Accumulation of epicardial adipose tissue (EAT) is associated with coronary artery disease (CAD) and increased risk of coronary events in asymptomatic subjects and low-risk patients, suggesting that EAT promotes atherosclerosis in its early stage. Recent studies have shown that the presence of CAD affects the properties of adjacent EAT, leading to dynamic changes in the molecular players involved in the interplay between EAT and the coronary arteries over the history of the disease. The role of EAT in late-stage CAD has not been investigated.
ObjectivesIn a comparative analysis with mediastinal and subcutaneous adipose tissue, we aim to investigate whether the volume of EAT assessed by computed tomography and its proteome assessed by SWATH-MS mass spectrometry are associated with late stages of CAD in an elderly cohort of severe aortic stenosis patients.
MethodsThe EPICHEART study (NCT03280433) is a prospective study enrolling patients with severe degenerative aortic stenosis referred for elective aortic valve replacement, whose protocol includes preoperative clinical, nutritional, echocardiographic, cardiac computed tomography and invasive coronary angiographic assessments. During cardiac surgery, samples of EAT and mediastinal and subcutaneous thoracic adipose tissue are collected for proteomics analysis by SWATH-MS. In addition, pericardial fluid and peripheral and coronary sinus blood samples are collected to identify circulating and local adipose tissue-derived biomarkers of CAD.
ConclusionWe designed a translational study to explore the association of EAT quantity and quality with advanced CAD. We expect to identify new biochemical factors and biomarkers in the crosstalk between EAT and the coronary arteries that are involved in the pathogenesis of late coronary atherosclerosis, especially coronary calcification, which might be translated into new therapeutic targets and imaging tools by biomedical engineering.
Acumulação de tecido adiposo epicárdico (TAE) tem sido associado a doença coronária aterosclerótica (DC) e aumento do risco de eventos coronários em indivíduos assintomáticos e doentes de baixo risco, sugerindo que o TAE pode promover fases precoces da DC. Estudo recentes mostraram que a presença de DC afeta as características do TAE adjacente levando a modificações dinâmicas nos mediadores envolvidos na comunicação entre o TAE e as artérias coronárias ao longo da história da DC. O papel doTAE nas fases avançadas da aterosclerose coronária não foi investigado.
ObjetivosAtravés de análise comparativa com o tecido adiposo mediastínico e subcutâneo, pretendemos investigar se o volume do TAE, avaliado por tomografia computadorizada (TC), e o seu proteoma, avaliado por espectrometria de massa técnica de SWATH, estão associados a estadios avançados da DC numa coorte de estenose aórtica grave.
MétodosO estudo EPICHEART (NCT03280433) é um estudo prospetivo que inclui doentes com estenose aórtica grave referenciados para substituição eletiva da válvula aórtica, cujo protocolo envolve avaliação pré-operatória clínica, nutricional, ecocardiográfica, por TC e angiografia coronária invasiva. Durante a cirurgia cardíaca, colhemos amostras de tecido adiposo epicárdico, mediastínico e subcutâneo para análise do seu proteoma por espectrometria de massa técnica de SWATH. Adicionalmente, colhemos líquido pericárdico, sangue venoso periférico e do seio coronário para investigar mediadores de DC derivados do TAE na circulação sistémica e local.
ConclusãoDesenhámos um estudo de translação para explorar a associação da quantidade e qualidade do TAE com a DC tardia. Esperamos identificar mediadores da comunicação recíproca entre o TAE e as artérias coronárias que estão envolvidos na patogénese das fases avançadas da DC, especialmente, calcificação coronária, os quais podem servir como novos alvos terapêuticos e soluções de engenharia biomédica para visualização da DC.
More than 25 years have passed since Agatston et al.1 introduced coronary artery calcification (CAC) quantification on computed tomography (CT) as a marker of subclinical coronary artery disease (CAD) and predictor of coronary events, independently of standard risk factors or risk scores.2 Recently, epicardial adipose tissue (EAT) has emerged as a new marker of CAD that complements the prognostic information provided by the Agatston CAC score and increases the predictive value of cardiac CT without additional radiation exposure or contrast administration.3–5
EAT is the visceral fat of the heart, located between the visceral pericardium and the myocardium. In physiological conditions, EAT exerts metabolic (source of free fatty acids), thermogenic (similar to brown fat) and mechanical cardioprotective properties.6 However, dysfunctional EAT has been associated with coronary atherosclerosis, atrial fibrillation and diastolic dysfunction, due to a shift in its secretome profile. In patients with CAD, compared with subcutaneous adipose tissue, EAT presented higher expression of proinflammatory, pro-oxidant, and angiogenic regulatory genes, and greater infiltration by immune cells, particularly proinflammatory M1 macrophages.7–9
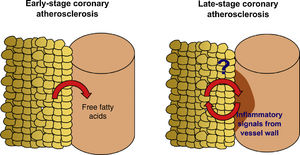
EAT can be quantified in clinical practice by non-invasive imaging techniques.10,11 In a contemporary systematic review and meta-analysis,12 we demonstrated that EAT volume assessed by CT is associated with the presence of coronary stenosis (lumen reduction ≥50% for obstructive stenosis and ≥70% for significant stenosis), myocardial ischemia and incident coronary events, independently of traditional cardiovascular risk factors. Most of the studies analyzed, however, were based on community-derived cohorts4,13–19 composed of subjects at very low cardiovascular risk. Similarly, Mahabadi et al.20 showed that EAT is associated with CAC progression, particularly in young subjects, suggesting that EAT has a role in the early stages of coronary atherosclerosis. How EAT volume is linked to CAD in high-risk patients and what EAT-derived molecular players are involved in the pathophysiology of advanced stages of atherosclerosis are still unknown (Figure 1).
Study hypothesis. Accumulation of epicardial adipose tissue (EAT) is associated with non-calcified, vulnerable coronary artery disease (CAD) and increased risk of coronary events in asymptomatic subjects and low-risk patients. Recent studies have shown that the presence of CAD affects the properties of the adjacent EAT, and that the molecular players involved in the interplay between EAT and the coronary arteries may undergo dynamic changes over the history of the disease. We designed the EPICHEART study (NCT03280433) to investigate how EAT volume and proteome are linked to the presence of coronary stenosis and coronary calcification in an elderly cohort of severe aortic stenosis patients who underwent elective aortic valve replacement.
In a high-risk cohort of patients with symptomatic severe aortic stenosis (AS) undergoing surgical aortic valve replacement, we aim to determine the association between the quantity and quality of EAT and coronary stenosis and calcification, taking as comparison the mediastinal and subcutaneous thoracic fat depots. Specifically, our aims are to investigate the association of EAT volume assessed by CT with the extent, distribution and complexity of coronary stenosis assessed by invasive coronary angiography, to compare the proteomics of EAT with that of mediastinal and subcutaneous adipose tissue obtained by sequential windowed acquisition of all theoretical fragment ion mass spectra (SWATH-MS) mass spectrometry, and to identify the EAT-derived proteins linked to advanced coronary atherosclerosis.
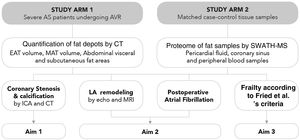
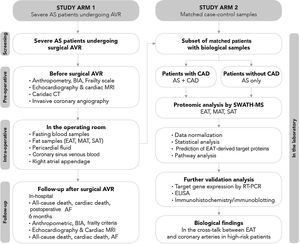
MethodsStudy populationThe Influence of EPICardial Adipose Tissue in HEART diseases (EPICHEART) study is a translational study designed to investigate the association between EAT and CAD, left atrial remodeling, postoperative atrial fibrillation, and frailty syndrome in patients with symptomatic severe AS. The study is composed of two arms: Arm 1 includes patients with severe AS referred for aortic valve replacement in whom we assessed clinical associations between EAT volume and CAD, and Arm 2 includes adipose tissue samples collected during cardiac surgery from a subset of Arm 1 patients (Figure 2).
EPICHEART study aims. The EPICHEART study is a prospective translational study enrolling severe aortic stenosis (AS) patients referred for aortic valve replacement, in which we will provide clinical associations between epicardial adipose tissue (EAT) volume assessed by computed tomography and different outcomes of heart disease, namely coronary artery disease, left atrial remodeling and incidence of postoperative atrial fibrillation, and frailty syndrome in severe AS patients referred to aortic valve replacement (Arm 1). In addition, the proteomic profile of EAT and mediastinal and subcutaneous thoracic fat samples collected from matched case-control pairs will be investigated and compared (Arm 2). In this paper, we focused on the methodology employed to address Aim 1. AVR: aortic valve replacement; CAD: coronary artery disease; CT: computed tomography; EAT: epicardial adipose tissue; echo: echocardiography; ICA: invasive coronary angiography; LA: left atrial; MAT: mediastinal adipose tissue; MRI: magnetic resonance imaging; SWATH-MS: sequential windowed acquisition of all theoretical fragment ion mass spectra mass spectrometry.
Patients with symptomatic severe AS (defined as aortic valve area of <1 cm2 or 0.6 cm2/m2 by transthoracic echocardiography) referred for aortic valve replacement were included. Patients have been recruited from the cardiology and cardiothoracic surgery departments at our institution since October 2014. Exclusion criteria are acute coronary syndrome in the previous three months, history of persistent or permanent atrial fibrillation or flutter, coexisting moderate to severe aortic valve regurgitation or moderate to severe mitral valve disease, bicuspid aortic valve, left ventricular dilatation (end-diastolic volume index >75 ml/m2), left ventricular ejection fraction <55%, stage 3-5 chronic renal failure (defined as glomerular filtration rate <30 ml/min/1.73 m2 estimated by the Cockcroft-Gault formula and adjusted for body surface area21), moderate to severe chronic obstructive pulmonary disease (defined as forced expiratory volume in one second <50% according to the 2011 Global Initiative for Chronic Obstructive Pulmonary Disease guidelines22), or active malignancy (no evidence of recurrence and no longer receiving active treatment23) (Table 1).
EPICHEART study population: inclusion and exclusion criteria.
| Inclusion criterion |
| Symptomatic severe AS (aortic valve area <1 cm2 or 0.6 cm2/m2 by transthoracic echocardiography) |
| Exclusion criteria |
| Acute coronary syndrome in the previous 3 months |
| History of persistent or permanent atrial fibrillation or flutter |
| Coexisting moderate to severe aortic valve regurgitation or moderate to severe mitral valve disease |
| Bicuspid aortic valve |
| LV dilatation (end-diastolic volume index >75 ml/m2) |
| LVEF <55% |
| Stage 3–5 CKD |
| Moderate to severe COPD |
| Active malignancy |
AS: aortic stenosis; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; LV: left ventricular; LVEF: left ventricular ejection fraction.
The study protocol includes patient screening, preoperative assessment, intraoperative sample collection, and in-hospital and six-month postoperative follow-up (Figure 3).
Study flowchart. Before surgery, beyond a detailed demographic, anthropometric and clinical characterization, patients’ body composition will be determined by bioelectrical impedance analysis and the presence of frailty assessed using Fried et al.’s criteria; cardiac computed tomography will be performed to quantify fat depots including epicardial adipose tissue (EAT) volume and total thoracic fat and abdominal subcutaneous or visceral fat area, and coronary artery calcification through Agatston calcium score; the degree, distribution and complexity of coronary artery disease (CAD) will be assessed invasively by cardiac catheterization; and cardiac structure and function will be characterized in detail by both transthoracic echocardiography and cardiac magnetic resonance imaging. On the morning of surgery, fasting peripheral venous blood samples will be obtained and during surgery, biopsies will be taken from three fat depots (EAT, mediastinal and subcutaneous thoracic fat) and the right atrial appendage; cardiac fluids (pericardial fluid and coronary sinus venous blood) will collected. Follow-up will be performed during hospitalization until hospital discharge and at six months after aortic valve replacement to assess the incidence of postoperative atrial fibrillation, cardiac remodeling and frailty change following cardiac surgery. The proteomic profile of the three fat samples (EAT, mediastinal and subcutaneous adipose tissue) will be investigated using SWATH-MS. After data normalization, a comparative analysis will be performed between the EAT proteome of patients with CAD and their matched controls without CAD, and between EAT, mediastinal adipose tissue and subcutaneous thoracic fat. Further validation of proteomic findings will be needed via quantitative polymerase chain reaction and Western blotting (and/or immunohistochemistry), and subsequently the pericardial fluid and coronary sinus blood (intracardiac local levels) and peripheral venous blood (systemic circulating levels) will be investigated by enzyme linked-immunosorbent assay in order to find potential target proteins secreted by EAT involved in the pathogenesis of late CAD. AF: atrial fibrillation; AS: aortic stenosis; AVR: aortic valve replacement; BIA: bioelectrical impedance analysis; CAD: coronary artery disease; CT: computed tomography; EAT: epicardial adipose tissue; ELISA: enzyme linked-immunosorbent assay; ICA: invasive coronary angiography; MAT: mediastinal adipose tissue; MRI: magnetic resonance imaging; PCR: polymerase chain reaction; SAT: subcutaneous adipose tissue; SWATH-MS: sequential windowed acquisition of all theoretical fragment ion mass spectra mass spectrometry.
All included patients undergo a prespecified examination including clinical, anthropometric and bioelectrical impedance analyses, echocardiographic, cardiac CT and invasive coronary angiography assessments. A subset of patients undergoes cardiac magnetic resonance imaging. Fasting peripheral blood samples are collected on the morning of the surgery.
Intraoperative assessmentDuring surgery and before the beginning of cardiopulmonary bypass, samples of approximately 0.5-10 g are collected from three different adipose tissue depots, namely EAT (taken from the anterior surface of the heart), mediastinal adipose tissue (MAT) (collected outside the pericardial cavity) and thoracic subcutaneous adipose tissue (taken from the incision at the chest wall). These specimens are rinsed with phosphate-buffered saline and divided into two portions: one imbedded in optimal cutting temperature compound and snap-frozen in liquid nitrogen at –80°C, and the other fixed in formaldehyde for histologic analysis. In addition to fat samples, samples of pericardial fluid, coronary sinus venous blood and the right atrial appendage are also collected. The right atrial appendage sample is collected immediately after cannulation of the right atrium.
In-hospital assessmentIn-hospital death and postoperative atrial fibrillation are recorded at six months after aortic valve replacement. The impact of surgery on body fat composition, recovery from frailty criteria, and cardiac remodeling assessed by transthoracic echocardiography and cardiac magnetic resonance imaging are analyzed.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Portuguese Data Protection Authority (CNPD) and by the Institutional Ethics Committee of Centro Hospitalar de Vila Nova de Gaia/Espinho. The study is registered at ClinicalTrials.gov (NCT03280433).
In this article, we focus on the methods used to study the relationship between EAT and coronary atherosclerosis. The variables are described in Table 2; for further details on the EPICHEART study's full endpoints see Supplementary Table 1.
EPICHEART study variables.
| Coronary artery disease |
| Cardiac computed tomography |
| Coronary artery calcification (coronary calcium score) |
| Invasive coronary angiography |
| Coronary artery stenosis (extent of CAD: obstructive stenosis, significant stenosis; distribution of CAD: number of diseased vessels, and complexity of CAD by SYNTAX score |
| Body fat quantity |
| Computed tomography |
| Thoracic fat: EAT volume, mediastinal fat volume |
| Abdominal fat: total, visceral and subcutaneous abdominal fat areas |
| Bioelectrical impedance analysis |
| Fat mass, fat mass percentage |
| Free fat mass, free fat mass percentage |
| Anthropometry |
| Weight, height, waist circumference, hip circumference |
| Body fat proteome |
| SWATH-MSFurther validation by qPCR and western blotting (and/or immunohistochemistry) |
| EAT, mediastinal fat, subcutaneous thoracic fat |
| Circulating blood |
| Levels of proteins with different expression between groups |
| Coronary sinus blood |
| Levels of proteins with different expression between groups |
| Pericardial fluid |
| Levels of proteins with different expression between groups |
CAD: coronary artery disease; EAT: epicardial adipose tissue; MS: mass spectrometry; qPCR: quantitative polymerase chain reaction; SWATH-MS: sequential windowed acquisition of all theoretical fragment ion mass spectra mass spectrometry.
The extent, distribution and complexity of coronary stenosis are characterized by invasive coronary angiography. An experienced invasive cardiologist (15 years performing and interpreting invasive coronary angiography) classifies the most severe stenosis within each coronary segment visually, and the anatomical complexity of CAD by the validated SYNTAX score. According to the standard definitions of flow-limiting stenosis, each patient is categorized according to (1) extent of CAD, classified by the degree of stenosis in obstructive CAD (i.e. ≥50% stenosis) and significant CAD (i.e. ≥50% stenosis in the left main coronary artery, and/or ≥70% or greater in any other coronary artery); (2) distribution of CAD, which is an ordered variable classified by CAD severity as one-, two- or three-vessel disease; and (3) complexity of CAD, which is a categorical variable defined as SYNTAX score ≥22.
Coronary artery calcificationAll patients undergo non-contrasted cardiac multidetector CT (SOMATOM Sensation Cardiac 64, Siemens, Forchheim, Germany) 1-3 months before cardiac surgery for CAC scoring and analysis of fat depots. A CAC scan is performed using a prospectively electrocardiogram-triggered scanning protocol with the following parameters: tube voltage 120 kV, tube current 190 mA, gantry rotation 330 ms, collimation 24 mm×1.2 mm, pitch 0.2, and image reconstruction 3 mm. CAC is reported as the Agatston score and calculated using a detection threshold of 130 Hounsfield units (HU) with semi-automated software (syngo Calcium Scoring, Siemens Medical Solutions). In addition, an abdominal single-slice acquisition is obtained between L4 and L5-S1 with the following radiographic factors: 120 kV and 216 mA with 5 mm thickness, resulting in an estimated radiation exposure of 0.06 mSv.
Adipose tissue assessmentAdipose tissue quantityFat depots including EAT, MAT, and abdominal visceral and subcutaneous adipose tissue are quantified by CT (syngo Volume, Siemens Medical Solutions), according to the predefined image display setting (window of -150 to -50 HU) to identify voxels that correspond to adipose tissue. To measure EAT volume, the pericardium is manually traced every 10 mm from the right pulmonary artery to the diaphragm to determine the region of interest. MAT is derived by the difference between total intrathoracic fat (defined as all fat located inside the thoracic cavity from first costal arch to diaphragm and from aorta to sternum) and EAT volumes. Total abdominal fat area is calculated as the total adipose tissue in the examined abdominal slice, and visceral abdominal fat area as adipose tissue located inside the region of interest defined by delineating the abdominal wall muscular layer. Subcutaneous adipose tissue is obtained by subtracting visceral abdominal fat from total abdominal fat areas.
Intra- and inter-rater reliability for each fat measurement was evaluated in 55 random patients as described previously.24
Adipose tissue qualityThe three fat samples (EAT, MAT and subcutaneous thoracic adipose tissue) will be phenotyped by SWATH-MS study of their proteome. A comparative analysis of EAT proteomics between patients with CAD and their matched controls without CAD, and between EAT, MAT and subcutaneous thoracic fat, will then be performed. Differently regulated proteins will be identified between tissues in patients with and without CAD through robust multivariate models. Finally, proteomics findings will be validated by immunohistochemistry and by determining transcript levels in EAT samples, and their availability explored in peripheral blood and pericardial fluid to unravel potential circulating or local biomarkers of CAD and CAC.
Statistical planPrevious studies investigating the association between EAT and CAD compared patients referred for coronary artery bypass grafting with those referred for valve replacement only (controls). Therefore, their control groups were very heterogeneous and differed from the CAD groups in factors other than CAD. In this study, we studied a homogenous cohort of symptomatic severe degenerative AS patients. Since AS shares the same pathophysiology as CAD and the prevalence of CAD in severe AS patients is about 50%,25 the groups AS+CAD (diseased group) and AS only (control group) are expected to present a balanced distribution of traditional cardiovascular risk factors. In addition, adjusted regression models were used to eliminate confounding by standard cardiovascular risk factors and drugs when assessing the association between EAT volume and coronary stenosis or calcification (Arm 1). Orthogonal projection to latent structures-discriminant analysis26–28 will be used to build models able to distinguish and interpret the observed differences between the three fat samples in the CAD and non-CAD groups (Arm 2). Partial least square regression will be used to study the multivariate correlations between EAT proteins and CAC score.
With regard to sample size calculation, as previously described, patients with ≥50% coronary stenosis are expected to have significantly higher EAT volume, with an estimated mean difference in EAT volume between patients with and without CAD of 17.33 ml.12 Assuming a beta of 0.20 and an alpha of 5%, it was estimated that a minimum of 294 patients will be needed to detect differences in EAT volume between patients with and without obstructive CAD. Additionally, it was estimated that at least 260 patients are needed to detect differences in EAT volume between patients with and without coronary calcification (CAC score >0), considering an overall mean difference in EAT volume of 19.15 ml, beta of 20% and alpha of 5%.
DiscussionIn a thoroughly phenotyped cohort of elderly patients with severe degenerative AS referred for aortic valve replacement, we aim to explore the association of EAT volume assessed by CT with the presence and severity of coronary stenosis and with coronary calcium score. Moreover, in a comparative analysis with mediastinal and subcutaneous thoracic adipose tissue, EAT will be phenotyped by the study of its proteomics using a highly sensitive mass spectrometry technique to identify EAT-derived proteins linked to the late stages of CAD.
Factors that modulate the link between epicardial adipose tissue and coronary artery diseaseSeveral factors may affect the crosstalk between EAT and the coronary arteries. Through a systematic review, we determined that the major factors of heterogeneity in the association between EAT volume and CAD are the pre-test probability of CAD, obesity, gender, and ethnicity. Increased EAT volume assessed by CT was independently associated with CAD in very low-to-intermediate risk subjects, but in the CORE320 Multicenter study, in which a third of included patients had previous myocardial infarction, EAT volume did not differ between patients with and without coronary stenosis or myocardial ischemia, and there was no significant correlation with CAC score.29 It has recently been discovered that, in the presence of coronary plaques, the release of inflammatory mediators and oxidation products from the diseased vessel wall modifies the phenotype of the adjacent EAT, affecting its morphology and adipokine secretome.30,31 It was shown that coronary inflammation blocks adipocyte maturation, leading to smaller adipocytes with a lower lipid content, changes that may have an impact on the total volume of EAT. Moreover, in an elderly population with a high prevalence of cardiovascular risk factors, conventional cardiovascular drugs, such as statins,32 oral antidiabetic agents,33 and aspirin34 also directly modulate the phenotype of visceral adipose tissue. Altogether this evidence suggests that the communication between EAT and the coronary vessel wall is reciprocal and leads to modifications at the morphological and functional level over the natural history of the disease. Consequently, the relationship between EAT and late CAD needs to be specifically addressed in a high-risk stable cohort, and both quantitative and qualitative aspects of fat biology should be explored to understand the role of EAT in advanced stages of coronary atherosclerosis.
Quantity versus quality of epicardial adipose tissueAlthough the volume, area and thickness of EAT on echocardiography, CT or cardiac magnetic resonance imaging are highly correlated with increased adipocyte metabolic activity and inflammatory cell infiltration, EAT quantity may not accurately reflect the phenotype of EAT. The phenotype (‘quality’) of EAT can only be investigated invasively through the study of its genome, transcriptome or proteome. Unlike the genome and transcriptome, the proteome is a direct measure of the determinants of cell phenotype, and can thus better elucidate the dynamic processes behind the manifestation and progression of disease.35 Mass spectrometry is the method of choice in proteomics approaches and SWATH-MS is recognized as a high-speed and high-resolution mass spectrometry acquisition method that accurately performs relative quantification of proteins even in complex samples.36 Furthermore, because little correlation has been found between EAT secretion and plasma adipocytokine levels, it is essential to investigate to what degree EAT-derived mediators are represented in cardiac fluids such as coronary sinus blood and pericardial fluid, to reveal molecular players involved in vasocrine and paracrine crosstalk between epicardial adipocytes and the coronary vessels.
ConclusionsIn this translational study, we investigate, for the first time in the same cohort, the quantitative and qualitative aspects of adipose tissue, and how they are linked to coronary atherosclerosis in high-risk patients. In a thoroughly phenotyped clinical cohort of severe AS patients, we will provide statistically powered associations between EAT volume and coronary stenosis and coronary calcification. Furthermore, we will generate an EAT bioresource that will help to create a library of independent markers of EAT biology, enabling correlations to be established between the EAT phenotype, its volume on CT, and the presence of disease. The results of our study will pinpoint potential EAT-derived targets for novel local therapies to slow down the progression and promote the stability of atherosclerotic plaques, and for the discovery of new CAD imaging tools by biomedical engineering.
FundingJ. Mancio is supported by the Fundação Portuguesa para a Ciência e Tecnologia (SFRH/BD/104369/2014) and by the Sociedade Portuguesa de Cardiologia (Bolsa de investigação João Porto). This article is a result of the DOCnet project (NORTE-01-0145-FEDER-000003), supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund.
Conflicts of interestThe authors have no conflicts of interest to declare.