Percutaneous coronary intervention (PCI) of severely calcified lesions is associated with a higher risk of procedural complications, suboptimal stent expansion, and in-stent restenosis. Lesion preparation with orbital atherectomy (OA) in severely calcified lesions has been shown to increase procedural success and decrease reintervention rates. In this study, we sought to report the procedural safety and efficacy of our initial experience with OA in a non-surgical center in Portugal.
MethodsPatients with severely calcified coronary lesions who were treated with intended intravascular ultrasound (IVUS) guided OA were included in a prospective single-center registry. We evaluated several endpoints, including: debulking success, defined <50% residual stenosis severity after OA; procedural success, defined as stent implantation according to Optimal-IVUS PCI criteria; use of additional calcium debulking strategies; and procedural complications, including coronary no-reflow, dissection, perforation or side branch occlusion. Patients were followed up for 30 days to assess early cardiovascular or procedure-related death, myocardial infarction, myocardial injury and reintervention.
ResultsBetween January 2023 and September 2023, 37 patients and 53 coronary arteries underwent OA. IVUS imaging was used in all cases. Debulking and procedural success were achieved in 90.5% and 97.3% of cases, respectively. In 26 (49.1%) lesions, additional calcium debulking techniques were needed. Procedural complications occurred in three cases and one patient died during hospitalization.
ConclusionOur initial experience with OA for heavily calcified coronary lesions demonstrated high procedural success and overall favorable clinical outcomes.
A intervenção coronária percutânea (ICP) de lesões severamente calcificadas está associada a maior risco de complicações procedimentais, expansão subótima de stent e reestenose intra-stent (RIS). A preparação com aterectomia orbital (AO) em lesões severamente calcificadas tem demonstrado aumentar o sucesso procedimental e diminuir as taxas de reintervenção. Neste estudo, procuramos relatar a segurança e a eficácia procedimental da experiência inicial com AO num centro não cirúrgico em Portugal.
MétodosDoentes com lesões coronárias severamente calcificadas tratadas com AO e ICP guiada por ultrassonografia intravascular (IVUS) foram incluídos num registo prospetivo unicêntrico. Foram avaliados vários desfechos, incluindo: sucesso de preparação da lesão, definido como gravidade de estenose residual <50% após AO; sucesso procedimental, definido com implantação de stent de acordo com os critérios de ICP Otimal-IVUS; necessidade de utilização de técnicas adicionais de modificação de cálcio; e complicações procedimentais, incluído fenómeno de no-reflow coronário, disseção, perfuração ou oclusão de ramo lateral. Os doentes foram acompanhados durante 30 dias com intenção de avaliar morte cardiovascular precoce ou relacionada com o procedimento, enfarte agudo do miocárdio, lesão miocárdica e re-intervenção.
ResultadosEntre janeiro de 2023 e setembro de 2023, 37 doentes e 53 artérias coronárias foram tratados com AO. A imagiologia intracoronária com IVUS foi utilizada em todos os casos. O sucesso de preparação da lesão e o procedimental foram alcançados em 90,5% e 97,3% dos casos, respetivamente. Em 26 (49,1%) lesões, foram necessárias técnicas adicionais de modificação de cálcio. Complicações procedimentais ocorreram em três casos e um doente morreu durante o internamento.
ConclusõesA nossa experiência inicial com AO para lesões coronárias severamente calcificadas demonstrou alta posibilidades de sucesso procedimental e segurança clínica.
Sustained technological innovations in the last decades have allowed interventionalists to tackle increasingly complex clinical and anatomical scenarios in coronary artery disease (CAD) and percutaneous coronary intervention (PCI). Of note, coronary artery calcification, a severe form of CAD, is known to be present up to three fourths of patients who undergo invasive coronary angiography and is associated with overall worse cardiovascular outcomes.1,2 Given the high prevalence of procedural complications and suboptimal stenting results in this scenario, calcium modification techniques are essential to prepare the target lesion adequately and to guarantee proper stent deployment, expansion and apposition.3–5
Orbital atherectomy (OA, Cardiovascaular Systems, Inc., St Paul, MN, USA) features a new approach to coronary calcium modification after its successful use in peripheral artery lesions.6 In the pivotal ORBIT I and II studies, OA was demonstrated to be safe and effective in treating severely calcified CAD lesions.7,8
ObjectivesWe aim to report the first series of consecutive patients with severely calcified CAD who underwent intravascular ultrasound (IVUS)-guided OA-facilitated PCI in Portugal.
MethodsStudy populationThis was a prospective single-center prospective registry to evaluate the safety and efficacy of OA in a non-surgical center in Portugal. All patients who underwent intended IVUS-guided OA between January and September 2023 were included. The evaluation of calcified lesions was initially based on the presence of radiopacities prior to contrast injection in angiography. Staged OA-facilitated PCI was performed in all cases and the presence of severe coronary calcification was confirmed by IVUS. The decision to use OA was left to operator discretion, without any specific inclusion criteria.
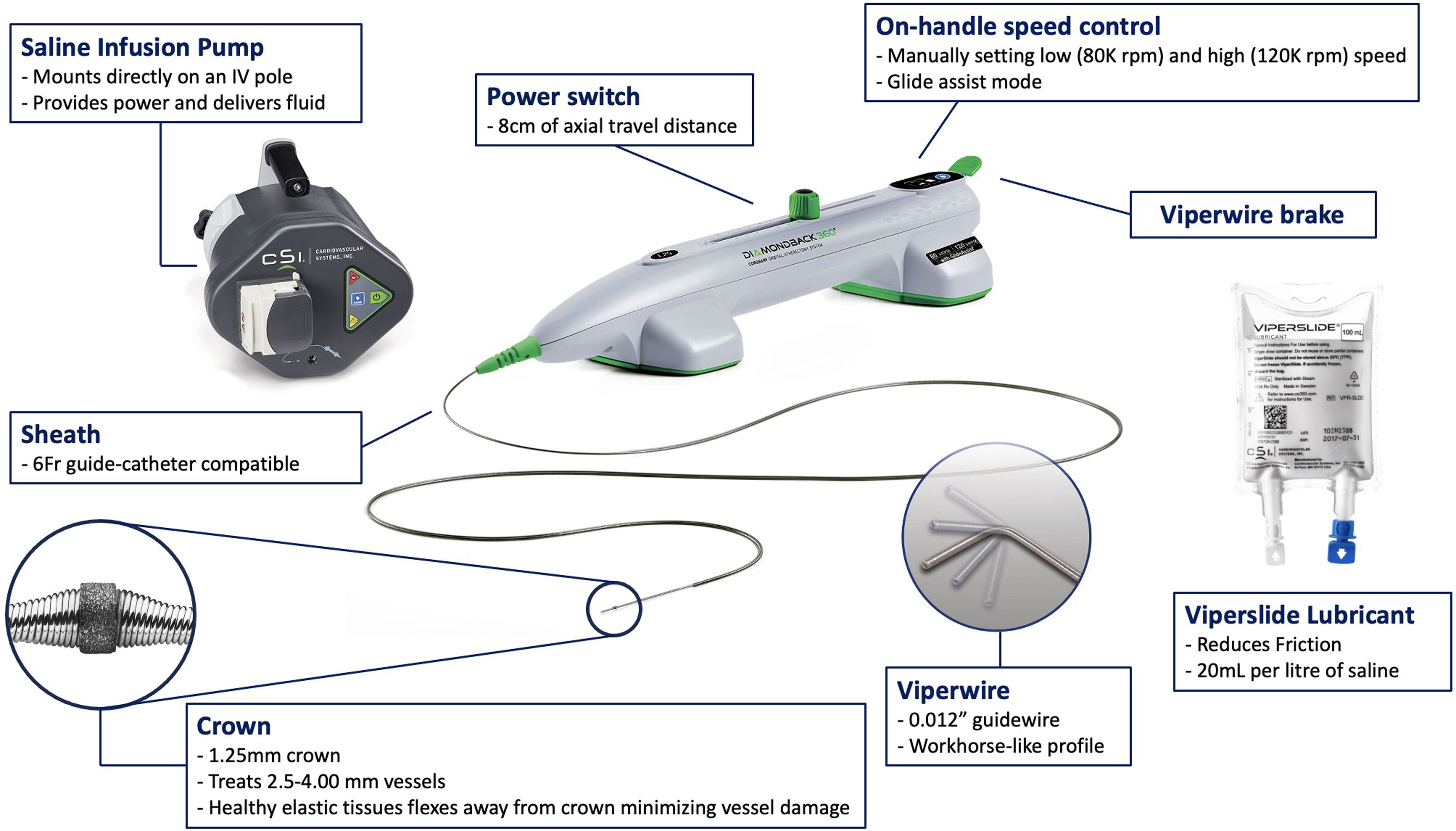
Study deviceThe Diamond Back 360 OA system is comprised of four main components (Figure 1), including: (1) a dedicated 0.012″ guidewire with a stainless steel core and nitinol coating (ViperWire; Cardiovascular Systems); (2) a 1.25 mm diamond-coated crown that is able to spin around its axis at two different pre-set speeds (80000 or 120000 rpm); (3) a power providing-pump; and (4) a dedicated lubricant solution (ViperSlide; Cardiovascular Systems) comprised of water, soybean oil, egg yolk phospholipids, glycerin and sodium hydroxide that is infused to the OA system to reduce friction between the OA device and the ViperWire.
Orbital atherectomy Diamondback 360 system. Marketed as the Diamondback 360°, it has some similarities to RA in that it is comprised of a stainless steel burr with embedded microscopic diamond chips, which allow differential atherectomy by sanding. Unlike RA's circulate tip spinning on a central axis, the OA device is an eccentrically orbiting, diamond-coated crown that goes against the plaque by of axis rotation via centrifugal force. As such, OA is able to ablate progressively larger cross-sectional areas. The crown, unlike that of RA, has diamond ablation surfaces on both its distal and proximal portions and is capable of ablation in an anterograde and retrograde fashion, reducing the risk of entrapment. IV: intravenous; RA: rotational atherectomy; OA: orbital atherectomy
The access site was left to the operator discretion, although they were incentivized to use the distal right radial artery. Temporary pacemaker insertion and atropine administration were also left at the discretion of the operator. Intravenous unfractionated heparin was administered at 100 U/kg to obtain a target activated clotting time >250 s. After obtaining standard angiography projections, the ViperWire was advanced to the distal segment of the target coronary artery and IVUS, using the Eagle Eye (Phillips Volcano, San Diego, CA, USA), was performed to determine lesion severity, length and proximal and distal landing zones. Balloon pre-dilatation was performed if the IVUS catheter was unable to cross the lesion. Direct OA without IVUS pre-evaluation was conducted if the lesion was deemed uncrossable or non-dilatable. The OA device was advanced using the ViperSlide feature, activated at the proximal edge of the target lesion and advanced back and forth at approximately 1 mm/s during 25–30 second intervals with a similar resting period in between. After performing OA, IVUS was repeated to evaluate adequate calcium debulking and to decide additional plaque preparation techniques. Stent selection and landing was made using IVUS co-registration. Device speed was set by default at 80000 rpm. In cases with particular high calcium burthen and whenever deemed necessary by the operator, the higher speed velocity could be utilized to obtain adequate luminal gain. Additional utilization of other calcium debulking modalities was left at the operator discretion, although they were incentivized to perform OA sanding at both velocities until no significant pitch differences were heard before other devices were considered. After stent implantation a final IVUS run was done to evaluate stent expansion and apposition and to exclude complications. Antithrombotic strategies were decided according to the European Society of Cardiology guidelines on myocardial revascularization and the update on dual antiplatelet therapy in CAD.9,10
EndpointsDebulking success was defined as proper device functioning and a <50% residual stenosis severity in the target lesion after OA. Procedural success was defined as adequate stent implantation using the Optimal IVUS-guided PCI criteria: (1) minimal cross-sectional area >5.0 mm2 (or >90% of the distal reference lumen cross-sectional area; (2) plaque burden at the proximal and distal stent edges <50%; and (3) no edge dissection involving media with length >3 mm.11 The need for additional calcium debulking modalities was recorded. Procedural complications included no-reflow phenomena, dissection, perforation and side branch occlusion. Safety outcomes included early (30 day) cardiovascular or procedure-related death, myocardial infarction, myocardial injury and reintervention and classified as by the fourth universal definition of myocardial infarction.12
Statistical analysisContinuous variables with normal distribution were expressed as mean and standard deviation. Categorical variables were expressed as absolute count and respective percentages. All statistical analyses were performed using commercially available software (SPSS 28.0, IBM).
ResultsStudy populationBetween January and September 2023, a total of 37 patients with 53 severely calcified vessels were treated with IVUS-guided OA-facilitated PCI. Clinical and angiographic characteristics of the study population are shown in Table 1. Median age was73 [68–78.5], 87.2% (n=33) were male. The majority of patients presented with chronic coronary syndromes (56.7%, n=21) and 16.2% (n=6) of patients had reduced left ventricle ejection fraction.
General characteristics of the study population.
| Population (n=37) | |
|---|---|
| Baseline demographics | |
| Age (years) | 73 [68–78.5] |
| Male (%) | 89.2 |
| BMI (kg/m2) | 26.5 [24.6–29.5] |
| Medical history | |
| Prior MI (%) | 70.3 |
| Prior PCI (%) | 46.9 |
| Prior CABG (%) | 5.4 |
| Hypertension (%) | 86.5 |
| Dyslipidaemia (%) | 78.4 |
| Congestive heart failure (%) | 16.2 |
| Diabetes mellitus (%) | 40.5 |
| Chronic kidney disease (%) | 27.0 |
| Pulmonary disease (%) | 13.5 |
| Peripheral artery disease (%) | 5.4 |
| Atrial fibrillation (%) | 16.2 |
| Severe aortic stenosis (%) | 13.5 |
| Clinical presentation | |
| STEMI (%) | 0.0 |
| NSTEMI (%) | 18.9 |
| Unstable angina (%) | 2.7 |
| Stable angina (%) | 27.0 |
| Silent ischemia (%) | 29.7 |
BMI: body mass index; CABG: coronary artery bypass graft; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction.
Procedural characteristics are shown in Table 2. Distal radial access was used most cases (54.1%, n=20). Guide catheter sheath size was 6Fr in 78.4% (n=29) of patients. Mechanical circulatory support was not used either upfront or in bailout in none of the cases. Transitory administration of inotropic and vasopressors were used in two patients. Median procedure time was 115 [84–150] minutes and median contrast volume utilization was 118 [93–160] mL.
Procedural characteristics.
| Population (n=37) | |
|---|---|
| Arterial access | |
| Distal radial (%) | 54.1 |
| Radial (%) | 21.6 |
| Femoral (%) | 21.6 |
| Brachial (%) | 2.7 |
| Guide catheter | |
| 6Fr (%) | 78.4 |
| 7Fr (%) | 24.3 |
| Other | |
| Procedural time (min) | 115 [84–150] |
| Fluoroscopy time (min) | 33.5 [23.1–42.9] |
| Contrast administrated (mL) | 118 [93–160] |
Overall, 53 coronary arteries were treated with OA, including 4 (10.8%) lesions in the left main (LM), 29 (45.9%) left anterior descending (LAD) artery, 14 (26.4%) in the left circumflex (LCx) artery and 17 (32.1%) in the right coronary artery (RCA) (Table 3). Most lesions were classified as type C (60.3%, n=32). IVUS imaging was performed in all patients. All lesions were treated at the lower speed setting of 80000 rpm. A total of three lesions were treated with both 80000 and 120000 rpm.
Pre-orbital and lesion characteristics.
| Population (n=53) | |
|---|---|
| Target coronary artery | |
| LM (%) | 10.8 |
| LAD (%) | 45.9 |
| LCx (%) | 27.0 |
| RCA (%) | 32.4 |
| Treated lesions per patient | 1.0 [1.0–1.0] |
| Lesion characteristics | |
| Type B (%) | 39.6 |
| Type C (%) | 60.3 |
| CTO (%) | 16.2 |
| Balloon uncrossable lesion (%) | 35.8 |
| Non-dilatable lesion (%) | 56.6 |
| Risk scores | |
| Euroscore II | 1.50 [0.93–2.81] |
| SYNTAX I | 21.5 [13.5–30.7] |
| SYNTAX II | 42.6 [29.2–58.9] |
| IVUS evaluation | |
| Maximum calcium angle (degrees) | 270 [120–360] |
| Length extension of calcification (mm) | 43.4±17.1 |
| Calcium nodule (%) | 60.3 |
| Need for balloon pre-dilatation prior to IVUS (%) | 54.7 |
IVUS: intravascular ultrasound; LAD: left anterior descending; LCx: left circumflex; RCA: right coronary artery.
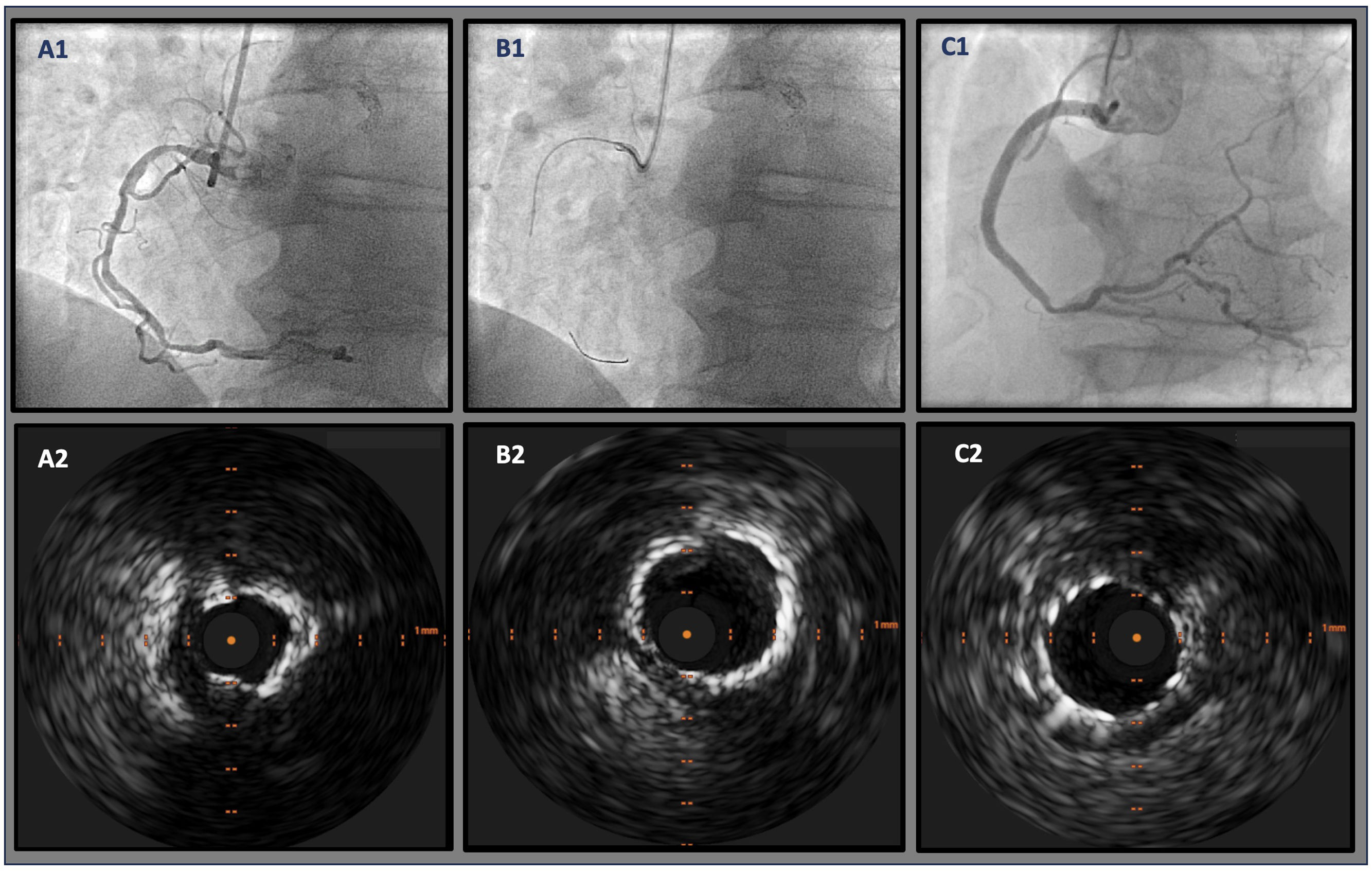
Calcium debulking success was achieved in 48 lesions (90.5%), in the form of superficial sanding (86.7%, n=46) and fracture (47.2%, n=25). Figure 2 illustrates examples of superficial sanding and medial calcium fracture in one patient. In 26 (49.1%) lesions, additional calcium modification techniques were used, the majority of which were cutting balloon. Intravascular lithotripsy (IVL) was used in 4 (7.5%) lesions. Additional non-compliant balloon dilatation preceded stent implantation in all cases. A median 2.0 [1.0–3.0] stents were implanted per patient (mean diameter 3.12±0.45 mm and mean length 28.2±7.9). Procedural success was 97.3% as assessed by IVUS imaging (Table 4).
Illustrative example of IVUS-guided, OA-facilitated PCI of a calcified RCA. A1: severe diffuse calcified lesion on the proximal and mid segments; B1: OA placed in the mid segment of the RCA and several advancements and retreatments were made; C1: final angiographic result after stenting from the mid-distal segment trough the ostium; A2: severe 360° calcified lesion of the RCA; B2: revaluation after OA showing adequate sanding (echo reverberations) and luminal gain; C2: final imaging after stenting showing overall good apposition and expansion. IVUS: intravascular ultrasound; OA: orbital atherectomy; PCI: percutaneous coronary intervention; RCA: right coronary artery
Post-orbital atherectomy and stenting characteristics.
| Population (n=53) | |
|---|---|
| Calcium debulking | |
| Debulking success (%) | 90.5 |
| Superficial sanding (%) | 86.7 |
| Calcium fracture (%) | 47.2 |
| Other calcium modification techniques | |
| Cutting balloon (%) | 45.2 |
| Intravascular lithotripsy (%) | 7.5 |
| Stent characteristics | |
| Number of stents per patient | 2.0 [1.0–3.0] |
| Stent length (mm) | 28.2±7.9 |
| Stent diameter (mm) | 3.12±0.45 |
Procedural complications occurred in 8.1% (n=3) of patients. A total of 11 clinical safety events occurred during 30 days of follow-up (Table 5), the majority were minor myocardial injuries, defined as high-sensitivity troponin T (hs-TnT) over the 99th percentile of the upper reference limit (URL) and major myocardial injury, defined as hs-TnT ≥5 times de URL. One patient suffered periprocedural myocardial infarction (Type 4a). A total of two patients had clinically manifest no-reflow phenomena. Both cases were in the context of acute coronary syndrome and poor left ventricular ejection fraction. One patient a particularly clinically severe no-reflow phenomena with cardiac arrest and need for mechanical ventilation and vasopressor support due to subsequent acute respiratory distress syndrome and died five days into hospitalization. No re-interventions were reported. One case of distal coronary perforation was reported due to distal advancement of a guidewire while retrieving a non-compliant balloon and was treated with fat embolization. No significant pericardial effusion occurred.
Procedural complications and safety outcomes at 30-day follow-up.
| Population (n=37) | |
|---|---|
| Procedure | |
| Procedural success (%) | 97.3 |
| Vascular complications | |
| Coronary perforation (%) | 2.7 |
| Coronary rupture (%) | 0.0 |
| No-reflow phenomena (%) | 5.4 |
| Coronary dissection (%) | 2.7 |
| Patient related complications | |
| Severe AV block (%) | 0.0 |
| Cardiogenic shock (%) | 2.7 |
| Cardiorespiratory arrest (%) | 2.7 |
| Hospitalization events | |
| Median hospital stay (days) | 2.0 [1.0–3.0] |
| Vasopressor/inotropic support (%) | 2.7 |
| Mechanic circulatory support (%) | 0.0 |
| Safety events at 30-days follow-up | |
| Death (%) | 2.7 |
| Myocardial infarction (%) | 8.1 |
| Major myocardial injury (%) | 18.8 |
| Minor myocardial injury (%) | 29.7 |
| Reintervention (%) | 0.0 |
AV: atrioventricular.
Our initial experience with OA to modify severely calcified coronary lesions confirmed excellent calcium debulking and procedural success with overall favorable clinical outcomes at early 30-day follow-up.
A wide arsenal of tools is available for modifying calcium deposits in the coronary arteries, ranging from balloons to more advanced technologies such as lasers, lithotripsy, rotational atherectomy (RA), and OA.13 Specifically, RA uses a rotating diamond-coated burr to abrade atherosclerotic plaques at speeds reaching 180000 rpm, and relies primarily on superficial sanding as the main mechanism of action, as heathy endothelium is able to deflect from the rotating burr while the calcified plaque cannot. This results in smoothing of the luminal surface without an increase in luminal size.14 Several potential upsides are observed when using OA instead of RA: (1) the ability of backward ablation that not only greatly reduces the probability of device entrapment but also allows to perform sanding in the inward vessel curve when retracting the crown; (2) OA creates more calcium modification in lesions with larger lumen area without the need for changing device size15; (3) substantial lower probability of bradycardia and need for pacing when performing ablation in the RCA when using OA16; and (4) the ViperWire has better profile than the current RotaWire, allowing it to be advanced directly into the distal segment of target vessel without the need for microcatheter exchange.
Potential downsides of OA when comparing it to RA include: (1) absence of evidence regarding the use of OA in ablating stented lesions and (2) being more time-consuming, as the crown must be advanced slowly and usually multiple passages are needed to achieved adequate sanding. Furthermore, ostial lesions, unprotected left main CAD and chronic total occlusions are yet to be studied in a clinical trial with OA. The relatively high profile of the distal edge of the classic OA crown can make passage through severely tight lesions particularly challenging. To address this issue, a micro crown was built with a profile to access tight (0.5 mm) lesions.17 Nevertheless, a recent meta-analysis of five observational studies found that except for lower fluoroscopy time with OA, there were no significant differences between OA and RA regarding procedural, periprocedural and 30-day major adverse cardiovascular events among patients with calcified CAD undergoing PCI.18
Although it was an initial experience, the coronary lesions in our series were more complex than those reported in the ORBIT I and II registries,7,8 as 60.3% of lesions in our study were classified as type C. This can explain the higher rate of vascular complications in our series (5.4%, n=2 patients). Theoretically, the particles that are formed from superficial sanding when using OA are small (<2 μm) and alongside with the maintenance of continuous anterograde blood flow reportedly leads to less distal embolization and microvascular obstruction when compared with RA.8 Data from the PREPARE-CALC and ROTATAXUS trials suggest that RA before stent implantation is feasible and effective, but did not result in better angiographic outcomes when compared with balloon-based strategy.19,20 The ECLIPSE trial (NCT03108456) will randomize patients with severely calcified CAD to be treated with a OA-bases strategy vs conventional PCI. The primary endpoint is a composite outcome of cardiac death, target vessel related myocardial infarction or ischemia driven target vessel revascularization. Prospective randomized data comparing the efficacy of RA and OA is not yet available.
A pooled analysis of the HORIZONS-AMI and ACUITY trials showed significant projected cost savings when using OA that could reach 4.341EUR, which was enough to offset the cost of the device,1,21 as the cost-effectiveness of OA arises from reduced procedural complications, length of stay and readmission rates.
Of note, official recommendations from society guidelines restrict the use of OA/RA to uncrossable lesions.22 Nevertheless, a considerable amount of published evidence has demonstrated that the usefulness of these modalities can be spread to other scenarios, such as crossable but non-expandable lesions and in severely diffused and calcified disease to facilitate stent expansion.23 In addition, patients enrolled in both ORBIT I and II registries, which validated the safety and effectiveness of OA in a controlled setting, did not require an uncrossable lesion to be included.7,8
Our study has important limitations. Its findings result from an early experience with OA at a single center in Portugal with probable patient selection and data reporting bias. Nevertheless, the treated coronary lesions were complex and heavily calcified, echoing the ones reported in the ORBIT I and II studies. Moreover, the high debulking and procedural success alongside the relatively low complication rate highlights the safe and rapid learning curve of the adoption of OA technology in daily practice.
ConclusionAdequate coronary lesion preparation before stenting is of fundamental importance to achieve short and long-term benefits when facing calcified coronary lesions. Our initial experience with OA demonstrated high procedural success and overall favorable clinical outcomes. Further randomized studies are needed to compare the long-term outcomes of both available atherectomy devices in this setting.
Conflict of interestsThe authors state that they have no conflict of interests.