Ischemic heart disease presents different features in men and women. We analyzed the relation between gender and prognosis in patients who had suffered a high-risk acute coronary syndrome (ACS).
MethodsThis was a prospective analytical cohort study performed at Lozano Blesa University Hospital, Zaragoza, Spain, of 559 patients diagnosed with high-risk ACS with and without ST-segment elevation according to the American College of Cardiology/American Heart Association guidelines. The sample was divided into two groups by gender and differences in epidemiologic, laboratory, electrocardiographic and echocardiographic variables and treatment were recorded. A Cox's proportional hazard model was applied and 6-month mortality was analyzed as the main variable.
ResultsThe median age was 65.2±12.7 years, and 21.8% were women. Baseline characteristics in women were more unfavorable, with higher GRACE scores, older age, higher prevalence of hypertension, diabetes and heart failure, lower ejection fraction and more renal dysfunction at admission. Women suffered more adverse cardiovascular events (27.9% vs. 15.8%, p=0.002). Sixty-four patients died, 18.9% of the women vs. 9.4% of the men (p=0.004). After multivariate analysis, female gender did not present an independent relation with mortality. Hemoglobin level, renal function, ejection fraction and Killip class >1 presented significant differences.
ConclusionsAcute syndrome coronary in women has a worse prognosis than in men. Their adverse course is due to their baseline characteristics and not to their gender.
A doença cardíaca isquémica apresenta características diferentes em homens e mulheres. Foi analisada a relação entre o sexo e o prognóstico, em pacientes vítimas de síndrome coronária aguda (SCA) de alto risco.
MétodosEstudo analítico prospetivo, de coorte, realizado no Hospital Universitário Lozano Blesa, Zaragoza, Espanha. A população em estudo é constituída por 559 pacientes com SCA, com e sem elevação do segmento ST, de acordo com a American College of Cardiology/American Heart Association. Esta população foi dicotomizada pelo sexo e realizado um estudo comparativo analisando variáveis epidemiológicas, laboratoriais, eletrocardiográficas, ecocardiográficas e de procedimento de tratamento. Aplicou-se o método de Cox para cálculo do risco proporcional e analisou-se a taxa de mortalidade como variável principal nos seis meses após o evento.
ResultadosA idade média foi de 65,2±12,7 anos. 21,8% dos doentes eram do sexo feminino. Quando comparadas com o sexo masculino, as características da população feminina eram mais desfavoráveis, apresentando um score GRACE mais elevado, uma idade superior e uma maior prevalência de hipertensão arterial, diabetes mellitus e de insuficiência cardíaca. A fração de ejeção era inferior e apresentavam grau maior de insuficiência cardíaca e de disfunção renal na admissão. As mulheres sofreram mais eventos cardiovasculares adversos (27,9% versus 15,8%, p=0,002). Sessenta e quatro pacientes morreram, sendo a mortalidade no grupo das mulheres quando comparada com o grupo dos homens significativamente superior (18,9% versus 9,4%, p= 0,004). Na análise multivariada, o sexo feminino não apresenta relação independente com a mortalidade. Por outro lado o valor sérico de hemoglobina, a função renal, a fração de ejeção e Killip >1 foram variáveis identificadas como preditoras de mortalidade.
ConclusõesA síndrome coronária agudo no grupo das mulheres tem pior prognóstico em relação ao dos homens. A evolução adversa deve-se às características iniciais e não ao facto de ser do sexo feminino.
Cardiovascular disease (CVD) is the most common cause of death in women in developed countries and is responsible for more than the combined number of deaths due to the next seven causes and more than all types of cancers combined. In the United States, CVD mortality in women exceeds that of men. Coronary heart disease is mainly responsible with 24%,1–4 and although death from ischemic heart disease (IHD) has declined in men, its incidence is stable in women.5,6
Despite the burden of coronary disease in women, CVD is still considered a disease of men and there is a false perception that women are in some way protected. IHD in women appears to have its own characteristics, although women are relatively under-represented in studies and registries.1,7
This lack of representation in randomized clinical trials has delayed recognition of specific cardiovascular risk factors by extrapolating results from studies of the male population. There are significant gaps in knowledge of CVD in women. In an analysis of gender-specific differences in the characteristics, development, management, and prognosis of acute coronary syndrome (ACS) between 1994 and 2002 in the RISCI, PRIAMHO I and II, Descartes and TRIANA trials conducted by the Working Group on Ischemic Heart Disease and Coronary Care Units of the Spanish Society of Cardiology, only 24.3% of 48369 patients were women.8
On average, women suffer myocardial infarction (MI) 7–10 years later than men, and show a poorer prognosis and 20% higher short-term mortality than men, independently of age.9
The precise reasons for this gender difference in the prognosis of acute IHD are unknown. However, epidemiological studies suggest that differences in mortality cannot be explained by possible protective effects of hormones. They give more weight to elements of the metabolic syndrome, such as hyperlipidemia or diabetes, which appear to be more prevalent in women, and to differences in clinical presentation or disparities in the use of diagnostic and therapeutic resources. Furthermore, gender-linked genetic factors, which may be critical to the development of the disease, are even less well understood.10
It is important to promote education and discussion on the differences in presentation, development, and treatment of ACS in both sexes and to obtain new indications for IHD in women. Editors of medical journals have highlighted the need for studies specifically on women that will indicate whether gender influences prognosis, including complications and adverse effects, in female patients who have suffered a high-risk ACS.11
ObjectiveWe set out to determine the differences in epidemiology, presentation and evolution of high-risk ACS in men and women, analyzing the possible effect of gender on mortality in-hospital and during a 6-month follow-up period after the acute coronary event. We also studied the association between gender and adverse cardiovascular events including post-infarction angina, reinfarction, and heart failure in the same follow-up period.
MethodsThis was a prospective analytical cohort study of patients admitted consecutively to our hospital between January 2006 and December 2007 with a diagnosis of high-risk ACS with and without ST-segment elevation according to the criteria of the ACC/AHA (American College of Cardiology/American Heart Association).12,13 Patients were treated according to the ACC/AHA guidelines.
Exclusion criteria were the presence of solid organ or hematological malignancy, fever or sepsis at the time of the study, autoimmune and inflammatory diseases, stage 5 chronic renal failure according to the 2002 National Kidney Foundation guidelines,14 and the absence of reliable records. Of the 590 patients initially selected, 31 were excluded, and the final sample analyzed was thus 559 patients.
Study variablesClinical variables analyzed were age, gender, risk factors (hypertension, diabetes, dyslipidemia, smoking, heart failure, previous MI), physical examination at admission (heart rate, systolic blood pressure [BP], diastolic BP, and pulse pressure). Risk stratification was performed using the GRACE score, dividing the sample into three groups: low (≤88 points), medium (89–118 points), and high risk (>118 points).
Laboratory variables recorded within two hours of admission included hemoglobin, leukocyte count, blood glucose, fibrinogen, uric acid, glomerular filtration rate estimated by the 4-variable Modification of Diet in Renal Disease equation,14 creatine kinase (CK) and/or creatine kinase-MB fraction (CK-MB) and troponin I. The biochemical variables were obtained using the Synchron LX20 Pro analyzer.
Patients were classified on the basis of ST-segment characteristics on the electrocardiogram into three groups: elevated, normal, or depressed. The occurrence of cardiac arrhythmias during hospitalization was documented.
Left ventricular ejection fraction (EF) was determined by echocardiography at admission and was classified as normal if >50% or reduced if ≤50%. Patients with Killip class >1 at admission were considered to have acute heart failure.
Reperfusion therapy for patients with ST-segment elevation ACS was divided into fibrinolysis and percutaneous coronary intervention.
The endpoints recorded were all-cause mortality (in-hospital or within six months) and adverse cardiovascular events (post-infarction angina, re-infarction, and heart failure). Data were collected by clinical and/or telephone contact with patients or their families.
Statistical analysisContinuous variables were expressed as mean ± standard deviation and qualitative variables as frequency and percentage. Differences in quantitative variables between groups with a normal distribution were compared with the Student's t test for independent samples; in cases of non-normal distribution, a nonparametric Mann-Whitney test was used. Discrete variables were compared using the chi-square test or Fisher's exact test. The independent variable of the study was categorized according to gender. Cumulative survival for each category of the main variable was modeled using Kaplan-Meier curves, and these categories were compared with the log-rank statistic. To determine the independent role of the variables in predicting mortality, a Cox proportional hazards analysis was performed. The estimated coefficients were expressed as hazard ratio (HR) with 95% confidence intervals (95% CI). The final determination of independent predictors of 6-month mortality was performed in multivariate analysis including variables with p<0.05 in the univariate analysis. Values were considered significant at p<0.05. The statistics software used was SPSS 15.0.
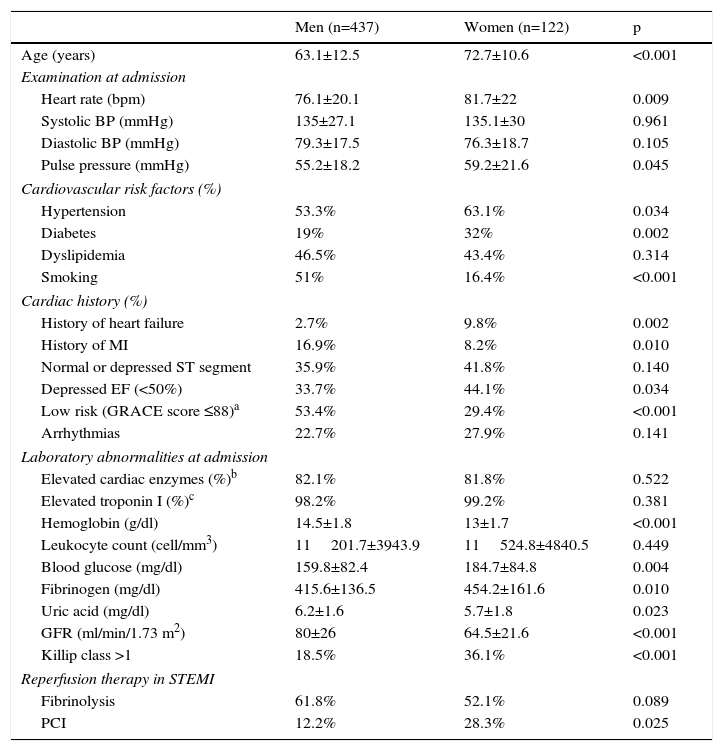
ResultsSample characteristicsThe baseline characteristics of the 559 patients with ACS enrolled in the study are shown in Table 1. The mean age was 65.2±12.7 years (33–93 years), and 21.8% were women. Females had lower diastolic BP and increased leukocyte count on admission, lower prevalence of dyslipidemia, increased frequency of normal or depressed ST segment on the electrocardiogram, and a greater tendency to arrhythmias. Female gender was also associated with older mean age, higher heart rate and pulse pressure on admission, increased prevalence of hypertension and diabetes but lower prevalence of smoking, more frequent history of heart failure but a lower incidence of previous stroke, lower EF, increased risk according to the GRACE score, and a higher frequency of heart failure (Killip >1) during hospitalization. Laboratory parameters at admission significantly associated with female gender were lower hemoglobin and uric acid levels and worse renal function, and higher blood glucose and fibrinogen concentrations. Women more often underwent primary angioplasty. Women clearly had a worse prognosis, with a higher rate of adverse cardiac events and higher mortality, both in-hospital and during follow-up (Table 2).
Demographic and clinical characteristics of the study population at admission by gender.
| Men (n=437) | Women (n=122) | p | |
|---|---|---|---|
| Age (years) | 63.1±12.5 | 72.7±10.6 | <0.001 |
| Examination at admission | |||
| Heart rate (bpm) | 76.1±20.1 | 81.7±22 | 0.009 |
| Systolic BP (mmHg) | 135±27.1 | 135.1±30 | 0.961 |
| Diastolic BP (mmHg) | 79.3±17.5 | 76.3±18.7 | 0.105 |
| Pulse pressure (mmHg) | 55.2±18.2 | 59.2±21.6 | 0.045 |
| Cardiovascular risk factors (%) | |||
| Hypertension | 53.3% | 63.1% | 0.034 |
| Diabetes | 19% | 32% | 0.002 |
| Dyslipidemia | 46.5% | 43.4% | 0.314 |
| Smoking | 51% | 16.4% | <0.001 |
| Cardiac history (%) | |||
| History of heart failure | 2.7% | 9.8% | 0.002 |
| History of MI | 16.9% | 8.2% | 0.010 |
| Normal or depressed ST segment | 35.9% | 41.8% | 0.140 |
| Depressed EF (<50%) | 33.7% | 44.1% | 0.034 |
| Low risk (GRACE score ≤88)a | 53.4% | 29.4% | <0.001 |
| Arrhythmias | 22.7% | 27.9% | 0.141 |
| Laboratory abnormalities at admission | |||
| Elevated cardiac enzymes (%)b | 82.1% | 81.8% | 0.522 |
| Elevated troponin I (%)c | 98.2% | 99.2% | 0.381 |
| Hemoglobin (g/dl) | 14.5±1.8 | 13±1.7 | <0.001 |
| Leukocyte count (cell/mm3) | 11201.7±3943.9 | 11524.8±4840.5 | 0.449 |
| Blood glucose (mg/dl) | 159.8±82.4 | 184.7±84.8 | 0.004 |
| Fibrinogen (mg/dl) | 415.6±136.5 | 454.2±161.6 | 0.010 |
| Uric acid (mg/dl) | 6.2±1.6 | 5.7±1.8 | 0.023 |
| GFR (ml/min/1.73 m2) | 80±26 | 64.5±21.6 | <0.001 |
| Killip class >1 | 18.5% | 36.1% | <0.001 |
| Reperfusion therapy in STEMI | |||
| Fibrinolysis | 61.8% | 52.1% | 0.089 |
| PCI | 12.2% | 28.3% | 0.025 |
BP: blood pressure; EF: ejection fraction; GFR: glomerular filtration rate by the 4-variable Modification of Diet in Renal Disease equation; MI: myocardial infarction; PCI: percutaneous coronary intervention (with or without stenting); STEMI: ST-segment elevation myocardial infarction.
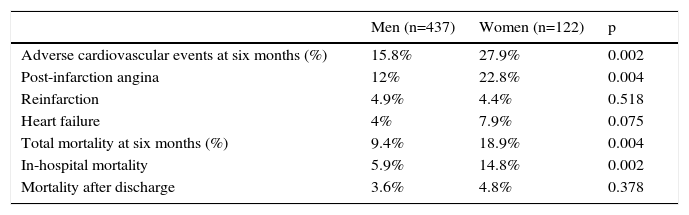
Adverse cardiovascular events and all-cause mortality at six months by gender.
| Men (n=437) | Women (n=122) | p | |
|---|---|---|---|
| Adverse cardiovascular events at six months (%) | 15.8% | 27.9% | 0.002 |
| Post-infarction angina | 12% | 22.8% | 0.004 |
| Reinfarction | 4.9% | 4.4% | 0.518 |
| Heart failure | 4% | 7.9% | 0.075 |
| Total mortality at six months (%) | 9.4% | 18.9% | 0.004 |
| In-hospital mortality | 5.9% | 14.8% | 0.002 |
| Mortality after discharge | 3.6% | 4.8% | 0.378 |
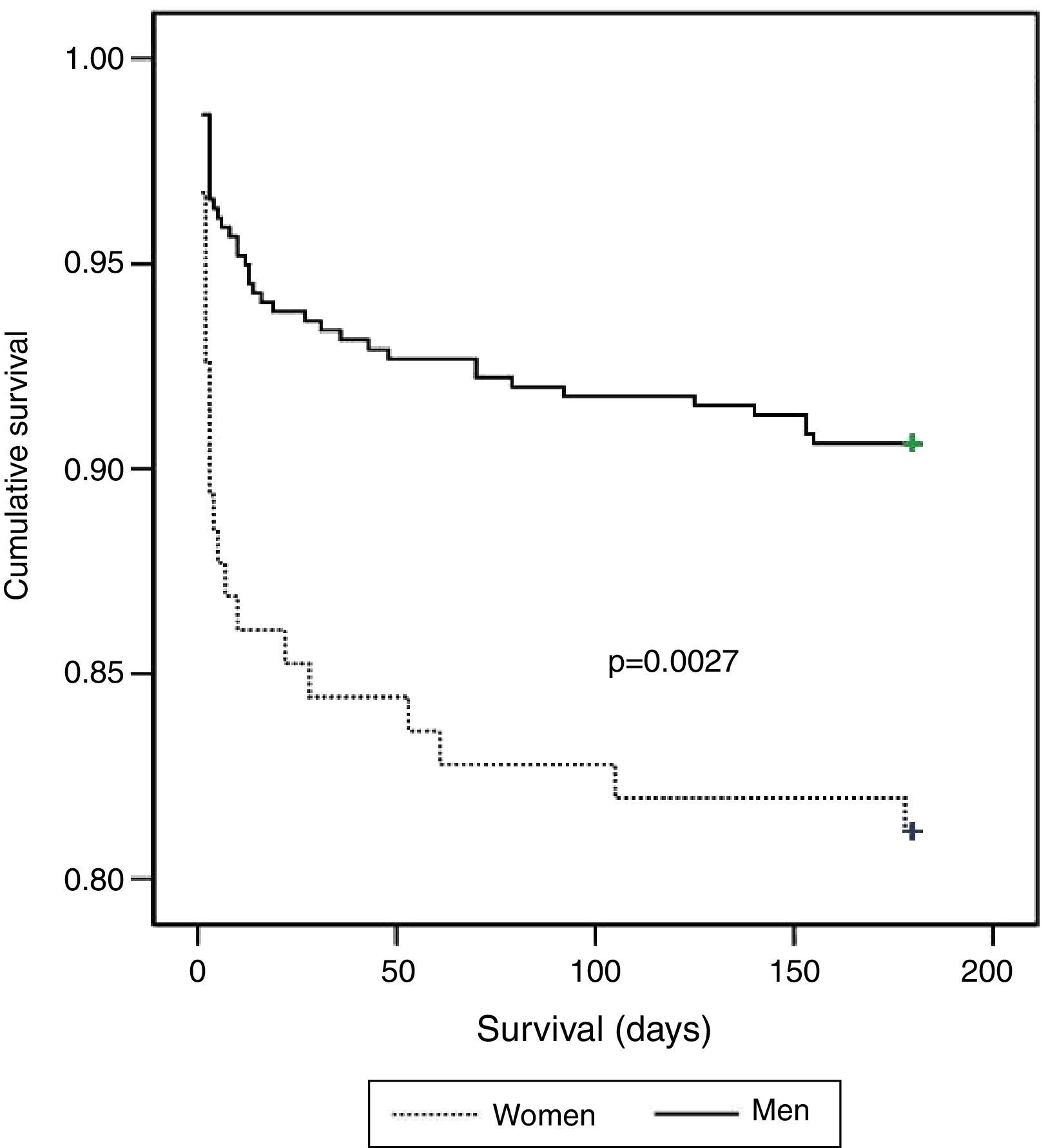
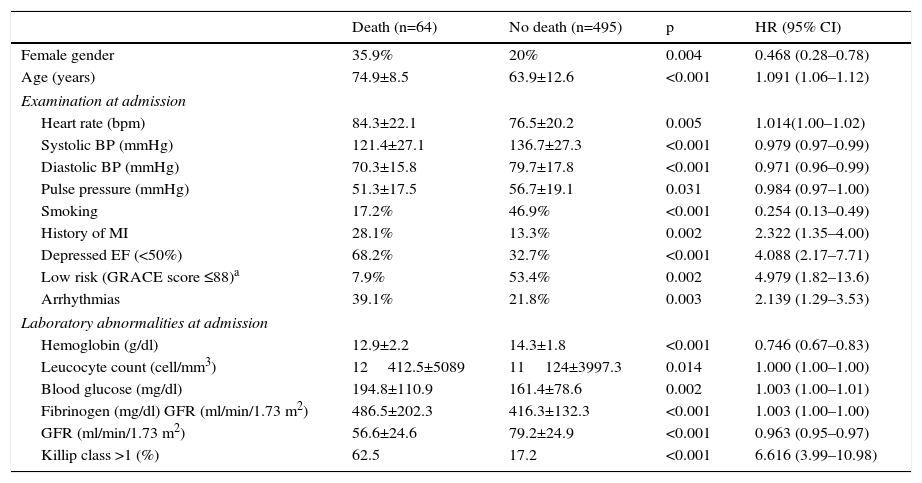
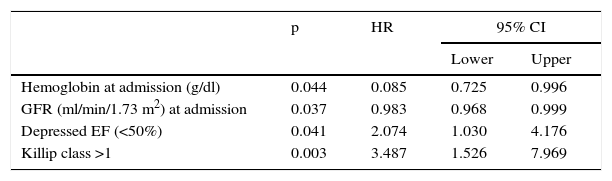
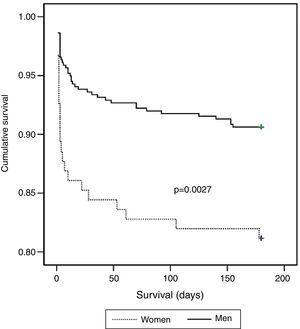
There were 64 deaths (11.4%), 44 in-hospital, the most frequent causes for which were cardiogenic shock (61.4%), heart failure (13.6%), and ventricular fibrillation (9.1%). The variables associated with mortality are shown in Table 3. In the deceased group 35.9% were women vs. 20% of the no-death group (p=0.004). Other predictors of mortality were age, hemodynamic measures at admission (decreased heart rate, systolic BP, diastolic BP, and pulse), laboratory tests at admission (lower hemoglobin concentration and glomerular filtration rate, increased blood glucose, fibrinogen and leukocyte count), history of MI and absence of smoking, low EF, Killip class >1, arrhythmias, and not being in the low-risk GRACE score group. The Kaplan-Meier curve shows poorer survival for women (Figure 1). Women had significantly higher mortality than men (18.9 vs. 9.4%, hazard ratio [HR] 0.468, 95% CI: 0.281–0.781, p=0.004). On multivariate analysis (adjusted for age, heart rate, systolic BP, diastolic BP, and pulse pressure at admission; history of smoking and previous infarction, EF, presence of arrhythmias, severity on GRACE score, hemoglobin, leukocytes, blood glucose, fibrinogen, and GFR at admission; and Killip class >1), gender as a variable was not a significant predictor of mortality. Independent predictors of mortality were hemoglobin level at admission (HR: 0.085; 95% CI: 0.725–0.996, p=0.044), renal function at admission (HR: 0.983, 95% CI: 0.968–0.999, p=0.037), depressed EF (HR: 2.074, 95% CI: 1.030–4.176; p=0.041), and heart failure during hospitalization as measured by Killip class >1 (HR: 3.487, 95% CI: 1.526–7.969, p=0.003), the latter being the main predictor factor of mortality (Table 4).
Individual variables associated with mortality at six months after acute coronary syndrome.
| Death (n=64) | No death (n=495) | p | HR (95% CI) | |
|---|---|---|---|---|
| Female gender | 35.9% | 20% | 0.004 | 0.468 (0.28–0.78) |
| Age (years) | 74.9±8.5 | 63.9±12.6 | <0.001 | 1.091 (1.06–1.12) |
| Examination at admission | ||||
| Heart rate (bpm) | 84.3±22.1 | 76.5±20.2 | 0.005 | 1.014(1.00–1.02) |
| Systolic BP (mmHg) | 121.4±27.1 | 136.7±27.3 | <0.001 | 0.979 (0.97–0.99) |
| Diastolic BP (mmHg) | 70.3±15.8 | 79.7±17.8 | <0.001 | 0.971 (0.96–0.99) |
| Pulse pressure (mmHg) | 51.3±17.5 | 56.7±19.1 | 0.031 | 0.984 (0.97–1.00) |
| Smoking | 17.2% | 46.9% | <0.001 | 0.254 (0.13–0.49) |
| History of MI | 28.1% | 13.3% | 0.002 | 2.322 (1.35–4.00) |
| Depressed EF (<50%) | 68.2% | 32.7% | <0.001 | 4.088 (2.17–7.71) |
| Low risk (GRACE score ≤88)a | 7.9% | 53.4% | 0.002 | 4.979 (1.82–13.6) |
| Arrhythmias | 39.1% | 21.8% | 0.003 | 2.139 (1.29–3.53) |
| Laboratory abnormalities at admission | ||||
| Hemoglobin (g/dl) | 12.9±2.2 | 14.3±1.8 | <0.001 | 0.746 (0.67–0.83) |
| Leucocyte count (cell/mm3) | 12412.5±5089 | 11124±3997.3 | 0.014 | 1.000 (1.00–1.00) |
| Blood glucose (mg/dl) | 194.8±110.9 | 161.4±78.6 | 0.002 | 1.003 (1.00–1.01) |
| Fibrinogen (mg/dl) GFR (ml/min/1.73 m2) | 486.5±202.3 | 416.3±132.3 | <0.001 | 1.003 (1.00–1.00) |
| GFR (ml/min/1.73 m2) | 56.6±24.6 | 79.2±24.9 | <0.001 | 0.963 (0.95–0.97) |
| Killip class >1 (%) | 62.5 | 17.2 | <0.001 | 6.616 (3.99–10.98) |
BP: blood pressure; CI: confidence interval; EF: ejection fraction; GFR: glomerular filtration rate by the 4-variable Modification of Diet in Renal Disease equation; HR: hazard ratio; MI: myocardial infarction.
Factors predicting all-cause mortality at six months after acute coronary syndrome by Cox regression analysis.
| p | HR | 95% CI | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Hemoglobin at admission (g/dl) | 0.044 | 0.085 | 0.725 | 0.996 |
| GFR (ml/min/1.73 m2) at admission | 0.037 | 0.983 | 0.968 | 0.999 |
| Depressed EF (<50%) | 0.041 | 2.074 | 1.030 | 4.176 |
| Killip class >1 | 0.003 | 3.487 | 1.526 | 7.969 |
CI: confidence interval; EF: ejection fraction; GFR: glomerular filtration rate by the 4-variable Modification of Diet in Renal Disease equation; HR: hazard ratio.
The study collected data prospectively on a consecutive series of patients who suffered high-risk ACS treated with an up-to-date therapeutic protocol for unstable IHD. The women in the study population were older and had a more unfavorable clinical profile, with poorer initial prognosis according to the GRACE risk score, and suffered a greater number of adverse cardiovascular events including post-infarction angina, reinfarction and heart failure, as well as increased in-hospital and all-cause mortality, in comparison to men. However, on multivariate analysis, these gender differences disappeared, which suggests that more or less favorable evolution depends to a large extent on the baseline characteristics of the groups.
Women had older mean age than men (63.1 vs. 72.7 years) and began to suffer coronary acute events a decade later, which is attributable, in part, to the protective effect of estrogens, according to some studies.15 Elevated total cholesterol, LDL cholesterol, and triglyceride levels, and lower HDL-C levels during menopause may contribute to the increased risk, especially from the sixth decade of life.16 In addition, estrogens, through the nitric oxide pathway, induce smooth muscle cell dilation and inhibit vascular proliferation.17,18 For years, these data were considered to suggest that exogenously administered estrogen could have a cardioprotective effect. However, the results of clinical trials on both primary (WHI and WISDOM) and secondary prevention (HERS and ESPRIT), and in angiographic endpoints (WAS, WELL-HART and WAVE), advised against the use of hormone replacement therapy, showing negative results and even greater progression of atherosclerosis and increased adverse effects such as CVD, deep vein thrombosis, pulmonary embolism and stroke, not to mention the potential carcinogenic effect on the breast, ovaries, and endometrium.19,20 Furthermore, studies focused on elderly patients with MI and a difference in age of less than one year between women and men have shown that hospital mortality in women is higher than in men (40% and 25%, respectively), which leads us to conclude that the age factor alone does not explain a worse prognosis. Also, if age is associated with a higher prevalence of risk factors for cardiovascular and other disease, then this confers a greater early mortality in women with MI.21
Cardiovascular disease is characterized by multifactorial etiology. The increased coronary risk is mainly related to risk factors that increase with age. Aging, hypertension, dyslipidemia, diabetes, smoking, physical inactivity, obesity, and family history are important and reinforce each other. In our results, a history of hypertension and diabetes was associated significantly with female gender.
The prevalence of hypertension in the adult population is 28% and more women are affected from the fifth or sixth decades of life (52% vs. 48% in men).22 Its impact in the form of left ventricular hypertrophy is associated with an increased risk of cardiovascular events, conferring a worse prognosis in women than in men.16 Diabetes, especially type 2, is more prevalent in women than in men, particularly after 65 years of age, and is the single most potent cardiovascular risk factor for coronary heart disease. Its impact appears to be even higher than in men.23
We observed higher mortality associated with greater renal dysfunction and this association was significant on admission in women. Renal failure is a poor prognostic factor in patients suffering from an acute coronary event; it is estimated that one third of these patients present renal dysfunction on admission.24 The above factors probably contributed to the increased renal dysfunction in women in our study and the fact that women had more prior heart failure, higher Killip class on admission and more episodes of cardiac decompensation after discharge. We also observed lower EF values in women and an increased frequency of arrhythmias during hospitalization. Although women had fewer previous episodes of IHD than males, their prognosis was less favorable, as also seen in the BADAPIC registry.25
With regard to laboratory tests on admission, anemia, hyperglycemia, and elevated fibrinogen levels were associated with increased mortality in women; in the case of anemia, even after multivariate analysis. The inflammatory mechanism underlying vascular stress in acute coronary events appears to contribute to these laboratory abnormalities. Elevated inflammatory markers predict cardiovascular risk and inflammation may act not only by promoting atherogenesis but also by destabilizing vulnerable plaque. In a study by Arant et al.,26 anemia, defined as a hemoglobin level of less than 12 g/dl, was a predictor of cardiovascular events following an ACS during a follow-up of 3.3 years. In addition, various studies have noted a relationship between the occurrence and severity of hyperglycemia and increased morbidity and mortality in ACS, independently of previously diagnosed diabetes.27
Some studies have found an association, at least in part, between the poorer prognosis in women and gender bias observed in the therapeutic measures applied, especially in reperfusion therapy, whose benefit would be even higher because women are higher-risk patients.28 The CRUSADE study data demonstrate that although IHD in women continues to be associated with older age, as well as with a higher prevalence of diabetes and hypertension, the therapeutic approach continues to be less aggressive, with fewer coronary angiograms and heparin treatments.29 We did not find these differences in the group of patients with ST-segment elevation (62.8% of the sample), and there were no significant differences in fibrinolytic therapy and reperfusion with primary angioplasty, even in women who were more likely to benefit from these treatments.
ConclusionsWe believe that all the risk factors known to be associated more closely with female gender may be responsible for their worse outcome. The significance of gender as such is diluted in multivariate analysis. The importance of classical variables as prognostic markers, which are independently associated with higher mortality (EF <50%, Killip class >1, renal dysfunction and anemia on admission) is increasing. Women have a high-risk stratification according to the GRACE score, suffer more adverse cardiovascular events, and have higher mortality, especially in the short term. We are aware that IHD in women is a major problem whose importance is greater than other conditions already known to be leading causes of mortality in women, such as breast cancer. However, we believe that before considering female gender per se an independent predictor of mortality and resigning ourselves to the existence of genetic differences that are not yet well known, we should first increase our efforts to include women in scientific studies and discussion forums rather than merely extrapolate research findings focused mostly on men. We should also promote the implementation of prevention campaigns because, although we deal with the risk factors of men, the prevalence of diabetes, hypertension, dyslipidemia, obesity, and smoking is still increasing in women.
Our work has several limitations: the sample size is small and therefore so is the number of deaths. A longer follow-up period would have provided more information, and it would also have been interesting to investigate the causes of mortality during follow-up.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.