Coronary vasomotion disorders (CVDs), including microvascular angina (MVA) and vasospastic angina (VSA), account for significant morbidity among patients with non-obstructive coronary artery disease (NOCAD). However, protocols for CVD assessment in clinical practice are seldom standardized and may be difficult to implement.
PurposeTo assess the safety and feasibility of a comprehensive coronary function test (CFT) protocol for assessment of CVD and the prevalence of different phenotypes of CVD in patients with angina and NOCAD (ANOCA).
MethodsPatients with persistent angina referred for invasive coronary angiogram and found to have NOCAD were prospectively recruited and underwent a CFT. Functional parameters (fractional flow reserve, coronary flow reserve and index of myocardial resistance) and coronary vasoreactivity were assessed in all patients.
ResultsOf the 20 patients included, the mean age was 63±13 years and 50% were females. Most patients had persistent typical angina and evidence of ischemia in noninvasive tests (75%). The CFT was successfully performed in all subjects without serious complications. Isolated MVA was found in 25%, isolated VSA in 40%, both MVA and VSA in 10% and noncardiac chest pain in 25% of patients. Antianginal therapy was modified after the results of CFT in 70% of patients.
ConclusionA coronary function test was feasible and safe in a cohort of patients with ANOCA. CVD were prevalent in this selected group of patients, and some presented mixed CVD phenotypes. CFT may provide a definitive diagnosis in patients with persistent angina and prompt the stratification of pharmacological therapy.
Os distúrbios vasomotores coronários (DVC), incluindo a angina microvascular (AMV) e a angina vasospástica (AV), resultam em morbilidade significativa para doentes sem doença coronária obstrutiva (DCO). Contudo, os protocolos de avaliação dos DVC na prática clínica variam substancialmente e poderão não ser de fácil implantação.
ObjetivoTestar a segurança e exequibilidade de um protocolo de estudo da função coronária (EFC) e avaliar a prevalência de diferentes fenótipos de DVC em doentes com angina mas sem DCO (ANOCA).
MétodosDoentes referenciados para angiografia coronária invasiva (ACI) com angina persistente e sem evidência de DCO foram prospetivamente incluídos e submetidos a EFC. Foi realizada avaliação de parâmetros funcionais (fractional flow reserve, fluxo de reserva coronária e resistência microvascular) e de vasoreatividade em todos os doentes.
ResultadosVinte doentes foram incluídos, a média de idade foi de 63±13 anos e 50% eram do sexo feminino. A maioria dos doentes apresentava angina persistente e evidência de isquemia em teste não invasivo (75%). O EFC foi bem-sucedido em todos os doentes e não se verificaram complicações major. A AMV foi diagnosticada em 25%, a AV em 40%, AMV e VSA em simultâneo em 10% e dor torácica não cardíaca em 25% dos doentes. Houve necessidade de modificação da terapêutica antianginosa após os resultados do EFC em 70% dos doentes.
ConclusãoO EFC demonstrou ser exequível e seguro numa população de doentes com ANOCA. Os DVC foram prevalentes nesse grupo selecionado e uma proporção de doentes apresentou simultaneamente diferentes fenótipos de DVC. O EFC possibilita um diagnóstico definitivo a doentes com ANOCA, permite o ajuste da terapêutica farmacológica.
Up to half of all patients undergoing coronary angiography with symptoms and/or signs of angina have no obstructive epicardial coronary artery disease (ANOCA).1
These patients rarely receive a definitive diagnosis and are frequently labeled and managed inappropriately.2 Depending on the patient population studied and the techniques used, up to two-thirds are found to have an underlying coronary vascular function disorder.3,4 The most common causes are microvascular angina (MVA), vasospastic angina (VSA) or both. These functional conditions cannot be excluded by anatomic tests such as non-invasive coronary computed tomography (CT) or invasive coronary angiogram (ICA).5
Coronary vasomotion disorders (CVDs) cause a relative supply-demand mismatch of myocardial blood flow and nutrients relative to their requirements, inducing myocardial ischemia that may be transient, recurrent, and/or chronic.1 This umbrella term incorporates a wide range of pathophysiological abnormalities, such as endothelial dysfunction, microvascular remodeling, microvascular and epicardial spasm and enhanced cardiac pain.2
Persistent symptoms frequently lead to subsequent repeated ICA, emergency room visits and hospitalizations, and has been associated with a reduced quality of life and substantial economic burden.6,7
The Coronary Vasomotion Disorders International Study Group (COVADIS) diagnostic criteria8,9 and the recent European consensus document on ischemia with no obstructive coronary artery disease (INOCA)10 were designed to overcome divergences in diagnostic criteria by providing standardized definitions of INOCA endotypes along with guidance on the diagnostic approach and management of these clinical entities. Furthermore, the CorMica trial underlined the importance of invasive diagnosis by demonstrating improvement in symptoms and quality of life comparing standard care with CFT guided treatment.11
Few real-world studies in Europe12–14 have prospectively evaluated multiparametric and sequential invasive coronary function test (CFT) protocols. Hence, we aimed to assess the safety and feasibility of CFT, as well as the prevalence and type of CVD in patients with ANOCA, according to the European consensus document.10
MethodsThis study was approved by the Ethics Committee of Centro Hospitalar Universitário de Lisboa Central. The study was conducted according to the Declaration of Helsinki. All participants signed written informed consent.
ParticipantsPatients presenting with persistent ischemic symptoms (chest pain or other potential ischemic equivalent) with suspected or documented myocardial ischemia who were referred for ICA and were found to have NOCAD, were selected for participation in this prospective registry.
After obstructive CAD was ruled out by ICA, the CFT was performed ad hoc. Those with a previous history of percutaneous coronary intervention (PCI), but with a patent stent and normal fractional flow reserve (FFR) were included. Exclusion criteria included report of significant allergy to contrast, severe anemia, end stage liver or renal disease, or inability to consent. All data were prospectively recorded using a dedicated questionnaire and an electronic patient data record. Health status was assessed by a validated questionnaire for quality of life using the visual analog scale of EQ 5D-3L, whereby higher scores represent a better quality of life (from 0 to 100).
Invasive coronary angiographyDiagnostic ICA was performed to confirm the absence of obstructive CAD, which was defined as a stenosis of more than 50% in combination with an abnormal FFR (≤0.80). All lesions with stenosis between 30 and 50%, including in non-target vessel for CFT, were assessed by FFR (except in one patient with a 50% lesion in a small diagonal branch – Table S1). Administration of intracoronary nitrates was avoided during the procedure if possible.
Coronary function testPressure indicesA pressure-temperature sensor guidewire (PressureWire X, Abbott Vascular, Santa Clara, USA) was used. After pressure equalization, the wire sensor was positioned in the distal third of the coronary artery, followed by at least three intracoronary injections of saline (3 ml) at room temperature. The mean transit time (MTT) was measured with each bolus and averaged to calculate the resting MTT. Then, an intravenous infusion of adenosine (140 μg/kg/min for 1–2 min) was administered via a large peripheral or central vein to induce steady-state maximal hyperemia. The transit time was remeasured after at least 3 injections and averaged to calculate the hyperemic MTT. Resting and hyperemic MMT were only accepted if the variability between the measurements was <20%. Simultaneous measurements of mean aortic pressure (by guiding catheter) and mean distal coronary pressure (DCP) were also made during maximal hyperemia, thus obtaining FFR.
Dedicated analysis software (Coroventis®, Uppsala, Sweden) was used for an automated calculation of coronary flow reserve (CFR) and index of microcirculatory resistance (IMR). The CFR was calculated as the resting MTT divided by hyperaemic MTT; an abnormal CFR was defined as a ratio of ≤2. Microvascular resistance, evaluated by IMR, was calculated as the DCP at maximal hyperemia multiplied by the hyperemic MTT.15 Increased IMR (≥25) indicated microvascular dysfunction.
Acetylcholine provocation testThe acetylcholine (Ach) provocation test was performed in accordance with the standardized protocol (Ong et al).16 Left anterior descending (LAD) was the default target vessel. If LAD was unsuitable either left circumflex (LCX) or right coronary artery (RCA) were used as alternatives.
Assessment of endothelium-dependent coronary vasomotor function was performed by manual infusion of incremental doses (2, 20, 100 and 200 μg) of ACh over a period of 3 min into the coronary artery through a guiding catheter. An evaluation of symptoms and a 12-lead electrocardiogram (ECG) were performed before and repeated after each infusion period. The ICA was also performed at each time point in an identical projection that delineates the artery without foreshortening. In case of persistent coronary spasm (CS), dinitrate isosorbide was administered.
DefinitionsThe CVD diagnoses were established according to standardized COVADIS diagnostic criteria.8,9 MVA is defined by the presence of symptoms of myocardial ischemia, NOCAD and proven coronary microvascular dysfunction (any of abnormal IMR, CFR or microvascular spasm to Ach).
A diagnosis of VSA requires the following three conditions to be satisfied during ACh testing: (1) focal or diffuse epicardial coronary diameter reduction ≥90%, (2) reproduction of symptoms and (3) ischemic ECG changes. Ischemic ECG changes were defined as transient ST segment elevation or depression of ≥0.1 mV, or ischemic T-wave changes, in at least two contiguous leads. Focal constriction is defined as a circumscribed vessel narrowing within the borders of one isolated or two neighboring coronary segments. Diffuse constriction is diagnosed when the vessel narrowing is observed in ≥2 adjacent coronary segments. A diagnosis of coronary microvascular spasm requires provocation and reproduction of anginal symptoms, ischemic ECG shifts, but no epicardial spasm during ACh testing. Noncardiac chest pain requires NOCAD (FFR >0.80) and normal CFT (CFR >2.0, IMR <25 and negative ACh testing).
Statistical analysesContinuous data are presented as mean±standard deviation (SD), or median and interquartile interval, as appropriate. We used the Kolmogorov-Smirnov test to check for normal distribution of data. Categorical data are presented as frequencies (%). All analyses were performed using SPSS Statistics version 25 (SPSS Inc., Chicago, USA).
ResultsBaseline characteristicsBetween February and October of 2021, a total of 20 patients were prospectively included (Table 1). The mean age was 63.4±12.5 years and 50.0% were female. Classical cardiovascular risk factors (CVRF) was highly prevalent. Although 20.0% had previous PCI, all had patent stents with NOCAD as defined by inclusion criteria. Global left ventricular systolic function was preserved in 85.0% of patients. Of the three patients with mild reduced left ventricle ejection fraction (LVEF), two had prior myocardial infarction and one had non-ischemic cardiomyopathy.
Baseline characteristics of patients.
| Baseline characteristics | Total(n=20) | MVA(n=7a) | VSA(n=10a) | Non-cardiac(n=5) |
|---|---|---|---|---|
| Age, years | 63.4±12.5 | 65.6±6.9 | 63.2±12.0 | 61.0±18.3 |
| Female | 10 (50.0%) | 3 (42.9%) | 6 (60.0%) | 2 (40.0%) |
| Body mass index, kg/m2 | 27.5±4.4 | 28.3±4.6 | 25.5±4.5 | 29.2±3.7 |
| Cardiovascular risk factors | ||||
| Hypertension | 15 (75.0%) | 5 (71.4%) | 7 (70.0%) | 4 (80.0%) |
| Dyslipidemia | 13 (65.0%) | 4 (57.1%) | 7 (70.0%) | 3 (60.0%) |
| Diabetes | 8 (40.0%) | 3 (42.9%) | 5 (50.0%) | 1 (20.0%) |
| Smoking habits | 8 (40.0%) | 3 (42.9%) | 3 (30.0%) | 2 (40.0%) |
| Previous history of obstructive CAD | 4 (20.0%) | – | 3 (30.0%) | 1 (20.0%) |
| Prior PCI | 4 (20.0%) | – | 3 (30.0%) | 1 (20.0%) |
| Prior myocardial infarction | 3 (15.0%) | – | 2 (20.0%) | 1 (20.0%) |
| Stroke | 1 (5.0%) | 1 (14.3%) | – | – |
| Left ventricular ejection fraction | ||||
| <35% | – | – | – | – |
| 35–54% | 3 (15.0%) | 1 (16.7%) | 1 (10.0%) | 1 (20.0%) |
| >55% | 17 (85.0%) | 6 (85.7%) | 9 (90.0%) | 4 (80.0%) |
| Clinical presentation | ||||
| Previous admissions for chest pain | 8 (40.0%) | 1 (14.3%) | 6 (60.0%) | 2 (40.0%) |
| Previous diagnostic angiography/cardiac CT | 11 (55.0%) | 1 (14.3%) | 7 (70.0%) | 3 (60.0%) |
| Typical angina | 15 (75.0%) | 5 (71.4%) | 10 (100.0%) | 2 (40.0%) |
| Atypical angina | 2 (10.0%) | 1 (14.3%) | – | 1 (20.0%) |
| Anginal equivalent | 3 (15.0%) | 1 (14.3%) | – | 2 (40.0%) |
| CCS angina classification | ||||
| Class II | 10 (50.0%) | 3 (42.9%) | 5 (50.0%) | 3 (60.0%) |
| Class III | 7 (35.0%) | 3 (42.9%) | 5 (50.0%) | – |
| Troponin-positive ACS | 1 (5.0%) | – | – | 1 (20.0%) |
| Non-invasive stress test | 16 (80.0%) | 7 (100.0%) | 8 (80.0%) | 3 (60.0%) |
| Exercise treadmill test | 4 (20.0%) | 2 (28.6%) | 3 (30.0%) | – |
| Myocardial perfusion scintigraphy | 12 (60.0%) | 5 (71.47%) | 5 (50.0%) | 3 (100.0%) |
| Positive stress test | 12 (75.0%) | 5 (71.4%) | 5 (50.0%) | 3 (100.0%) |
| Cardiovascular medication | ||||
| Beta-blocker | 12 (60.0%) | 5 (71.4%) | 5 (50.0%) | 3 (60.0%) |
| ACE-inhibitor/ARBs/ARNi | 13 (65.0%) | 4 (57.1%) | 7 (70.0%) | 3 (60.0%) |
| Calcium channel blocker | 7 (35.0%) | 4 (57.1%) | 4 (40.0%) | – |
| Long-acting nitrates | 8 (40.0%) | 3 (42.9%) | 5 (50.0%) | – |
| Ranolazine | 2 (10.0%) | – | 2 (20.0%) | – |
| Trimetazidine | 1 (5.0%) | 1 (14.3%) | 1 (10.0%) | – |
| Statin | 13 (65.0%) | 5 (71.4%) | 8 (80.0%) | 2 (40.0%) |
| Laboratory | ||||
| Hemoglobin, g/L | 13.4 (12.8–14.5) | 13.3 (13.1–14.5) | 13.2 (11.7–13.8) | 13.8 (12.0–14.7) |
| Creatinine, mg/dL | 0.9 (0.8–1.1) | 1.0 (0.8–1.1) | 0.9 (0.8–1.1) | 0.8 (0.7–0.8) |
| Glomerular filtration rate, ml/min/1.73 m2 | 79.0 (59.0–93.8) | 76.0 (58.0–80.0) | 72.0 (58.8–93.3) | 104.0 (84.0–106.5) |
| High sensitivity – cardiac troponin, ng/ml | 0.5 (0.0–2.9) | 0.0 (0.0–2.9) | 0.0 (0.0–1.4) | 2.9 (1.1–49.2) |
| NT-BNP, pg/ml | 156.5 (64.0–701.7) | 147.0 (82.0–193.0) | 156.6 (56.0–268.5) | 224.0 (68.5–857.5) |
| Low-density lipoprotein, mg/dL | 101.0 (75.0–131.0) | 99.0 (68.0–148.0) | 101.0 (68.0–131.0) | 105.0 (78.5–145.5) |
| Hemoglobin A1c, % | 5.9 (5.3–6.6) | 6.0 (5.9–6.0) | 5.8 (5.1–6.9) | 5.5 (5.2–6.3) |
| Coronary angiography | ||||
| No lesions | 11 (55.0%) | 5 (71.4%) | 5 (50.0%) | 3 (60.0%) |
| Nonsignificant lesions | 9 (45.0%) | 2 (28.6%) | 5 (50.0%) | 2 (40.0%) |
| LVEDP, mmHg | 11.0 (6.5–14.0) | 10.0 (5.0–18.0) | 12.0 (9.0–14.0) | 7.0 (6.5–11.5) |
| LVEDP >15 mmHg | 2 (10.0%) | 2 (28.6%) | – | – |
ACE: angiotensin-converting enzyme; ACS: acute coronary syndrome; ARBs: angiotensin II receptor blockers; ARNi: angiotensin receptor-neprilysin inhibitors; CAD: coronary artery disease; CCS: Canadian Cardiovascular Society; CT: computed tomography; LVEDP: left ventricle end-diastolic pressure; PCI: percutaneous coronary intervention. Data are expressed as mean±SD, median (IQR) or number (percentage).
Patients had angina with a median duration of 16 (interquartile range (IQR) 6.5–25.0) months before CFT and 55.0% had previous diagnostic angiography or CT due to anginal symptoms. Typical angina was the most prevalent form of angina (75.0%). Antianginal therapies more commonly prescribed were beta-blockers (60.0%), long-acting nitrates (40.0%) and calcium channel blockers (35.0%).
Before study inclusion, the attending interventionalist considered that angina was likely or very likely due to obstructive CAD in 75.0% of patients. Of patients who underwent non-invasive stress testing (80%), three-quarter had evidence of ischemia. No visual angiographic stenosis was found in 55.0% of patients and 45.0% had non obstructive lesions (Table S1). Median left ventricle end-diastolic pressure (LVEDP) was 11.0 (IQR 6.5–14.0) mmHg.
Procedural detailsThe CFT was successfully completed in all subjects without any serious adverse events (SAE).
During ACh infusions, three patients (15.0%) showed temporary and self-terminating AV-conduction disorders. One patient experienced self-limited paroxysmal atrial fibrillation without thromboembolic complications. In cases of coronary vasospasm during Ach provocation test, intracoronary dinitrate isosorbide was effective in normalizing coronary flow in all patients. Most CFT (95.0%) were performed using a radial access. There were no complications related to the access site. The median procedure time was 75.0 minutes. The median fluoroscopic time was 11.5 minutes and median contrast volume used was 190 ml (Table 2).
Coronary function test data.
| Total(n=20) | MVA(n=7a) | VSA(n=10a) | Non-cardiac (n=5) | |
|---|---|---|---|---|
| Vasorelaxation and resistance | ||||
| Baseline MTT, s | 0.79 (0.44–1.16) | 1.01 (0.87–1.89) | 0.55 (0.38–1.00) | 0.56 (0.41–1.17) |
| Hyperemic MTT, s | 0.25 (0.15–0.45) | 0.45 (0.31–1.02) | 0.28 (0.15–0.44) | 0.15 (0.13–0.22) |
| Hyperemic Pa, mm Hg | 77.0 (65.3–87.8) | 66.0 (57.0–78.0) | 79.0 (63.5–90.5) | 87.0 (75.5–94.5) |
| Hyperemic Pd, mm Hg | 72.0 (57.3–82.5) | 57.0 (52.0–77.0) | 73.5 (56.5–82.0) | 83.0 (69.5–87.5) |
| FFR | 0.92 (0.86–0.94) | 0.90 (0.83–0.97) | 0.91 (0.86–0.93) | 0.93 (0.89–0.98) |
| CFR | 2.95 (2.02–3.85) | 3.60 (1.0–4.40) | 2.75 (1.78–3.60) | 3.7 (2.35–7.80) |
| CFR <2.0 | 4 (20.0%) | 3 (42.9%) | 2 (20.0%) | 0 (0%) |
| IMR | 18.5 (13.0–25.8) | 26.0 (23.0–47.0) | 21.5 (12.5–24.3) | 13.0 (11.0–15.0) |
| IMR ≥25 | 6 (30.0%) | 5 (71.4%) | 2 (20.0%) | 0 (0%) |
| ACh provocation | ||||
| Ach maximal dosage, μg | 200 (100–200) | 200 (200–200) | 100 (100–200) | 200 (200–200) |
| Epicardial spasm | 10 (50.0%) | 0 (0%) | 10 (100.0%) | 0 (0%) |
| Procedural details | ||||
| Procedure time, min | 75.0 (60.3–97.5) | 85.0 (67.0–116.0) | 71.0 (62.3–100.5) | 60.0 (60.0–77.0) |
| Fluoroscopic time, min | 11.5 (9.3–16.8) | 10.0 (7.0–28.0) | 11.0 (8.0–16.5) | 12.0 (11.0–16.5) |
| Fluoroscopy dose, μGm2 | 1138.5 (584.5–1280.8) | 1182.0 (569.0–1359.0) | 600.0 (390.5–1097.8) | 1274.0 (1014–1469.0) |
| Contrast volume, ml | 190 (161.3–213.8) | 190.0 (150.0–215.0) | 200.0 (161.3–215.0) | 175.0 (150.0–225.0) |
Ach: acetylcholine; CFR: coronary flow reserve; FFR: fractional flow reserve; IMR: index of microcirculatory resistance; MTT: mean transit time; MVA: microvascular angina; Pa: aortic pressure; Pd: distal coronary pressure; VSA: vasospastic angina. Data are expressed as median (IQR) or number (percentage).
The LAD was the target artery in 90.0% and LCX in 10.0% of patients, due to severe tortuosity of the LAD. Median FFR was 0.92 (IQR 0.86–0.94). Overall, four patients had CFR <2.0 and six patients had an IMR ≥25. Three patients had simultaneously low CFR and abnormal IMR (Table 2).
Assessment for propensity for coronary vasoconstriction: acetylcholine provocationAll patients were tested in the LAD and one patient in the LAD and the RCA. In Ach provocation testing, 10 patients had epicardial CS accompanied by ischemic ECG changes and reproduction of anginal symptoms. Eight patients presented focal epicardial spasm and two patients showed diffuse epicardial spasm. One patient had angina and depression of ST segment in lateral leads without epicardial spasm during ACh provocation and therefore was diagnosed with coronary microvascular spasm.
Diagnosis and managementThe underlying abnormalities revealed by the CFT included: isolated MVA in 5 (25%) including coronary microvascular spasm in one patient, isolated VSA in 8 (40%), combined MVA and VSA in 2 (10%), and non-cardiac chest pain in 5 (25%) patients. Of the five patients with non-cardiac chest pain, three presented atypical angina or anginal equivalent. Figures 1–4 present illustrative cases of each CFT.
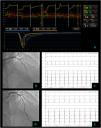
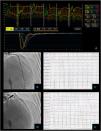
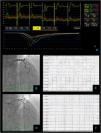
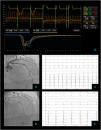
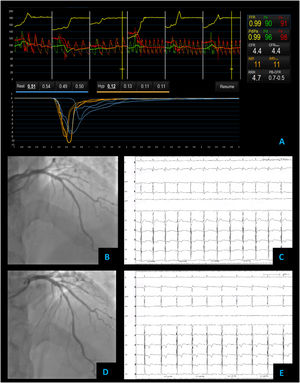
Non cardiac chest pain – coronary vascular function testing was performed using the thermodilution technique. Coronary flow reserve (4.4) and index of microvascular resistance (11) were both normal (A). Baseline coronary angiography with no obstructive CAD (B) and no epicardial vasospasm on maximal dose (200 mcg) acetylcholine provocation testing (D). The patient's electrocardiogram (E) also did not significantly change from baseline (C).
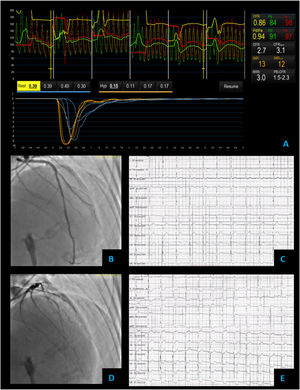
Vasospastic angina – coronary vascular function testing was performed in the LAD using the thermodilution technique. The LAD had no obstructive lesions – FFR 0.86 (A and B). Coronary flow reserve (3.0) and index of microvascular resistance (13) were both normal (A). In acetylcholine provocation testing, there was a significant diffuse reduction of diameter in the mid and distal LAD (D), accompanied by inferior and lateral ST depression (E), which were not present at baseline (C) and complaints of angina.
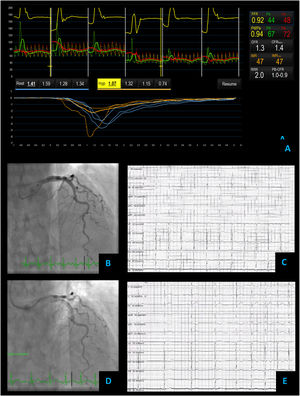
Microvascular angina – coronary vascular function testing was performed in the LAD using the thermodilution technique. The LAD had no obstructive lesions – FFR 0.92 (A and B). Both coronary flow reserve (1.3) and index of microvascular resistance (47) were abnormal high at 47 (A). There was no change in flow or vessel diameter on acetylcholine challenge (D) neither in the electrocardiogram comparing to baseline (C and E).
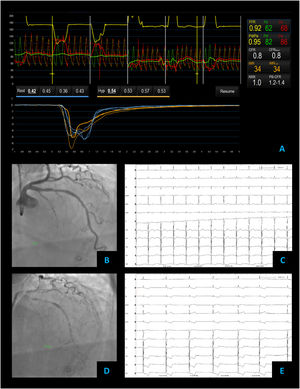
Combined microvascular and vasospastic angina – coronary angiography revealed tortuous coronary arteries with no epicardial obstruction – FFR 0.92 (A and B). Coronary vascular function testing was performed in the LAD using the thermodilution technique. Both coronary flow reserve (0.8) and index of microvascular resistance (34) were abnormal (A). On acetylcholine provocation testing, there was a severe and diffuse vasospasm in the LAD and LCX (D), accompanied by inferior and lateral ST depression (E), which were not present at baseline (C).
Based on the diagnosis, antianginal therapy adjustment was needed in 70% of patients, suspension of long-acting nitrates (in MVA cases) and/or beta-blockers (in VSA cases) and initiation of calcium channel blockers were the most common adjustments. At three-month follow-up, the quality of life as assessed by the EQ-5D-3L visual analog scale score improved in 15 patients (75%), with a median of 62.5 (IQR 50.0–70.0) from a baseline of 40.0 (IQR 20.0–42.5).
DiscussionWe present a case series of twenty patients with ANOCA who underwent a standardized CFT based on the European consensus document.10 To the best of our knowledge, it is the first prospective study in Portugal that comprehensively assessed coronary functional abnormalities for both epicardial coronary arteries and coronary microcirculation in such a population.
The major findings of our study are: (1) CFT is feasible and safe in clinical practice, providing definitive diagnosis of the underlying cause of angina, allowing stratified treatment of the distinct CVD entities and (2) in patients with ANOCA, coronary functional abnormalities, including epicardial CS, reduced microvascular vasodilatation and increased microvascular resistance are prevalent (75% in this selected cohort) and frequently coexist.
Most of ANOCA patients have a combination of functional and structural abnormalities of coronary microcirculation, including impaired dilatation and excessive coronary microvascular constriction.17,18 Impaired dilatation may be the consequence of abnormalities in endothelium-dependent mechanisms, frequently associated with diabetes, obesity, smoking, and other CVRF, and/or endothelium-independent mechanisms. The presence of an abnormal endothelium-dependent function may be diagnosed by Ach provocation test, where a limited microvascular vasodilatory response or even a paradoxical microvascular vasoconstriction is recorded.19 On the other hand, abnormal endothelium-independent function can be diagnosed as a reduced CFR and/or an increased IMR during a steady-state hyperemia induced by intravenous adenosine infusion, in the absence of significant epicardial stenosis.10,17
This single invasive procedure allows for CVD diagnosis, overcoming issues of specificity and sensitivity associated with non-invasive ischemia tests. CFT has a high diagnostic yield of identifying MVA and VSA with small additional procedural risks (up to 0.7%)20 and has been recently endorsed by European guidelines on chronic coronary syndromes.21
The CFT proved to be a safe procedure in our cohort, without related SAE. Furthermore, our CFT protocol through routine radial access enabled CVD evaluation at a reasonable expense of time and contrast volume.
Our study findings are consistent with prior studies,2,3,12,22 which have also reported on the high prevalence of coronary endothelial dysfunction and microcirculatory abnormalities in the population of patients with INOCA or ANOCA.
In our series, 75% of patients were found to have CVD. Isolated MVA was present in 25%, and 10% of the patients had both conditions (MVA and VSA). A similar prevalence of 30% was reported in a large study with invasive assessment of MVA.3 Traditional CVRF associated with increased risk for MVA23 were also noted in the majority of our patients with MVA diagnosis. Only two patients had a LVEDP >15 mmHg, both with LVEF > 55% and isolated MVA. Thus, the LVEDP was probably related to diastolic dysfunction associated with hypertension and/or diabetes. Isolated VSA was found in 40% of patients. The reported prevalence of CS in literature ranges between 33% and 62%, depending on the provocation test protocol and the population included.16,24 In this study we used a maximum Ach dose of 200 μg which is consistent with most contemporary protocols1,16 and has been shown to be safe.16
Interestingly, only 25% of patients who underwent CFT had completely normal testing, probably revealing selection bias (highly symptomatic population with significant CVRF burden justifying referral for ICA). Furthermore, most of the patients with final diagnosis of non-cardiac chest pain presented initially with atypical angina or anginal equivalent. This observation warrants further studies on cost-effectiveness of invasive CFT in patients presenting with non-typical angina.
Providing a diagnosis to patients with INOCA/ANOCA can be helpful in preventing repeated stress testing and ICA, and also on guiding therapy. Besides lifestyle interventions, first-line therapy for patients with MVA consists of a beta-blocker, unless microvascular spasm is concomitant. On the contrary, beta-blockers may potentiate coronary vasospasm and should be avoided in VSA and microvascular spasm. The significant difference in therapies according to CVD phenotypes mandates a definitive diagnosis. This has been corroborated in our study where 70% of patients required antianginal therapy adjustment after obtaining a definitive diagnosis with CFT. This new interventional diagnostic tool has the potential to change the paradigm of revascularization-oriented interventional cardiology and provide patients a truly individualized therapy, which in turn may lead to symptom improvement and has the potential to lower the overall health care burden.
Nevertheless, more studies are needed to refine diagnostic and therapeutic strategies in order to improve CVD detection, to reduce disease progression and also to determine whether clinical benefits found in CorMica trial are reproducible in a real-world cohort of patients.
LimitationsThe present work has some limitations. This was a single-center study, and the sample size was small, not allowing for comparisons between groups and endotype characterization. Previous studies found that women are more likely to experience chest pain symptoms and have abnormal CFT results than men despite showing less extensive CAD. Due to our small sample, no gender associations could be proven. Furthermore, the results of the quality of life analysis, which was a secondary analysis, may not be applicable to other clinical contexts, given the sample size and the selected group of patients included. These issues warrant future prospective studies.
ConclusionThis case series highlights the different phenotypes of patients with ANOCA. CFT was feasible and safe in a cohort of patients with ANOCA. CVD were prevalent in this selected group of patients, and some presented mixed phenotypes of CVD. CFT may provide a definitive diagnosis in patients with persistent angina and tailor the stratification of pharmacological therapy.
Conflicts of interestThe authors have no conflicts of interest to declare.