Vascular endothelial growth factor (VEGF) inhibitors are widely used in oncology and ophthalmology. Although these agents have been shown to increase the risk of cardiovascular events in systemic use, the effect of local applications is unclear. In our study, we aimed to investigate the effects of anti-VEGF agents on left heart functions after intravitreal injection using speckle tracking echocardiography.
MethodsIn this prospectively designed study, 44 patients who were going to start intravitreal anti-VEGF treatment were included in the study. Patients were evaluated with speckle tracking echocardiography before the first anti-VEGF administration and at three months of anti-VEGF treatment.
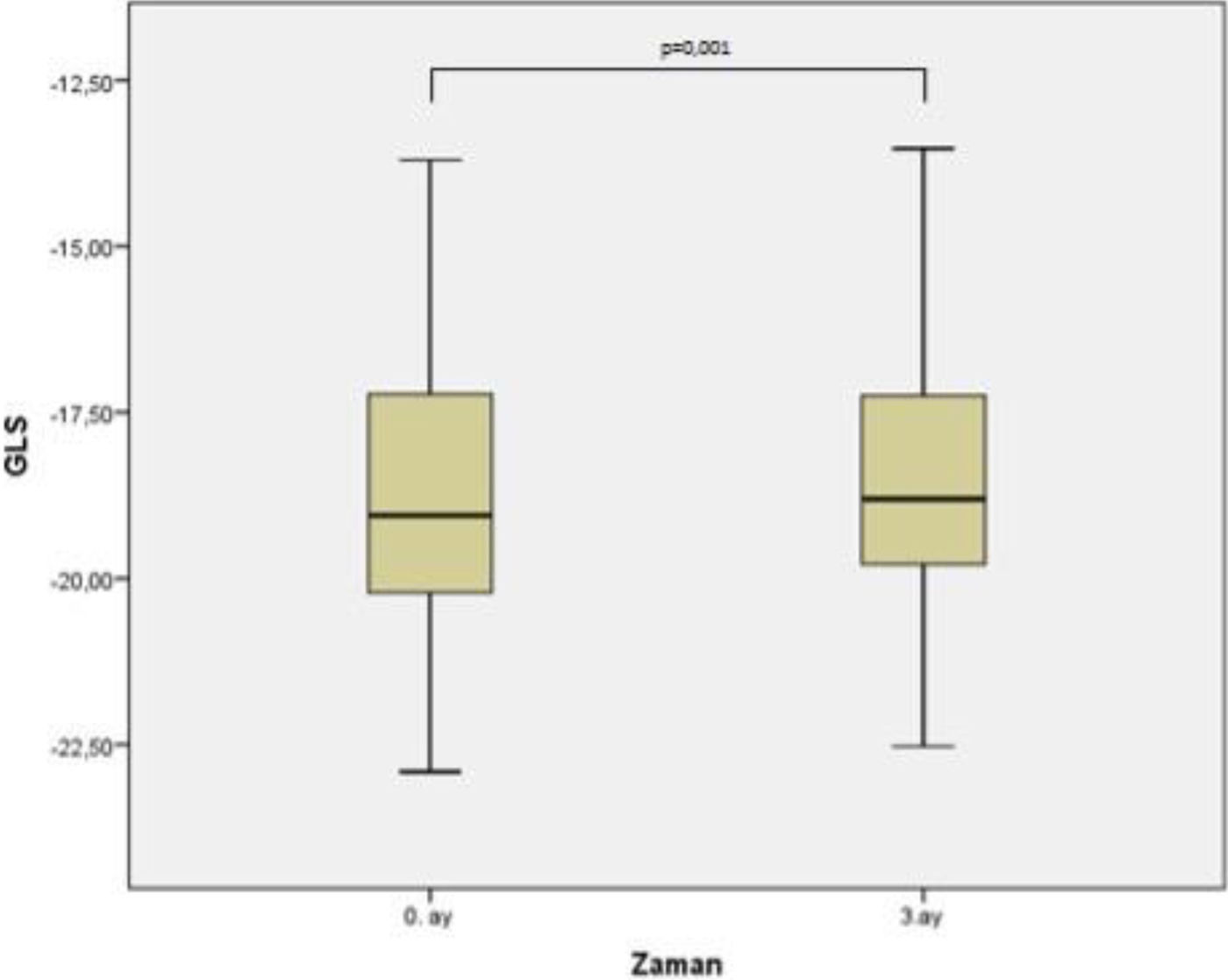
ResultsGlobal longitudinal strain (GLS) values at three months were lower in the patients who participated in the study and this was statistically significant (−18.77±2.17, −18.60±2.01, p=0.001). Also, there was a statistically significant decrease in the mean values of GLS (GLS4CH) obtained from apical four space image, GLS (GLSAPLAX) obtained from apical long axis image and GLS (GLS2CH) obtained from apical 2 space image at month 0 and month 3 (−19.08±2.39, −18.93±2.26, p=0.004; −18.81±2.29, −18.60±2.12, p=0.001; −18.44±2.31, −18.27±2.12, p=0.013, respectively).
ConclusionThe slight decrease in GLS in our study suggests that the use of intravitreal anti-VEGF agents may have cardiac effects.
Os inibidores do fator de crescimento endotelial vascular (FCEV) são amplamente utilizados em oncologia e oftalmologia. Embora tenha sido demonstrado que estes agentes aumentam o risco de eventos cardiovasculares no uso sistémico, o efeito das aplicações locais não é claro. No nosso estudo, o nosso objetivo foi investigar os efeitos dos agentes anti-FCEV nas funções do coração esquerdo após injeção intravítrea (IVI) por meio da ecocardiografia com speckle tracking.
MétodosNeste estudo prospetivo, 44 pacientes que iriam iniciar tratamento intravítreo anti-FCEV foram incluídos no estudo. Os pacientes foram avaliados com ecocardiografia com speckle tracking antes da primeira administração de anti-FCEV e aos três meses de tratamento anti-FCEV.
ResultadosOs valores do global longitudinal strain (GLS) aos três meses foram menores nos pacientes que participaram do estudo e isso foi estatisticamente significativo (−18,77 ± 2,17, −18,60 ± 2,01, p = 0,001). Além disso, houve uma diminuição estatisticamente significativa nos valores médios de GLS (GLS4CH) obtido da imagem do espaço apical 4, GLS (GLSAPLAX) obtido da imagem apical do eixo longo e GLS (GLS2CH) obtido da imagem do espaço apical 2 no mês 0 e mês 3 (−19,08 ± 2,39, −18,93 ± 2,26, p = 0,004; −18,81 ± 2,29, −18,60 ± 2,12, p = 0,001; −18,44 ± 2,31, −18,27 ± 2,12, p = 0,013, respetivamente).
ConclusãoA ligeira diminuição do GLS observada no nosso estudo sugere que o uso de agentes anti-FCEV intravítreos pode ter efeitos cardíacos.
Neovascular age-related macular degeneration (ARMD), diabetic macular edema (DME) and macular edema due to central retinal vein occlusion (RVO) are among the leading causes of blindness worldwide.1 Most retinal conditions associated with systemic diseases such as diabetes, hypertension and thrombosis are characterized by neovascularization resulting from vascular occlusion, hypoxia and ischemia.2 Hypoxia stimulates the transcription of the vascular endothelial growth factor (VEGF)-A gene by the endothelium, pericytes and retinal pigment epithelium via hypoxia inducible factor-1α (HIF-1α).3,4 VEGF plays a key role in the process of angiogenesis, both in physiological conditions such as the menstrual cycle and in proliferative diabetic retinopathy (DR), retinopathy of prematurity, retinal neovascularization and tumor growth.5 Anti-VEGF agents were first used in oncology to inhibit tumor-induced angiogenesis that feeds neoplastic tissues. Later, bevacizumab and other specific agents were developed for intravitreal administration in the treatment of AMD, high-risk DR and other conditions characterized by retinal edema and/or neovascularization.6,7
Intravitreal injections (IVI) have revolutionized the treatment of AMD, DME, proliferative DR, RVO, pathologic myopia, uveitis and many more. They are also currently considered the most viable treatment option for a variety of retinal and choroidal disorders, based on their ability to enhance the ocular therapeutic effects of many agents and reduce the incidence of serious systemic adverse events.8 Systemic administration of anti-VEGF agents in oncology has been associated with multiple events, including increased risk of arterial hypertension and embolism, whereas agents for ophthalmic indications are administered locally to the eyeball in much smaller amounts. However, clinical observations suggest that anti-VEGF agents may increase cardiovascular risk in elderly and/or diabetic patients.2
Speckle tracking echocardiography (STE) is an echocardiographic method that automatically tracks the movement of ultrasound signals, enables the measurement of myocardial tension, reduces measurement errors compared to tissue Doppler imaging, and evaluates all segments of the myocardium.9 Global longitudinal strain (GLS) has emerged as a new marker of subclinical ventricular dysfunction and has demonstrated a stronger association with prognosis than left ventricular ejection fraction (LVEF) in non-oncology heart disease populations.10,11 Previous studies have shown anti-VEGF left ventricular (LV) dysfunction has been demonstrated through GLS in metastatic colorectal and renal cell cancer patients treated with agents and it has been stated that it can be used to detect early LV dysfunction.12,13
ObjectivesIn our study, we aimed to investigate the effects of anti-VEGF agents on left heart functions after IVI via STE.
MethodsStudy protocol and populationIn this prospectively designed study, 44 patients who were going to start intravitreal bevacizumab treatment were included in the study. Patients were evaluated with STE before the first anti-VEGF administration and at 3 months of anti-VEGF treatment. Patients who received intravitreal bevacizumab once a month were included in the final analysis.
The exclusion criteria of the study were: being younger than 18 years of age, having been previously treated with anti-VEGF agents for any reason or receiving systemic chemotherapy, insufficient image quality in echocardiographic examination, atrial fibrillation or active arrhythmia during the examination, not attending the follow-up at 3rd month, dying and not giving informed consent. Also, patients diagnosed with new coronary artery disease (CAD), hypertension, acute renal failure, diabetes, heart failure, valvular disease that may alter GLS within 3 months following the first anti-VEGF injection were excluded from the study.
This study was approved by the ethics committee of the local university according to all ethical criteria for research on human subjects specified in the Second Declaration of Helsinki (CUTF: 2021-04/25). After obtaining informed consent from all participants in the study, detailed echocardiographic evaluations were performed.
The baseline characteristics, detailed anamnesis, comorbidities, and cardiac symptoms of all participants were evaluated in detail. At the end of echocardiographic examination, blood pressure and heart rate were determined. Basic biochemical parameters of the patients were recorded.
Echocardiographic evaluationThroughout our study, echocardiographic examinations were performed with the Vivid S70N (GE, Horten, Norway) using a 2.5 MHz transducer. Echocardiographic evaluation was performed by a cardiologist blinded to the study design. LV quantitative analysis was performed according to the American Society of Echocardiography recommendations.14 Patients of the study group were compared on echocardiographic parameters obtained at 0th and 3rd month.
Echocardiographic evaluation was performed in the left lateral decubitus position. Ejection fraction, left ventricular systolic and end-diastolic volume were calculated using the modified Simpson method with apical 4-chamber (A4C) and apical 2-chamber (A2C) images. Ascending aortic diameter, left atrium diameter, left ventricular end-diastolic diameter, interventricular septum wall thickness, and posterior wall thickness were measured from parasternal long axis images. Left atrial volume measurements were made from A4C and A2C images in systole and diastole and averaged separately, and the left atrial volume was indexed according to body surface area.14
Pulsed Doppler velocities and tissue Doppler traces of transmitral flow were recorded on A4C. Early (E) and atrial (A) pulsed peak velocities of the transmitral flow and tissue Doppler diastolic velocities (e′ and a′) were measured in the septal or lateral mitral annulus and averaged (e′ and a′ mean). Diastolic measurements were determined according to current recommendations.15
In our study, GLS evaluation was performed using the 2D-STE technique with the help of automated functional imaging (AFI), which is a special software package (GE Healthcare) included in the device.16
In patients who underwent electrocardiogram (ECG) monitoring, apical two-, three- and four-chamber images were recorded in consecutive cardiac cycles. The lowest possible depth setting was used to increase the reliability of the analysis. The software automatically tracked the endocardial contour and analyses were performed by manually correcting the software for poorly tracked segments rejected by the software. The left ventricle was divided into seventeen segments, and automated measurements of segmental longitudinal strain values on APLAX, A4C, and A2C views were then used to create a Bull's-eye map. Patients with insufficient echocardiographic window and insufficient tracking in ≥2 segments in strain analysis were excluded from the study. The GLS value automatically calculated by the software was recorded. An example of strain analysis performed with AFI is shown in Figure 1.
Statistical analysisThe data obtained from our study were uploaded to the Statistical Package for Social Sciences for Windows 22.0 (SPSS Inc) program and arithmetic mean, standard deviation, min–max and median values of the variables obtained by measurement or counting were calculated. Variables classified categorically were given as frequency and percentage.
The assumption of normal distribution was tested with the Shapiro–Wilk test. If it was suitable for normal distribution, the paired t-test and the significance test of the difference between two means, which are parametric tests, were used. For variables that did not show normal distribution, Wilcoxon T test and Mann–Whitney U test were used. p<0.05 was considered significant.
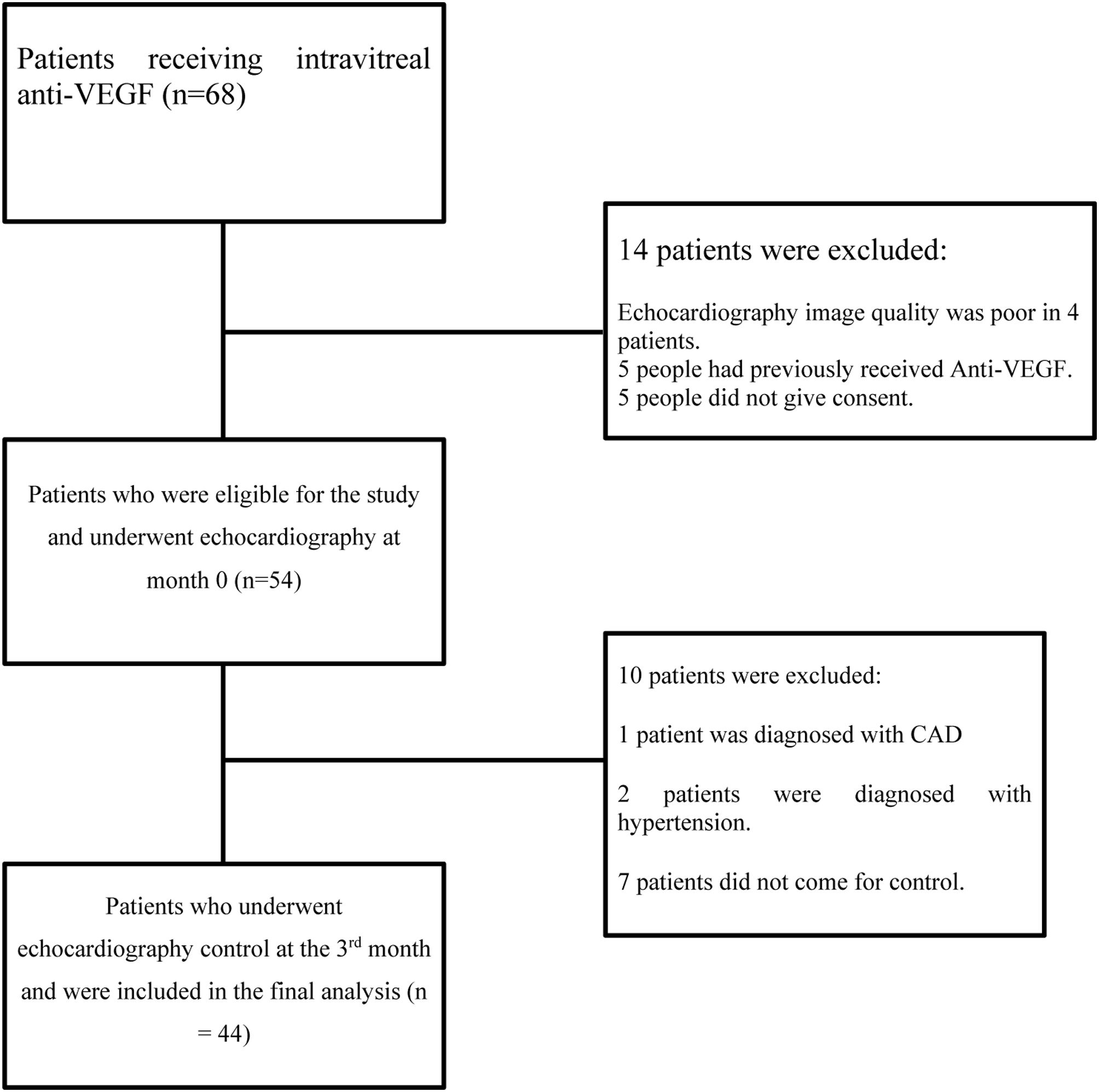
ResultFor our study, 68 patients were scanned between 01 May 2021 and 01 February 2022. Of these, four patients were excluded from the study because of poor echocardiographic image quality, five patients had been previously treated with anti-VEGF agents, and five patients did not give consent. Echocardiography was performed on 54 patients at month 0, just before anti-VEGF treatment was started. Up until the 3rd month of follow-up, one patient with a new diagnosis of CAD and two patients with hypertension were excluded from the study. Additionally, seven patients who did not come for the third month follow-up were excluded from the study. A total of 44 patients were included in the study and control echocardiography was performed at the 3rd month. The flow diagram of patient selection is shown in Figure 2.
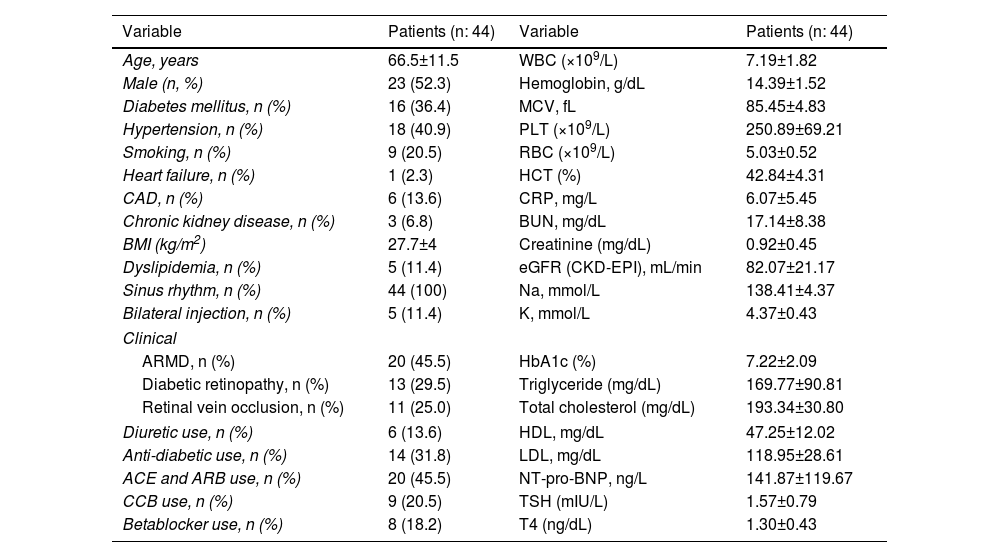
The study involved participants aged 45–88; 52.3% of the patients were male. 36.4% had diabetes, 40.9% had hypertension, and 13.6% had CAD. The indication for intravitreal bevacizumab application was determined as ARMD in 45.5% of the patients, DRP in 29.5%, and RVO in 25%. The demographic and clinical characteristics of the 44 patients included in the study are shown in Table 1.
Demographic and clinical characteristics of patients.
| Variable | Patients (n: 44) | Variable | Patients (n: 44) |
|---|---|---|---|
| Age, years | 66.5±11.5 | WBC (×109/L) | 7.19±1.82 |
| Male (n, %) | 23 (52.3) | Hemoglobin, g/dL | 14.39±1.52 |
| Diabetes mellitus, n (%) | 16 (36.4) | MCV, fL | 85.45±4.83 |
| Hypertension, n (%) | 18 (40.9) | PLT (×109/L) | 250.89±69.21 |
| Smoking, n (%) | 9 (20.5) | RBC (×109/L) | 5.03±0.52 |
| Heart failure, n (%) | 1 (2.3) | HCT (%) | 42.84±4.31 |
| CAD, n (%) | 6 (13.6) | CRP, mg/L | 6.07±5.45 |
| Chronic kidney disease, n (%) | 3 (6.8) | BUN, mg/dL | 17.14±8.38 |
| BMI (kg/m2) | 27.7±4 | Creatinine (mg/dL) | 0.92±0.45 |
| Dyslipidemia, n (%) | 5 (11.4) | eGFR (CKD-EPI), mL/min | 82.07±21.17 |
| Sinus rhythm, n (%) | 44 (100) | Na, mmol/L | 138.41±4.37 |
| Bilateral injection, n (%) | 5 (11.4) | K, mmol/L | 4.37±0.43 |
| Clinical | |||
| ARMD, n (%) | 20 (45.5) | HbA1c (%) | 7.22±2.09 |
| Diabetic retinopathy, n (%) | 13 (29.5) | Triglyceride (mg/dL) | 169.77±90.81 |
| Retinal vein occlusion, n (%) | 11 (25.0) | Total cholesterol (mg/dL) | 193.34±30.80 |
| Diuretic use, n (%) | 6 (13.6) | HDL, mg/dL | 47.25±12.02 |
| Anti-diabetic use, n (%) | 14 (31.8) | LDL, mg/dL | 118.95±28.61 |
| ACE and ARB use, n (%) | 20 (45.5) | NT-pro-BNP, ng/L | 141.87±119.67 |
| CCB use, n (%) | 9 (20.5) | TSH (mIU/L) | 1.57±0.79 |
| Betablocker use, n (%) | 8 (18.2) | T4 (ng/dL) | 1.30±0.43 |
ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; ARMD: age-related macular degeneration; BC: white blood cell; BMI: body mass index; BUN: blood urea nitrogen; CAD: coronary artery disease; CCB: calcium channel blocker; CRP: C reactive protein; eGFR: glomerular filtration rate; HbA1c: hemoglobin A1c; HCT: hematocrit; HDL: high density lipoprotein; K: potassium; LDL: low density lipoprotein; MCV: mean corpuscular volume; Na: sodium; PLT: platelet; RBC: red blood cell; TSH: thyroid stimulating hormone; T4: thyroxine hormone.
There was no significant difference in the systolic blood diastolic blood pressure values recorded before the echocardiographic evaluation in month 0 and the third month in the study group (p=0.29 and p=0.96, respectively). Heart rate was also similar (p=0.19). In addition, no patient required a change in medical treatment at 0 and 3 months. Clinical assessment and the NT-pro-BNP values were used specifically to monitor participants’ loading condition on a case-by-case basis, and there was no change over the study period.
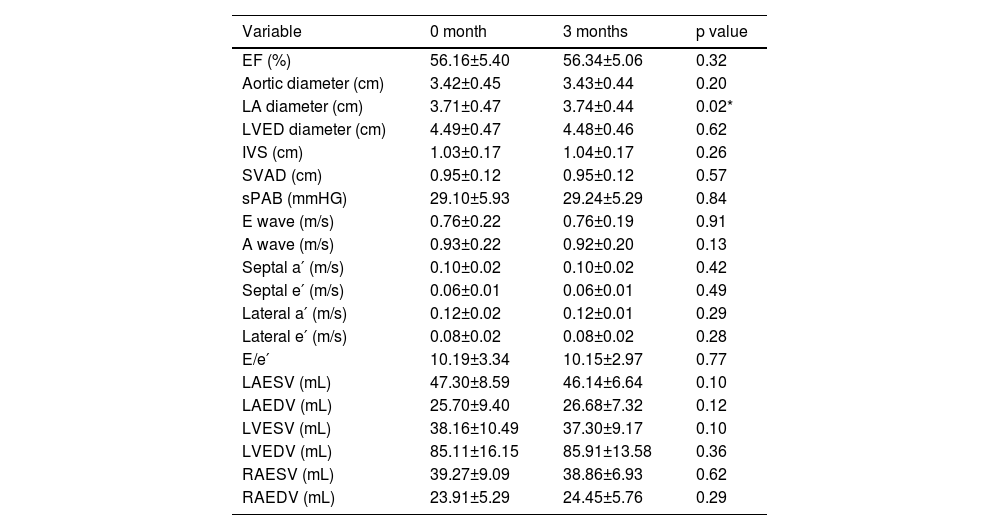
The two-dimensional (2D) echocardiography revealed a statistically significant difference only in LA diameter at 0 and 3 months (3.71±0.47, 3.74±0.44, p=0.02). When E and A waves, E/A, lateral e′ and a′, septal e′ and a′ and E/e′ measurements obtained by Doppler echocardiography were compared, no significant change was found at 0 and 3 months. Doppler and 2D echocardiography findings of the patients are shown in Table 2.
Echocardiographic findings of the patients.
| Variable | 0 month | 3 months | p value |
|---|---|---|---|
| EF (%) | 56.16±5.40 | 56.34±5.06 | 0.32 |
| Aortic diameter (cm) | 3.42±0.45 | 3.43±0.44 | 0.20 |
| LA diameter (cm) | 3.71±0.47 | 3.74±0.44 | 0.02* |
| LVED diameter (cm) | 4.49±0.47 | 4.48±0.46 | 0.62 |
| IVS (cm) | 1.03±0.17 | 1.04±0.17 | 0.26 |
| SVAD (cm) | 0.95±0.12 | 0.95±0.12 | 0.57 |
| sPAB (mmHG) | 29.10±5.93 | 29.24±5.29 | 0.84 |
| E wave (m/s) | 0.76±0.22 | 0.76±0.19 | 0.91 |
| A wave (m/s) | 0.93±0.22 | 0.92±0.20 | 0.13 |
| Septal a′ (m/s) | 0.10±0.02 | 0.10±0.02 | 0.42 |
| Septal e′ (m/s) | 0.06±0.01 | 0.06±0.01 | 0.49 |
| Lateral a′ (m/s) | 0.12±0.02 | 0.12±0.01 | 0.29 |
| Lateral e′ (m/s) | 0.08±0.02 | 0.08±0.02 | 0.28 |
| E/e′ | 10.19±3.34 | 10.15±2.97 | 0.77 |
| LAESV (mL) | 47.30±8.59 | 46.14±6.64 | 0.10 |
| LAEDV (mL) | 25.70±9.40 | 26.68±7.32 | 0.12 |
| LVESV (mL) | 38.16±10.49 | 37.30±9.17 | 0.10 |
| LVEDV (mL) | 85.11±16.15 | 85.91±13.58 | 0.36 |
| RAESV (mL) | 39.27±9.09 | 38.86±6.93 | 0.62 |
| RAEDV (mL) | 23.91±5.29 | 24.45±5.76 | 0.29 |
EF: ejection fraction; IVS: interventricular septum; LA: left atrium; LAEDV: left atrial end-diastolic volume; LAESV: left atrium end-systolic volume; LVAD: left ventricular posterior wall; LVED: left ventricular end diastolic; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; RAEDV: right atrium end-diastolic volume; RAESV: right atrium end-systolic volume; sPAP: mean pulmonary arterial pressure.
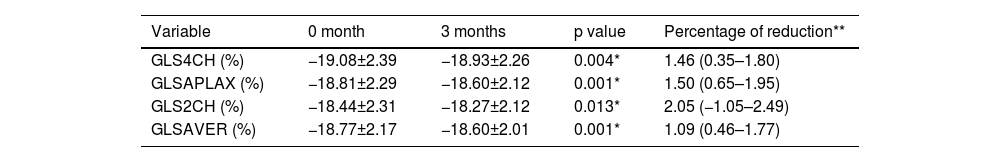
The third month measurements revealed there was a statistically significant decrease in the mean values of GLS (GLS4CH) obtained from the apical 4 space image, GLS (GLSAPLAX) obtained from apical long axis image and GLS (GLS2CH) obtained from apical 2 space image at month 0 and month 3 (−19.08±2.39, −18.93±2.26, p=0.004; −18.81±2.29, −18.60±2.12, p=0.001; −18.44±2.31, −18.27±2.12, p=0.013, respectively). Accordingly, global longitudinal strain (GLSAVER) values, which are the average of GLS4CH, GLSAPLAX and GLS2CH values, were also lower and this was statistically significant (−18.77±2.17, −18.60±2.01, p=0.001). The change in GLS values is shown in Table 3. The change in average GLS values is shown in Figure 3.
Global longitudinal strain change at month 0 and month 3.
| Variable | 0 month | 3 months | p value | Percentage of reduction** |
|---|---|---|---|---|
| GLS4CH (%) | −19.08±2.39 | −18.93±2.26 | 0.004* | 1.46 (0.35–1.80) |
| GLSAPLAX (%) | −18.81±2.29 | −18.60±2.12 | 0.001* | 1.50 (0.65–1.95) |
| GLS2CH (%) | −18.44±2.31 | −18.27±2.12 | 0.013* | 2.05 (−1.05–2.49) |
| GLSAVER (%) | −18.77±2.17 | −18.60±2.01 | 0.001* | 1.09 (0.46–1.77) |
GLSAPLX: global longitudinal strain obtained from apical long axis image; GLSAVER: average global longitudinal strain; GLS2CH: global longitudinal strain obtained from apical 2-chamber view; GLS4CH: global longitudinal strain obtained from apical 4-chamber view.
In our study designed to evaluate early evidence of cardiotoxicity and left heart function in patients treated with intravitreal bevacizumab, we found a significant decrease in mean GLS and GLS in apical long axis, two space and four space images using the STE method. According to our results, intravitreal anti-VEGF applications are not cardiac innocent and may cause increased cardiovascular outcomes in the future.
Anti-VEGF therapy is effective in inhibiting tumor angiogenesis, an integral mechanism that promotes the continued growth and metastasis of tumors.17 One of the most widely used anti-VEGF agents is bevacizumab, a monoclonal antibody used in the treatment of patients with metastatic colorectal cancer.18 Despite their benefits in reducing morbidity and mortality in patients with metastatic colorectal and renal cell carcinoma, these drugs have been associated with a number of cardiotoxic side effects including hypertension, left ventricular systolic dysfunction and heart failure.19 Hypertension is the most commonly reported adverse event under anti-VEGF therapy. It occurs within hours or days, is dose-dependent and usually reverses with drug withdrawal.20,21 During the course of our study, the newly diagnosed hypertension in two patients was thought to be drug-related and these patients were not included in the final analysis.
Cancer treatment-related cardiac dysfunction (CTRCD) was defined as a 10% decline in LVEF to a final value of less than 53% or a >15% relative decline in GLS compared with baseline, confirmed on subsequent imaging performed 2–3 weeks after the initial measurement.22 CTRCD has traditionally been assessed using clinical symptoms and reductions in LVEF. This assessment is disadvantaged by the high interobserver variability of traditional echocardiographic analyzes and the fact that LVEF decline is relatively insensitive for diagnosing cardiotoxicity at an early stage. A detectable LVEF decline occurs only after the loss of a large amount of myocardial tissue; therefore, a reduced LVEF after chemotherapy is usually a sign of already extensive myocardial damage and overt HF.23–25 Recently, 2D-STE analysis has been investigated with cancer patients. STE may offer a more sensitive approach than LVEF to measure cardiotoxicity because of its advantage in detecting subtle wall motion abnormalities of regional function that do not reduce overall LVEF. It has also been recognized as a potential measure to identify early subclinical myocardial damage.22,26,10
Twenty-five patients were included in the study designed by Sonaglioni et al. to investigate whether 2D-STE could noninvasively detect early evidence of cardiotoxicity in metastatic colorectal cancer patients treated with bevacizumab. Echocardiographic control was performed on the patients at baseline, 3rd and 6th months. The primary endpoint was defined as a decrease in GLS of >15%, and the secondary endpoint was newly diagnosed hypertension. The number of patients with more than 15% decrease in GLS was 9.13 In a study by Nhola et al. involving 40 patients with metastatic colon cancer and metastatic renal cell carcinoma, echocardiographic control was performed at the 1st, 3rd and 6th months of anti-VEGF therapy. Eight percent of patients developed clinically asymptomatic cancer therapeutic-induced cardiac dysfunction; 30% of patients developed clinically significant decreases in GLS, which is a marker for early subclinical cardiac dysfunction.27 In our study, although there was a statistically significant decrease in mean GLS values at the 3rd month controls, no patient had a GLS decrease of more than 15%. There were no significant changes in LV size or LVEF, consistent with our study, and no cases of clinically symptomatic heart failure were observed in these bevacizumab-treated patients. The use of E/e′ to estimate left ventricular filling pressures is valuable and recommended.15 There was no difference in the E/e′ value in our findings, but in our study the lack of change may be related to the fact that there was no decrease in GLS by >15%.
Although anti-VEGF agents were first used in oncology, they also have great importance in ophthalmology practice today. Ranibizumab is the only monoclonal antibody approved in Europe and the United States for the treatment of AMD, RVO, and DME. Bevacizumab is approved for the treatment of metastatic solid cancers but is widely used as an off-label treatment for AMD, DME, and RVO. Because of its lower cost and comparable efficacy compared with other treatments, its off-label use is valuable.28 However, the systemic side effect profile of these intravitreal agents is unknown. Bevacizumab concentration remains close to plasma IC50 (concentration required to inhibit 50% of VEGF biological activity) for 30 days after intravitreal administration; this makes cardiovascular side effects potentially possible, as they are associated with detectable levels in systemic circulation.29,30 Although intravitreal bevacizumab is administered at a dose of 1.0–2.5 mg (150 times less than the systemic dose used in cancer), it has been suggested that VEGF inhibition may cause systemic adverse effects that may be serious for patients with diabetes or elderly patients at high risk for cardiovascular adverse events.31,32 In Hanhart et al.’s retrospective study of 5385 patients with AMD who received bevacizumab as monotherapy, 1063 (19.7%) died during follow-up after bevacizumab compared with 1298 (12.1%) in the control group (p<0.001). After adjusted Cox survival regression, mortality differed significantly between groups (OR=1.69, 95% CI 1.54–1.84; p<0.001).33 In the study of Maloney et al., which included 23348 patients treated with a diagnosis of DME, 13365 patients were treated with laser, 9219 patients were treated with intravitreal anti-VEGF, and 764 patients were treated with intravitreal corticosteroid. There was an increased risk of all-cause hospitalization in the group treated with anti-VEGF compared with macular laser (HR, 1.17; 95% CI, 1.05–1.30; p=0.01).34
Our study was conducted due to the lack of information on detailed evaluation of the cardiac effects of intravitreal anti-VEGF applications. Intravitreal administration of bevacizumab reduced GLS. However, the 15% absolute GLS decrease determined in previous studies was not observed. It is important to note that lower GLS has been associated with higher cardiovascular risk.35,36 Although a 15% decrease was not observed in the study group, further GLS decreases may be observed in larger scale studies or in longer follow-up periods after repeated applications. Intravitreal anti-VEGF applications may not be innocent. Large-scale studies evaluating cardiovascular outcomes with longer follow-up periods are needed.
Limitations of the studyOur study was conducted at a single center. The number of participants included in the study was limited and the follow-up period was short, which may have prevented us from obtaining the reduction in GLS. Since only the AFI method can be used in strain analysis in our clinic, only longitudinal axis was used, and radial and circumferential strain could not be examined. Additionally, strain could not be measured in the right ventricle and atrium, and strain calculations were limited to the left ventricle. However, it should not go unnoticed that the only parameter considered clinically significant among these is GLS, which we focused on in our study.
ConclusionWe examined the change in GLS in patients administered intravitreal anti-VEGF using the STE method and detected a statistically significant decrease, although low in percentage, in the GLS values obtained from the mean GLS and apical long axis, 2-chamber and 4-chamber images separately.
Although it is known that a serious decrease in GLS is associated with an increase in the risk of cardiovascular events, the slight decrease in GLS in our study suggests that the use of intravitreal anti-VEGF agents may have cardiac effects. However, more comprehensive randomized controlled studies are needed to obtain more information on this subject and to guide treatment.
Conflicts of interestThe authors have no conflicts of interest to declare.