: confidence interval; DASI: Duke Activity Status Index; DLP: dyslipidemia; EBC: early breast cancer; echo: echocardiographic; FEC: 5-fluorouracil plus epirubicin plus cyclophosphamide; HF: heart failure; HR: hazard ratio; ICD-9: International Classification of Diseases, Ninth Revision; LVEF: left ventricular ejection fraction; MBC: metastatic breast cancer; NYHA: New York Heart Association functional class; OR: odds ratio; RR: risk ratio; SD: standard deviation; T: trastuzumab.
Of the 39 articles included in the qualitative analysis, most were Italian31–36,49–51,57,58 (n=9) or American29,38,41,48,54,55,63 (n=7), and all were published between 200555 and 2020.25,30,33,35 The shortest follow-up time was six months47,61 and the longest was 9.5 years.33 Seven studies were prospective and observational,30,43,45–47,59 27 were retrospective and observational,29,31–34,36,37,39,41,42,44,49–53,56–58,60–63 and five were subanalyses of large trials35,38,48,54,55 (of the ALTTO trial,35 NSABP B-31,38,48,55 and HERA.54 The studies included a total of 21079 patients (17998 excluding those based on the same population32,38,46,48,50,51,57), with ages ranging from 2027 to 9253 years, and one study focused on the elderly population.53 No mentions of male patients were found. The median sample size was 179 patients, ranging from 4553 to 4190.35
Patients were treated in both (neo)adjuvant and palliative settings. Only 11 studies did not analyze HER-2 confirmed breast cancer.26,34,39,40,42,43,49,51,52,58,62 Some studies had higher rates of anthracycline use and lower rates of trastuzumab therapy. This was most evident in Kaboré et al.43 (43% patients treated with trastuzumab) and Yoon et al.32 (15.7%). We tried to keep this bias to a minimum by excluding three articles in which trastuzumab use was less than 5% and were therefore judged to have inadequate intervention. These characteristics and other several variations in the treatment for adjuvant/neoadjuvant therapy, dosing, and regimen are further described in Table 1.
DyslipidemiaThe definition of dyslipidemia was similar between studies. Alghafar et al.25 and Farolfi et al.36 defined it as total plasma cholesterol >5.2 mmol/l or use of lipid-lowering medications, while Ganz et al.,38 Romond et al.48 and Tan-Chiu et al.55 used only lipid medications as the marker for dyslipidemia. Matos et al.45 defined dyslipidemia as a combination of low-density lipoprotein cholesterol >3 mmol or total cholesterol >5 mmol/l. Piotrowski et al.,47 Russo et al.50,51 and Tarantini et al.57 defined dyslipidemia as total serum cholesterol >190 mg/dl or lipid-lowering therapy or triglycerides >150 mg/dl. Bonifazi et al.34 used International Classification of Diseases, Ninth Revision (ICD-9) codes to identify patients diagnosed with dyslipidemia.
In our systematic review, after excluding articles dealing with the same populations,32,38,46,48,50,51,57 lacking information on dyslipidemia prevalence,60,62 and large subanalyses of RCTs,35,38,48,54,55 we found the overall prevalence of dyslipidemia to be 11.32%, ranging between studies from 1.75%39 to 43.9%.33 In the meta-analysis this figure was 15.84% and in RCTs only, it was 7.01%.
Trastuzumab-induced cardiotoxicityMost studies included in this systematic review defined cardiotoxicity as a ≤10–16% decline in LVEF, a decline in LVEF to <50–55%, or patients exhibiting signs and symptoms of heart failure.25–33,35–38,40–46,48–63 All these studies used either echocardiography or multigated acquisition (MUGA) scan as a tool to serially assess LVEF. However, outcome definitions and assessment of results differed widely between different studies, with a vast array of descriptive terminology being used. Bonifazi et al.34 defined the primary outcome as ICD-9 code reports referring to possibly drug-induced cardiac hospitalization in the main diagnosis, while Gong et al.39 and Rossi et al.49 defined it as hospitalizations due to cardiac events, and it was defined as use of medication in Ganz et al.,38 Romond et al.,48 and Tan-Chiu et al.55 We also included other studies in which there were slight variations in the percentages of LVEF decrease29,36,44,46,62,63 (Table 1), such as asymptomatic cardiotoxicity defined as LVEF drop ≥16%29,63 or LVEF drop ≥15%,36,46 or for symptomatic cardiotoxicity, LVEF drop ≥10% to LVEF <55%62,63 or LVEF <53%.44
A detailed characterization of cardiotoxicity-related parameters is presented in Table 2. In this systematic review, excluding RCTs,35,38,48,54,55 we found the overall incidence of TIC to be 11.94%, with 5.59% of patients presenting symptoms of heart failure. Performing the same calculation only for RCTs, we found these values to be 9.18% and 2.4%, respectively. In the primary meta-analysis, the cardiotoxicity rate was 13.55%.
Characterization of cardiotoxicity events.
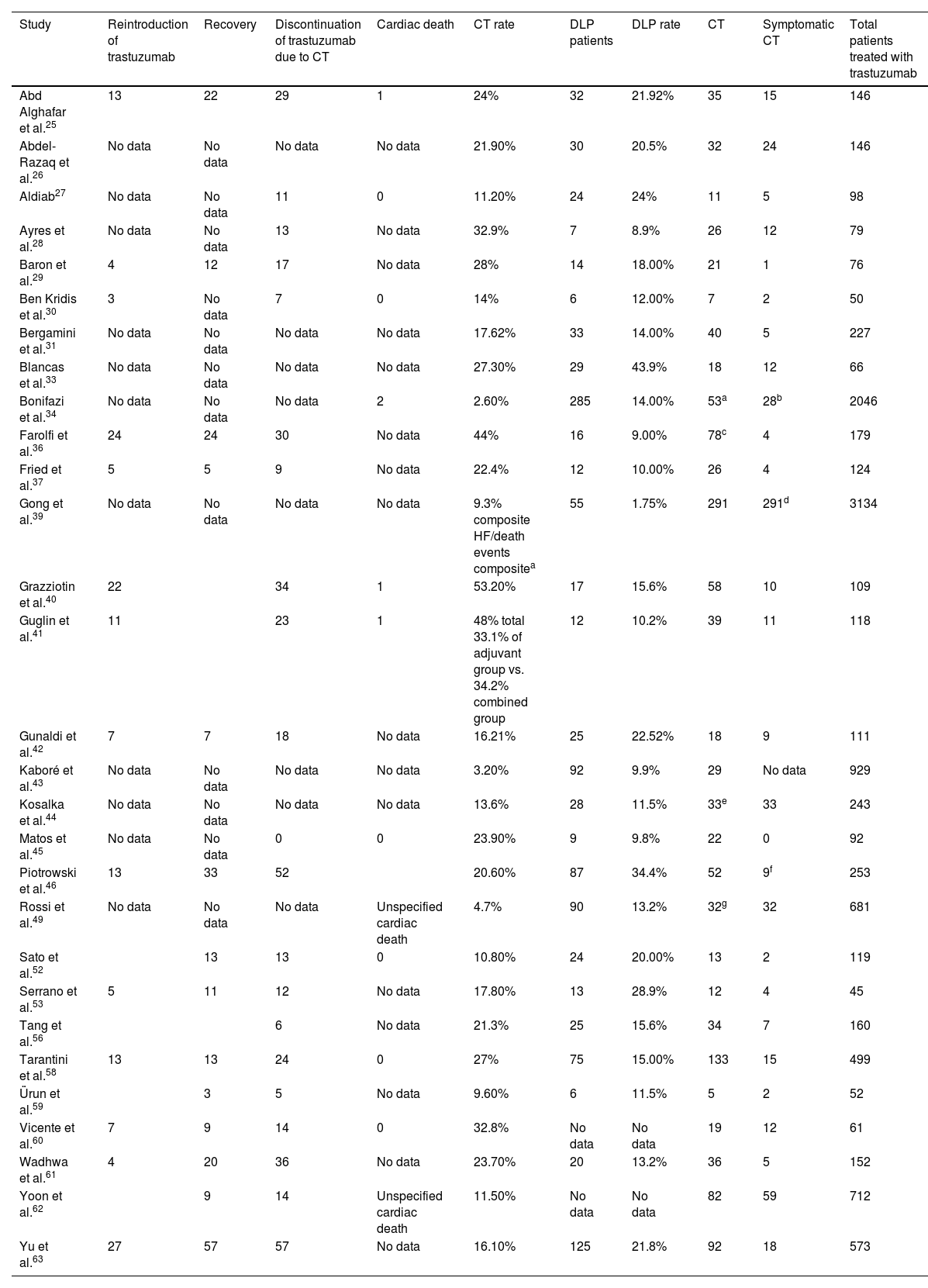
| Study | Reintroduction of trastuzumab | Recovery | Discontinuation of trastuzumab due to CT | Cardiac death | CT rate | DLP patients | DLP rate | CT | Symptomatic CT | Total patients treated with trastuzumab |
|---|---|---|---|---|---|---|---|---|---|---|
| Abd Alghafar et al.25 | 13 | 22 | 29 | 1 | 24% | 32 | 21.92% | 35 | 15 | 146 |
| Abdel-Razaq et al.26 | No data | No data | No data | No data | 21.90% | 30 | 20.5% | 32 | 24 | 146 |
| Aldiab27 | No data | No data | 11 | 0 | 11.20% | 24 | 24% | 11 | 5 | 98 |
| Ayres et al.28 | No data | No data | 13 | No data | 32.9% | 7 | 8.9% | 26 | 12 | 79 |
| Baron et al.29 | 4 | 12 | 17 | No data | 28% | 14 | 18.00% | 21 | 1 | 76 |
| Ben Kridis et al.30 | 3 | No data | 7 | 0 | 14% | 6 | 12.00% | 7 | 2 | 50 |
| Bergamini et al.31 | No data | No data | No data | No data | 17.62% | 33 | 14.00% | 40 | 5 | 227 |
| Blancas et al.33 | No data | No data | No data | No data | 27.30% | 29 | 43.9% | 18 | 12 | 66 |
| Bonifazi et al.34 | No data | No data | No data | 2 | 2.60% | 285 | 14.00% | 53a | 28b | 2046 |
| Farolfi et al.36 | 24 | 24 | 30 | No data | 44% | 16 | 9.00% | 78c | 4 | 179 |
| Fried et al.37 | 5 | 5 | 9 | No data | 22.4% | 12 | 10.00% | 26 | 4 | 124 |
| Gong et al.39 | No data | No data | No data | No data | 9.3% composite HF/death events compositea | 55 | 1.75% | 291 | 291d | 3134 |
| Grazziotin et al.40 | 22 | 34 | 1 | 53.20% | 17 | 15.6% | 58 | 10 | 109 | |
| Guglin et al.41 | 11 | 23 | 1 | 48% total 33.1% of adjuvant group vs. 34.2% combined group | 12 | 10.2% | 39 | 11 | 118 | |
| Gunaldi et al.42 | 7 | 7 | 18 | No data | 16.21% | 25 | 22.52% | 18 | 9 | 111 |
| Kaboré et al.43 | No data | No data | No data | No data | 3.20% | 92 | 9.9% | 29 | No data | 929 |
| Kosalka et al.44 | No data | No data | No data | No data | 13.6% | 28 | 11.5% | 33e | 33 | 243 |
| Matos et al.45 | No data | No data | 0 | 0 | 23.90% | 9 | 9.8% | 22 | 0 | 92 |
| Piotrowski et al.46 | 13 | 33 | 52 | 20.60% | 87 | 34.4% | 52 | 9f | 253 | |
| Rossi et al.49 | No data | No data | No data | Unspecified cardiac death | 4.7% | 90 | 13.2% | 32g | 32 | 681 |
| Sato et al.52 | 13 | 13 | 0 | 10.80% | 24 | 20.00% | 13 | 2 | 119 | |
| Serrano et al.53 | 5 | 11 | 12 | No data | 17.80% | 13 | 28.9% | 12 | 4 | 45 |
| Tang et al.56 | 6 | No data | 21.3% | 25 | 15.6% | 34 | 7 | 160 | ||
| Tarantini et al.58 | 13 | 13 | 24 | 0 | 27% | 75 | 15.00% | 133 | 15 | 499 |
| Ürun et al.59 | 3 | 5 | No data | 9.60% | 6 | 11.5% | 5 | 2 | 52 | |
| Vicente et al.60 | 7 | 9 | 14 | 0 | 32.8% | No data | No data | 19 | 12 | 61 |
| Wadhwa et al.61 | 4 | 20 | 36 | No data | 23.70% | 20 | 13.2% | 36 | 5 | 152 |
| Yoon et al.62 | 9 | 14 | Unspecified cardiac death | 11.50% | No data | No data | 82 | 59 | 712 | |
| Yu et al.63 | 27 | 57 | 57 | No data | 16.10% | 125 | 21.8% | 92 | 18 | 573 |
CT: cardiotoxicity; DLP: dyslipidemia; HF: heart failure.
The table does not include studies in which different analyses of the same samples were performed.32,38,46,48,50,51,57
In observational studies that provided data concerning discontinuation of trastuzumab, 11.13% of 3808 patients discontinued treatment temporarily or permanently due to cardiac complications. The figure was 6.61% when only RCTs were considered.
Furthermore, in studies that provided data concerning recovery of cardiac function after trastuzumab discontinuation, recovery was observed in 72.12% of patients discontinuing trastuzumab due to cardiac reasons.
Also, in studies reporting reintroduction of trastuzumab after discontinuation, we observed that 43.65% presented significant recovery of cardiac function that enabled continuation of therapy.
Association between dyslipidemia and trastuzumab-induced cardiotoxicitySix of the included studies28,31,36,43,52,53 presented univariate estimations of ORs concerning the role of dyslipidemia in trastuzumab-induced cardiotoxicity, while another six presented multivariate data.26,28,35,38,47,53 In all other studies, we calculated ORs based on demographic data found in the reports.
Of the latter studies, only Bonifazi et al.34 found a significant association (OR=2.28, 95% CI 1.22–4.26, p=0.01). The study's original summary measure was the hazard ratio (HR). This study identified the rate of severe cardiac adverse events among 2046 women treated with trastuzumab for early breast cancer, through a record linkage between health care databases and searches for records concerning ICD-9 codes referring to cardiac events and cardiovascular risk factors. A cumulative risk of cardiotoxicity was then estimated using the Kaplan-Meier method over a follow-up of three years. The predictors found were age and history of cardiac disease.
Other studies were close to the significance threshold. Gunaldi et al.42 retrospectively assessed a sample of 111 women, however, a significant association between hyperlipidemia and LVEF decrease was not found (OR=2.65, 95% CI 0.90–7.79, p=0.08). Kaboré et al.43 used prospective data in a French national multicenter study aiming to examine the association of body mass index (BMI) and cardiotoxicity defined as a decrease in LVEF in a total of 929 patients. In multivariate analysis, obesity was independently associated with cardiotoxicity. However, no significant association was found for dyslipidemia on univariate analysis (OR=2.46, 95% CI 0.97–6.23, p=0.05). This proximity to significance may also be in part explained by the low rates of trastuzumab use (43%).
Furthermore, studies that performed subanalyses of large RCTs,35,38,48,54,55 including studies reporting multivariate measures,35,38 did not observe such an association either: OR=0.90, 95% CI 0.60–1.36, p=0.63 as observed in Eiger et al.35 and OR=1.92, 95% CI 0.85–4.36, p=0.12 for the association between baseline elevated lipid profile medication use and low Duke Activity Index Status score in Ganz et al.38
Other studies were not included in the meta-analysis because of unavailability of data to calculate OR. These studies used other summary measures such as risk ratio (RR)41,55,62 or HR,39,40,48 however, they also failed to find an association. Of note that one of these studies performed rigorous adjustments for HR.39
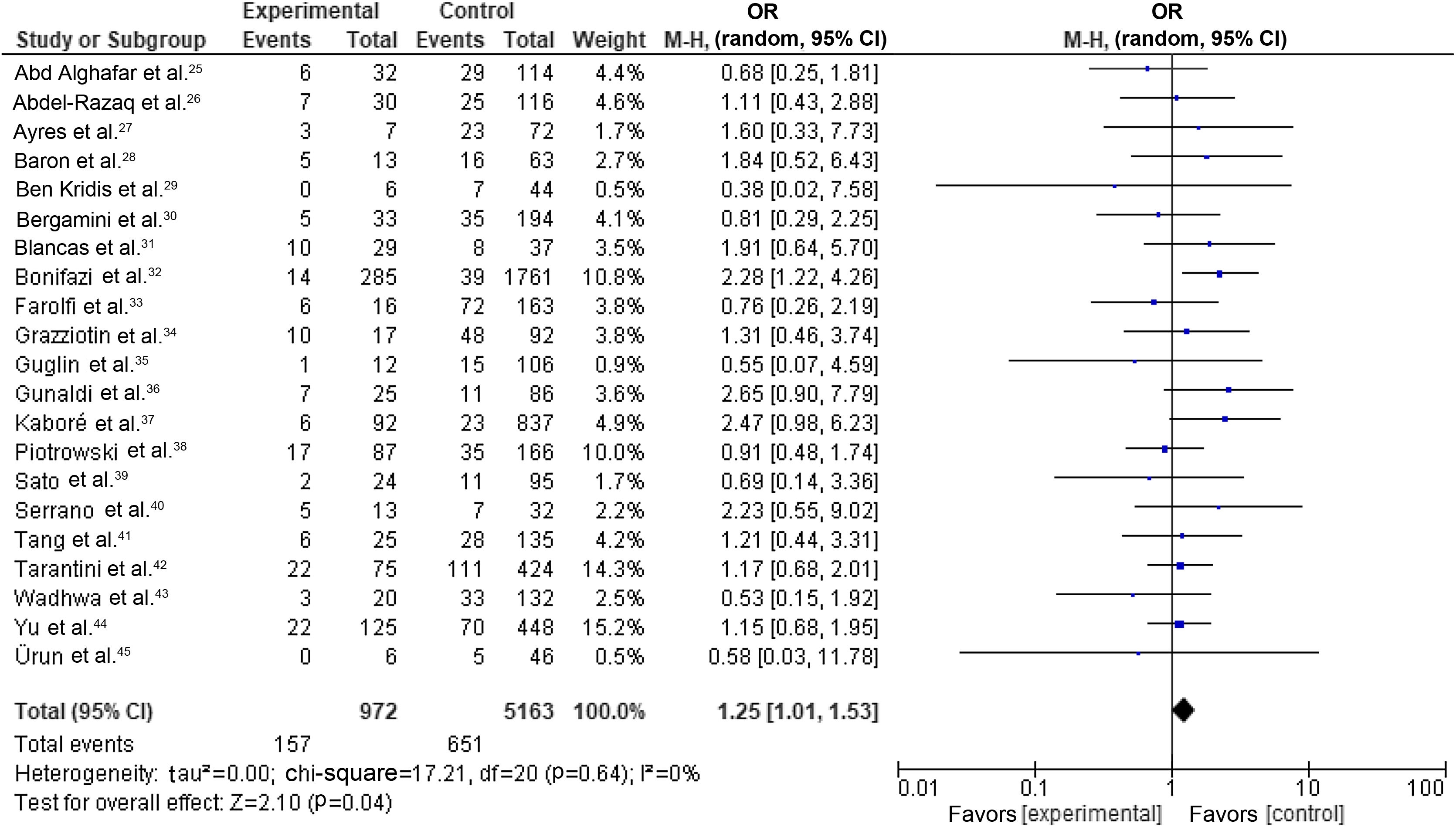
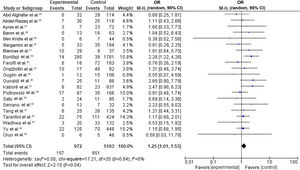
Our random-effects meta-analysis encompassed 21 studies5,25,26,28–31,33,34,36,40–43,46,53,56,58,59,61,63 and included 6135 patients. The prevalence of dyslipidemia was 15.84%. The pooled estimate for the OR of cardiotoxicity for individuals with dyslipidemia undergoing trastuzumab treatment for breast cancer was 1.25 (95% CI 1.01–1.53, p=0.04, I2=0%) (Figure 2).
Forest plot representing the effect on cardiotoxicity of dyslipidemia compared with the absence of a diagnosis of dyslipidemia in trastuzumab-based breast cancer treatment (odds ratio with 95% confidence interval, random effects meta-analysis). CI: confidence interval; OR: odds ratio; SE: standard error.
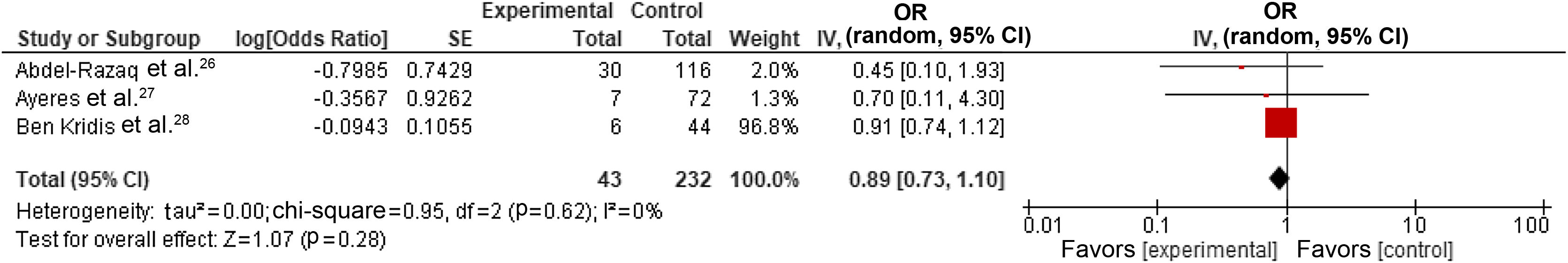
We also performed a subgroup analysis by selecting only studies reporting the most adjusted results,26,28,30 which resulted in a pooled estimate for the OR of cardiotoxicity for individuals with dyslipidemia undergoing trastuzumab treatment for breast cancer of 0.89 (95% CI 0.73–1.10, p=0.28, I2=0%) (Figure 3). However, one study30 demonstrated a disproportionate weight in the pooled result, but it did cause significant heterogeneity.
Forest plot representing the effect on cardiotoxicity of dyslipidemia compared with the absence of a diagnosis of dyslipidemia in trastuzumab-based breast cancer treatment only in studies reporting adjusted data (odds ratio with 95% confidence interval, random effects meta-analysis). CI: confidence interval; OR: odds ratio; SE: standard error.
There were no significant differences between individual studies in the magnitude of the association between dyslipidemia and cardiotoxicity in our primary meta-analysis, as indicated by the statistical test for heterogeneity (tau2=0.00; chi-square=17.21, df=20 [p=0.64]; I2=0%).
However, across studies, the treatment was heterogeneous due to the use of different regimens (Table 1), which makes it difficult to define the effect specifically caused by dyslipidemia in the setting of trastuzumab therapy.
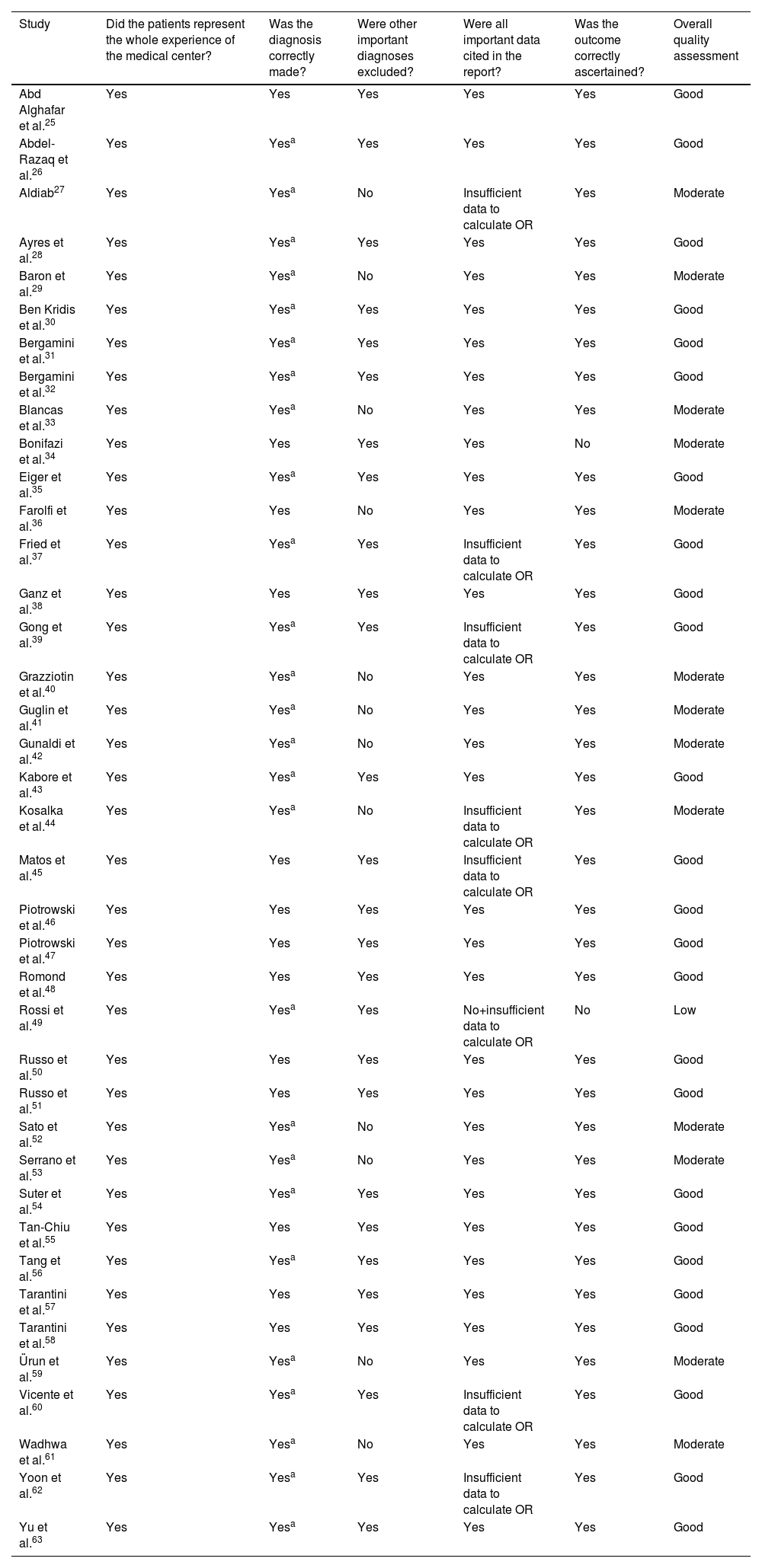
The quality of the studies and the risk of bias were assessed at the study level using the method of Haffar et al.18 Overall, we judged 13 studies to be of moderate quality, 1227,29,33,36,40–42,44,52,53,59,61 due to incomplete exclusion of pre-existing LVEF impairment and one due to lack of specification of the tool used for outcome assessment.34 One study was deemed to be of low quality49 because of failure to collect diagnosis details and unclear diagnostic method. The results of this assessment are shown in Table 3.
Assessment of article quality according to the method used by Haffar et al.18
| Study | Did the patients represent the whole experience of the medical center? | Was the diagnosis correctly made? | Were other important diagnoses excluded? | Were all important data cited in the report? | Was the outcome correctly ascertained? | Overall quality assessment |
|---|---|---|---|---|---|---|
| Abd Alghafar et al.25 | Yes | Yes | Yes | Yes | Yes | Good |
| Abdel-Razaq et al.26 | Yes | Yesa | Yes | Yes | Yes | Good |
| Aldiab27 | Yes | Yesa | No | Insufficient data to calculate OR | Yes | Moderate |
| Ayres et al.28 | Yes | Yesa | Yes | Yes | Yes | Good |
| Baron et al.29 | Yes | Yesa | No | Yes | Yes | Moderate |
| Ben Kridis et al.30 | Yes | Yesa | Yes | Yes | Yes | Good |
| Bergamini et al.31 | Yes | Yesa | Yes | Yes | Yes | Good |
| Bergamini et al.32 | Yes | Yesa | Yes | Yes | Yes | Good |
| Blancas et al.33 | Yes | Yesa | No | Yes | Yes | Moderate |
| Bonifazi et al.34 | Yes | Yes | Yes | Yes | No | Moderate |
| Eiger et al.35 | Yes | Yesa | Yes | Yes | Yes | Good |
| Farolfi et al.36 | Yes | Yes | No | Yes | Yes | Moderate |
| Fried et al.37 | Yes | Yesa | Yes | Insufficient data to calculate OR | Yes | Good |
| Ganz et al.38 | Yes | Yes | Yes | Yes | Yes | Good |
| Gong et al.39 | Yes | Yesa | Yes | Insufficient data to calculate OR | Yes | Good |
| Grazziotin et al.40 | Yes | Yesa | No | Yes | Yes | Moderate |
| Guglin et al.41 | Yes | Yesa | No | Yes | Yes | Moderate |
| Gunaldi et al.42 | Yes | Yesa | No | Yes | Yes | Moderate |
| Kabore et al.43 | Yes | Yesa | Yes | Yes | Yes | Good |
| Kosalka et al.44 | Yes | Yesa | No | Insufficient data to calculate OR | Yes | Moderate |
| Matos et al.45 | Yes | Yes | Yes | Insufficient data to calculate OR | Yes | Good |
| Piotrowski et al.46 | Yes | Yes | Yes | Yes | Yes | Good |
| Piotrowski et al.47 | Yes | Yes | Yes | Yes | Yes | Good |
| Romond et al.48 | Yes | Yes | Yes | Yes | Yes | Good |
| Rossi et al.49 | Yes | Yesa | Yes | No+insufficient data to calculate OR | No | Low |
| Russo et al.50 | Yes | Yes | Yes | Yes | Yes | Good |
| Russo et al.51 | Yes | Yes | Yes | Yes | Yes | Good |
| Sato et al.52 | Yes | Yesa | No | Yes | Yes | Moderate |
| Serrano et al.53 | Yes | Yesa | No | Yes | Yes | Moderate |
| Suter et al.54 | Yes | Yesa | Yes | Yes | Yes | Good |
| Tan-Chiu et al.55 | Yes | Yes | Yes | Yes | Yes | Good |
| Tang et al.56 | Yes | Yesa | Yes | Yes | Yes | Good |
| Tarantini et al.57 | Yes | Yes | Yes | Yes | Yes | Good |
| Tarantini et al.58 | Yes | Yes | Yes | Yes | Yes | Good |
| Ürun et al.59 | Yes | Yesa | No | Yes | Yes | Moderate |
| Vicente et al.60 | Yes | Yesa | Yes | Insufficient data to calculate OR | Yes | Good |
| Wadhwa et al.61 | Yes | Yesa | No | Yes | Yes | Moderate |
| Yoon et al.62 | Yes | Yesa | Yes | Insufficient data to calculate OR | Yes | Good |
| Yu et al.63 | Yes | Yesa | Yes | Yes | Yes | Good |
OR: odds ratio.
aNot specified, probably adequate.
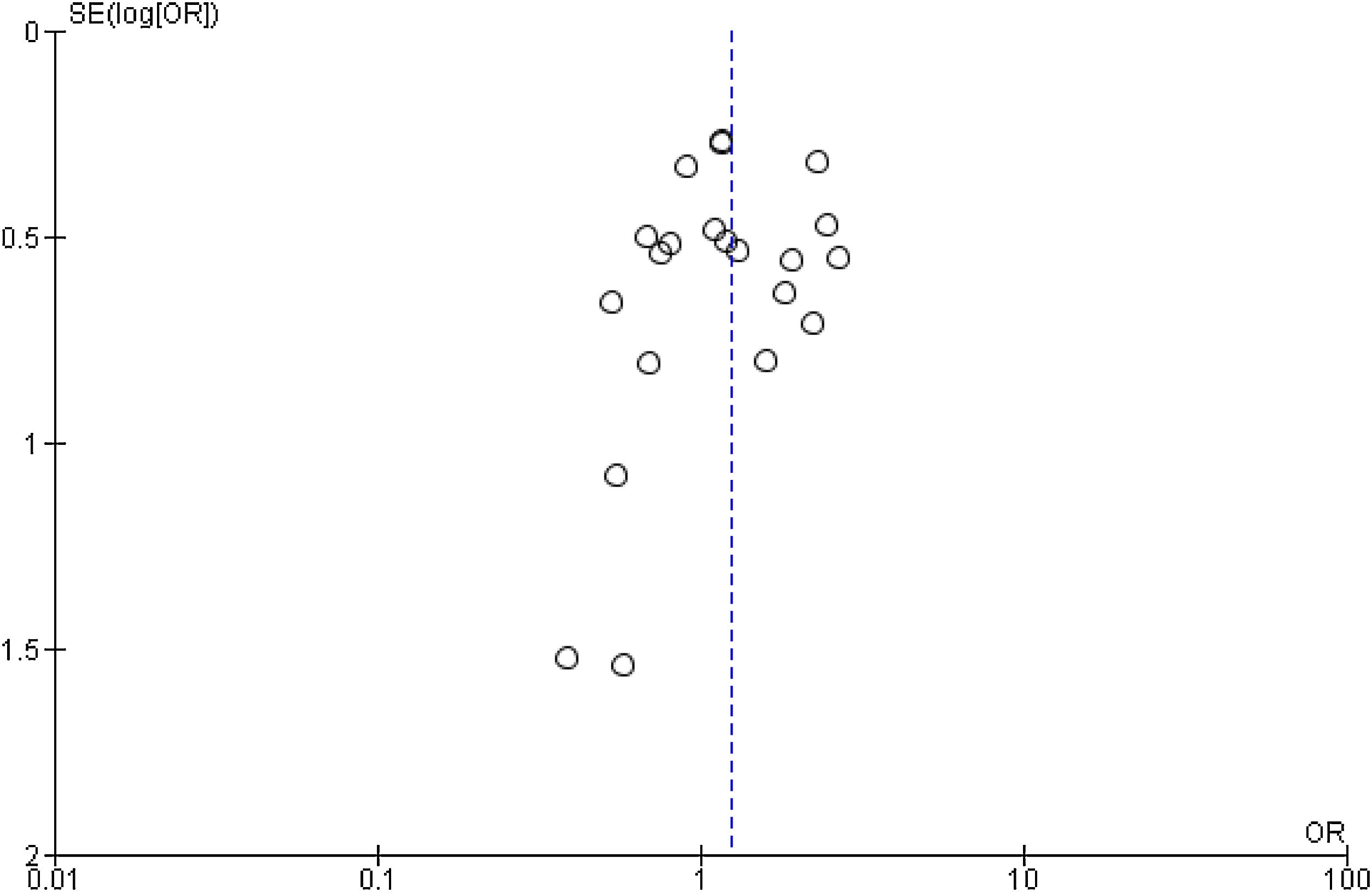
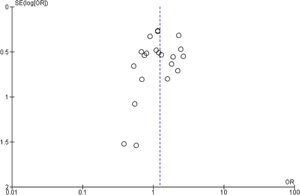
Publication bias and small-study effects were assessed for all collected variables, as demonstrated by the funnel plot of data from the 21 studies included in the meta-analysis of raw ORs, which was asymmetrical (Figure 4). These data indicate that included studies with small sample sizes appear to underestimate the effect of dyslipidemia.
DiscussionTo our knowledge, this is the most comprehensive and up-to-date systematic review and meta-analysis studying the effect of dyslipidemia on TIC. Overall, most studies (38 out of 39), adjusted or otherwise, did not observe an association between dyslipidemia and cardiac events. The quantitative analysis using random-effects model meta-analysis of raw demographic data yielded an OR of 1.25 (95% CI 1.01–1.53, p=0.04, I2=0%). However, subgroup analysis using only the most adjusted measures failed to observe this association (OR=0.89, 95% CI 0.73–1.10, p=0.28, I2=0%).
Is dyslipidemia a predictor of trastuzumab-induced cardiotoxicity?Despite the existence of a plethora of studies on the mechanisms and characteristics of TIC, there remains ambiguity concerning robust clinical predictors for this complication. The systematic review by Jawa et al.13 identified hypertension, diabetes, age, and previous anthracycline use as risk factors for a cardiac event, but failed to prove other associations with known risk factors, including dyslipidemia. Attempts have also been made to develop risk scores that will predict rates of heart failure and cardiotoxicity to enable appropriate monitoring in this group,48,64 considering such factors as baseline LVEF,48 age,48,64 adjuvant chemotherapy, coronary artery disease, atrial fibrillation or flutter, diabetes, hypertension, and renal failure.65
Dyslipidemia is linked with heart failure and coronary heart disease in the general population.14 There have been several animal and human studies that point to the beneficial effect of statins in the context of TIC,66–68 and a study has shown that rats fed a high-lipid diet are more sensitive to anthracycline-induced cardiotoxicity.65 We therefore hypothesized that dyslipidemia may play a role in TIC. A previous meta-analysis13 failed to show such an association; however, most of the literature had been based on small samples and there was a lack of studies presenting formal multivariate adjustment, hence the need for an up-to-date and comprehensive systematic review on this topic.
This systematic review was planned and designed to assess the association between dyslipidemia and TIC in breast cancer patients and included 39 studies found by a systematic search regarding this topic.
We found the overall prevalence in this systematic review of dyslipidemia in observational studies to be 11.32%, ranging between studies from 1.75%39 to 43.9%.33 This wide range may be explained because a large proportion of patients excluded in our primary analysis were in subanalyses of data from large RCTs,35,38,48,54,55 in which the dyslipidemia prevalence was 7.01%. These trials included relatively healthier and younger patients. Consequently, there is a risk that it may inadequately represent the real-world breast cancer population, who may present with a higher prevalence of risk factors, including dyslipidemia. Indeed, a major research concern in oncology is the lack of information on elderly populations.69 This question was addressed in a study included in our review that included a population with a median age of 75.9 years53 and that presented a higher prevalence of dyslipidemia (28.9%) than most included studies.
We documented a significant rate of TIC in this population, around 12.0% in the overall review of observation studies, which is consistent with previously reported data.13,70 A recent pooled analysis of adjuvant trials investigated the incidence of TIC and its impact on treatment completion.70 The incidence of symptomatic heart failure in our meta-analysis was 3.18%, which was slightly higher than the figure reported by these authors (2.3%). This suggests that cardiotoxicity may be more frequent outside clinical trials, as observed in recent retrospective cohorts.4
In the qualitative and quantitative synthesis of the data, only one study, Bonifazi et al.,34 showed a clear association between dyslipidemia and trastuzumab-induced cardiotoxicity (OR=2.28, 95% CI 1.22–4.26, p=0.01). This study had the third largest sample size (2046 patients); however, ICD codes were used for both identification of patients diagnosed with dyslipidemia and the incidence of cardiac events (defined as hospitalization). Hence, we judged this study to be at moderate risk of bias for our meta-analysis, and thus the results in the meta-analysis of unadjusted measures may be overestimated due to the weight of this study. Other studies appear to suggest an association; however, in none did it achieve statistical significance.33,42,43 These findings are further supported by the absence of association found in our subgroup analysis, which only took into consideration adjusted measures. However, it is of note that studies were also unclear about their methods of adjustment, with only Ayres et al.28 specifying that their adjustments controlled for age and BMI.
In our meta-analysis, an association of dyslipidemia with TIC was observed in the pooled data from raw ORs in observational studies. This association was not supported by a subgroup analysis of studies reporting the most adjusted measures. This suggests that the role of dyslipidemia may merit further attention, since it is a condition that often exists in interplay with other comorbidities in a synergistic interaction. Indeed, obesity,35,42,43,59,71 diabetes,13,35,59,65 and hypertension13,30,38,42,47,49 are factors reported to be associated with TIC, which suggests that metabolic syndrome as a whole could be linked to this complication. A study by Kosalka et al.44 found that, compared with any risk factor alone, the combination of two or three comorbidities (such as diabetes, dyslipidemia, and obesity) is associated with a significant increase in the incidence of symptomatic cancer therapy-related cardiotoxicity.
Strengths and limitationsThis systematic review was conducted according to the PRISMA guidelines.15,16 Data selection was rigorous, and the analysis was thorough. We were also conservative in our analysis, as undefined data were not considered and the most precise and adjusted measures were extracted for a subgroup analysis.
However, this study has all the limitations inherent to systematic reviews and meta-analyses, particularly of observational studies. Overall, we judged thirteen studies to be of moderate quality27,29,33,34,36,40–42,52,53,59,61 and one of low quality.49 There is considerable heterogeneity between the included studies concerning sample size, therapeutic regimens, cancer stages and molecular profiles, patient demographics, and inclusion/exclusion criteria and follow-up in each study.
The fact that advanced cancer predisposes to further doses of treatment should be taken into consideration, since it may enhance the risk for cardiac harm and consequently overestimate the incidence of cardiotoxicity. Furthermore, different imaging modalities were used, particularly MUGA scans as opposed to echocardiography, which is currently considered the preferred imaging modality for surveillance.72 We should also bear in mind that there is still no clear consensus on the correct definition of cardiotoxicity, hence our outcome aggregates subclinical and clinical cardiotoxicity as a single endpoint. This highlights the need to create a universally acceptable definition of cardiotoxicity in this setting that could be uniformly used in future trials.
Although we tried to minimize the risk of selection bias by performing a wider initial search for all possible risk factors for TIC, the studies included were not specifically designed to address this question and the definitions of dyslipidemia were not always stated and differed between studies. This led to bias in data collection, as we were only able to extract data from studies in which the authors considered dyslipidemia to be a relevant factor.
Clinical relevanceAlthough our systematic review and meta-analysis found an association between dyslipidemia and cardiotoxicity in a pooled estimation of unadjusted data, the same did not apply for the meta-analysis of multivariate measures. Hence, in the absence of other relevant cardiovascular risk factors, routine review of these patients’ lipid profile may not be as important as previously thought. Also, breast cancer patients who are candidates for trastuzumab therapy and who present isolated dyslipidemia should probably not be considered at high risk for the development of cardiotoxicity and can be managed in the same way as patients with no dyslipidemia, without referral for cardio-oncology assessment.
ConclusionTIC is responsible for a significant burden in breast cancer treatment, causing an increase in morbidity and mortality. Although there is concern about the role of dyslipidemia in TIC, our study was not able to provide conclusive evidence to identify dyslipidemia as a risk factor for TIC. These findings, however, should not be taken as definitive, as the data are insufficient and extracted from observational studies at risk of various biases.
As such, a low-bias, adequately powered RCT designed to clarify this question and additional systematic reviews on the topic of the real predictive value of cardiovascular risk factors in the development of TIC would be of significant scientific and clinical value.
Even so, this review may provide valuable support for stratifying the risk for this effect, and help to manage and avoid adverse outcomes in these patients, particularly if integrated into a system to predict the risk of this complication in each patient. These questions and the role of other specific factors should be addressed in future studies.
FundingNone declared.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors wish to thank Professor José Nunes for his assistance in the editing of the manuscript and advice given throughout the development of this project.
Our search was performed using the following queries:
((“trastuzumab”[MeSH Terms] OR “trastuzumab”[Title/Abstract] OR “pertuzumab”[Title/Abstract] OR “lapatinib”[Title/Abstract] OR “neratinib”[Title/Abstract]) AND (“cardiotoxicity”[MeSH Terms] OR “cardiotox*”[Title/Abstract] OR (“cardiac”[Title/Abstract] AND “toxi*”[Title/Abstract]) OR “LVEF”[Title/Abstract] OR “cardiomyopathy”[Title/Abstract])) NOT (animal[mh] NOT human [mh]) NOT ((Review[pt]) OR (meta-analysis[pt]) OR (practice-guideline[pt]))
(TITLE-ABS (trastuzumab) OR TITLE-ABS (pertuzumab) OR TITLE-ABS (lapatinib) OR TITLE-ABS (neratinib)) AND (TITLE-ABS (cardiotoxicity) OR TITLE-ABS (cardiotox*) OR (TITLE-ABS (cardiac) AND TITLE-ABS (toxi*)) OR TITLE-ABS (lvef) OR TITLE-ABS (cardiomyopathy)) AND NOT (TITLE-ABS (animal) AND NOT TITLE-ABS (human)) AND (LIMIT-TO (DOCTYPE, “ar”))
TS=(trastuzumab OR pertuzumab OR lapatinib OR neratinib) AND TS= (cardiotoxicity OR cardiotox* OR (cardiac AND toxicity) OR Cardiomyopathy OR LVEF) NOT TS=(animal NOT human) Refined by: DOCUMENT TYPES: (ARTICLE)