Inflammation plays an important role in several stages of the cardiovascular continuum. In recent decades a plethora of studies have provided new data highlighting the role of inflammation in atherogenesis and atherothrombosis in two-way interactions with various cardiovascular risk factors and further influencing these dynamic processes. The concept of targeting residual inflammatory risk among individuals with ischemic heart disease (IHD) is therefore gaining increasing attention. Recently, several landmark randomized controlled trials have assessed different pharmacological approaches that may mitigate this residual risk. The results of some of these studies, such as CANTOS with canakinumab and COLCOT and LoDoCo2 with colchicine, are promising and have provided data to support this concept. Moreover, though several aspects remain to be clarified, these trials have shown the potential of modulating inflammation as a new target to reduce the risk of cardiovascular events in secondary prevention patients. In the present review, we aim to present a pragmatic overview of the complex interplay between inflammation and IHD, and to critically appraise the current evidence on this issue while presenting future perspectives on this topic of pivotal contemporary interest.
A inflamação apresenta um papel de destaque ao longo do continuum cardiovascular. Ao longo das últimas décadas diferentes estudos reforçaram o papel da inflamação na aterogénese e na aterotrombose, interagindo com diversos fatores de risco cardiovascular de forma bidirecional, sendo capaz de influenciar estes processos dinâmicos. Neste contexto, o conceito de abordar o risco residual inflamatório em indivíduos com doença cardíaca isquémica (DCI) tem ganhado destaque. Recentemente, diversos ensaios clínicos aleatorizados avaliaram diferentes estratégias farmacológicas no sentido de mitigar este risco residual. Os resultados de alguns destes estudos, como o CANTOS com o canakinumab ou o COLCOT e LoDoCo2 com a colquicina, foram promissores e forneceram dados que corroboram este conceito. Adicionalmente, e apesar da necessidade de clarificação adicional de diversos pontos, estes estudos reforçaram o potencial da modulação inflamatória como um novo alvo na redução do risco de eventos cardiovasculares em prevenção secundária. O presente artigo tem por objetivo apresentar uma revisão pragmática referente à interação complexa entre a inflamação e a DCI e avaliar de forma crítica a evidência atual referente a este tópico, apresentando também uma perspetiva futura no âmbito desta temática de ampla relevância contemporânea.
Cardiovascular disease (CVD) is a major cause of morbidity and mortality and ischemic heart disease (IHD) is one of its most common manifestations.1,2 While significant improvements have been made in prevention, diagnosis, and management, patients with IHD still have a high residual risk of cardiovascular (CV) events.1–4 There is accordingly great interest in exploring new mechanistic pathways related to the development and overall expression of the disease, to mitigate this residual risk.5,6
Inflammation has long been recognized as being closely related to CVD and specifically to the atherosclerotic process.7–9 In the 19th century Virchow described the potential influence of inflammation on the atherosclerotic plaque.8 While the association between chronic inflammatory diseases such as rheumatoid arthritis and systemic lupus erythematosus and CVD has been extensively discussed,3,10,11 the idea that low-grade inflammation has a central role in atherogenesis and atherothrombosis has increasingly moved into the spotlight.12–14 In recent decades substantial advances have been made in our understanding of the complex and multidimensional interplay between inflammation and IHD,7,15–17 which led to the publication of several landmark clinical trials such as JUPITER, CANTOS, COLCOT and LoDoCo2, among others.13,18–22
In this article we aim to review the latest evidence linking inflammation with IHD and to explore the novel therapeutic strategies for reducing the risk of CV events by targeting residual inflammatory risk.
Inflammation and cardiovascular disease: a brief overview of pathophysiological mechanismsThe myriad pathways by which inflammation can influence the atherosclerotic process are highly complex and dynamic, involving both local and systemic mechanisms.5,15,23–26 These mechanisms can be influenced by conditions such as autoimmune diseases, infections, and changes in host microbiota, but also by ambient pollution, tobacco use, medications and other external factors.10,15,25–27 Moreover, there is evidence that this interplay is modulated by the genetic background.28 Interestingly, illustrating the potential importance of different contexts, several studies have reported an association between infectious agents and atherosclerosis, in diverse backgrounds ranging from periodontitis to Chlamydia pneumoniae infection.12,25,29 Importantly, while the direct effect of single pathogenic microorganisms on the atherosclerotic plaque itself does not appear to underlie this relationship, several pathways related to chronic inflammatory stimuli have been postulated as potentially involved in this association.12,25,29 Although trials involving anti-infectious agents such as macrolides have not shown a beneficial effect in terms of CV events, they have nonetheless provided additional insights into this complex interaction.12,24,29 Although an extensive review of the pathophysiology underpinning these interactions is beyond the scope of this article (see the elegant overview by Ketelhuth et al.15), a working knowledge of this issue is essential to understand the latest advances in this area.17,23–25
As mentioned, different stimuli can lead to the activation of various cell types such as lymphocytes and mast cells, leading to expression of proinflammatory cytokines, which in turn further modulate the activity of monocytes which migrate from the bloodstream to the vessel wall, as well as of other cell types.24–26 Notably, flow status and associated shear stress dynamics appear to play a central role in this interaction, with studies showing that specific patterns associated with atherosclerosis-prone segments can lead to differential expression of adhesion molecules by endothelial cells.7,12,23,24 As leukocytes migrate, the leukocytic infiltrate at the atheromatous plaque site can produce molecules such as proteases, procoagulant factors and inflammatory cytokines, further modulating thrombus formation and destabilization of the lesion.23,24 Among the most important cytokines involved, a delicate balance between anti-inflammatory (such as interleukin [IL]-10) and proinflammatory (such as IL-18 and IL-1 and, downstream, IL-6) signaling has a crucial role.9,16,18,25,26 In this balance, the NLR family pyrin domain containing 3 (NLRP3) inflammasome, a macromolecular protein complex which forms part of the innate immune system, has gained increasing prominence.16,30 NLRP3 can be activated in response to different stimuli (particularly pathogen-associated or damage-associated molecular patterns, some of which can be induced due to cholesterol accumulation, hypoxia or dysregulation in autophagy), and lead to a proinflammatory cytokine shift and cell death (via pyroptosis, a form of programmed cell death via specialized caspases).7,16,30–32
Another issue to be considered is the production of autoantibodies (including cardiac autoantibodies).10,25,33 These may be related to background autoimmunity (as in the case of systemic lupus erythematosus) and further amplify the immune response,10 but can also be found in individuals with CVD as well as in the general population, thus further illustrating the overlap between mechanisms.25,34–36 In addition, data support the notion that there are changes in the leukocyte profile in CVD.37 Although the full scope of these findings remains to be fully ascertained, a proinflammatory imbalance as expressed by changes in the neutrophil-to-lymphocyte ratio, suggesting a shift towards increased inflammatory mediators via neutrophils and a reduction in anti-inflammatory signaling via lymphocytes, has been proposed as among the mechanisms underlying the association between changes in blood cell profiles and CVD.38 Furthermore, in parallel with the intense crosstalk between cell types (promoted by a proinflammatory imbalance in the cytokine milieu), oxidative stress may also have an important role, affecting different cellular components and further promoting the evolving atherogenic process.15,16,31 Mitochondrial dysfunction can also lead to activation of the NLRP3 inflammasome by way of reactive oxygen species, while altered mitochondrial homeostasis can perpetuate this maladaptation.7,16,30
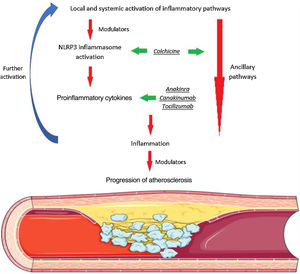
Given these diverse and multifaceted mechanisms, various strategies have been investigated to modulate the inflammatory cascade, as detailed in Figure 1.17,31,39
Inflammation and atherosclerosis. Both systemic (ranging from traditional cardiovascular risk factors to infection, autoimmune disease, and air pollution) and local factors (such as ischemia and cholesterol accumulation) can lead to activation of different inflammatory pathways. Among these complex phenomena, activation of the NLRP3 inflammasome has a pivotal role in leading to a proinflammatory cytokine imbalance with activation of various cell types and ensuing progressive inflammatory signaling. Several drugs with potential to modulate different steps of this cascade have been assessed. Among these, colchicine and canakinumab have shown promising results in large cardiovascular outcome trials.
Given the evidence supporting an association between inflammation and CVD, the concept that inflammation could be considered a risk marker or risk factor has continued to evolve in recent years.13,18,32,40,41 C-reactive protein (CRP) has provided important evidence on this issue.42–45 This protein, mainly synthesized in hepatocytes and whose mRNA transcription is influenced by IL-6 and IL-1β, is released in response to a plethora of stimuli, such as infections and trauma, forming part of a non-specific innate defense mechanism.42,43 The sole determinant of its plasma concentration is its rate of synthesis, making it an interesting biomarker for assessing the intensity of the stimuli which lead to its production.42,43 Even so, CRP is a downstream marker that is probably not directly related to the atherogenic process itself.46,47
Notwithstanding, high-sensitivity CRP (hs-CRP) assays, which are able to assess lower levels of CRP (such as those associated with low-grade inflammation), have emerged as important ancillary tools for understanding the association between inflammation and ischemic events.13,45,47,48 Several studies have shown that hs-CRP levels can predict CV events, both in the general population and in individuals with previous CVD.47–49 Various reports have shown that hs-CRP level can discriminate CV risk independently of lipid parameters, highlighting its potential role as a risk marker.41,47,49,50
While data have shown an association between several CV risk factors and inflammation,12,51–54 recent studies have progressively refined this relationship.13,41 The seminal JUPITER trial, which assessed the effect of rosuvastatin on individuals without CVD who had LDL cholesterol (LDL-C) <130 mg/dl and elevated hs-CRP (≥2 mg/l), provided important insights13 (Table 1). In this study, rosuvastatin was associated with significant reductions in the primary composite endpoint, while also reducing all-cause death.13 Importantly, rosuvastatin was associated with significant reductions in both LDL-C and hs-CRP.13 Since this study, trials assessing proprotein convertase subtilisin/kexin type 9 inhibitors, which can reduce LDL-C to levels below those achieved with statin therapy and which do not substantially reduce hs-CRP levels, showed important reductions in CV events.32,41,47,55 At the other end of the spectrum, in the landmark CANTOS trial, canakinumab, which lowered hs-CRP without affecting LDL-C levels, reduced CV event rates, providing further arguments for the potential of targeting inflammation in CV prevention strategies.9,18,25,39 Current data thus reinforce the complementarity between inflammation and traditional CV risk factors in atherosclerosis,41,47,56 although historically a putative dichotomy between these components had at times been perceived.8,56
Overview of landmark studies on inflammation and atherosclerosis.
| Study (year) | Study drug | Design | Inclusion criteria | n | Follow-up (median) | Primary (efficacy) outcome (definition) | Primary outcome (results) | Comments |
|---|---|---|---|---|---|---|---|---|
| JUPITER13 (2008) | Rosuvastatin 20 mg daily | Randomized, double-blind, placebo-controlled, multicenter | Males ≥50 years old, females ≥60 years old plus no CVD plus baseline LDL-C <130 mg/dl and hs-CRP ≥2 mg/l | 17 802 | 1.9 years | Composite of MI, stroke, hospitalization for UA, arterial revascularization, or CV death | Significantly reduced (HR 0.56 [0.46-0.69], p<0.00001) | Significant reduction in all-cause deathIncrease in physician-reported diabetes |
| CANTOS18 (2017) | Canakinumab, different doses (50 mg, 150 mg, 300 mg) every three months | Randomized, double-blind, placebo-controlled, multicenter | Patients ≥18 years old plus history of MI plus hs-CRP ≥2 mg/l | 10 061 | 3.7 years | Composite of MI, stroke, or CV death | Significantly reduced for canakinumab 150 mg (HR 0.85 [0.74-0.98], p=0.021) | No reduction in CV or all-cause mortalityNo reduction in cancer incidence, but significant reduction in cancer mortalitySignificant increase in fatal infections or sepsis |
| CIRT14(2019) | Methotrexate 15-20 mg weekly (target dose) | Randomized, double-blind, placebo-controlled, multicenter | Patients ≥18 years old plus history of MI or multivessel coronary disease plus type 2 diabetes or metabolic syndrome | 4786 | 2.3 yearsa | Composite of MI, stroke, or CV death (original).Composite of MI, stroke, CV death or hospitalization for UA that lead to urgent revascularization (final) | No significant difference in original (HR 1.01 [0.82-1.25], p=0.91) or final (HR 0.96] 0.79-1.16], p=0.67) | No reduction in CV or all-cause mortalitySignificant increase in cancer incidence (driven by non-basal cell skin cancer) |
| COLCOT19 (2019) | Colchicine 0.5 mg daily | Randomized, double-blind, placebo-controlled, multicenter | Patients ≥18 years old plus MI within 30 days of enrolment (who had completed any planed percutaneous revascularization procedures and were treated according to guidelines including intensive use of statins) | 4745 | 22.6 months | Composite of MI, stroke, CV death, resuscitated CA, or urgent hospitalization for angina that lead to coronary revascularization | Significantly reduced (HR 0.77 [0.61-0.96], p=0.02) | No reduction in CV or all-cause mortalityNo increase in cancer or septic shockIncrease in pneumonia |
| LoDoCo221 (2020 | Colchicine 0.5 mg daily | Randomized, double-blind, placebo-controlled, multicenter | Patients 35-82 years old with chronic CAD (calcium score ≥400 Agatston units, CAD on CT or invasive coronary angiography) | 5522 | 28.6 | Composite of MI, ischemic stroke, CV death, or ischemia-driven coronary revascularization | Significantly reduced (HR 0.69 [0.57-0.83], p<0.001) | No reduction in all-cause mortalityNon-significant increase in non-CV mortalityNo increase in cancer or hospitalization for infection or pneumonia |
| COPS22 (2020) | Colchicine 0.5 mg twice daily for 1 month, once daily for 11 months | Randomized, double-blind, placebo-controlled, multicenter | Patients 18-85 years old who presented with ACS, and had CAD | 795 | 1.0 years | Composite all-cause mortality, ACS, non-cardioembolic ischemic stroke, or urgent revascularization | No significant difference (p=0.09, log-rank test)b | Increase in non-CV mortality (p=0024, log-rank test) |
ACS: acute coronary syndrome; CA: cardiac arrest; CAD: coronary artery disease; CT: computed tomography; CV: cardiovascular; CVD: cardiovascular disease; HR: hazard ratio; hs-CRP: high-sensitivity C-reactive protein; LDL-C: low-density lipoprotein cholesterol; MI: myocardial infarction; UA: unstable angina.
Finally, regarding the role of hs-CRP as a marker to refine CV risk assessment, the current US guidelines on the management of blood cholesterol state that it could have a role as a risk enhancer in selected individuals when assessing therapeutic strategies in primary prevention.11 By contrast, the European guidelines on CV prevention and on dyslipidemias, although addressing some of the data concerning hs-CRP, do not provide a specific recommendation for this biomarker in this context (particularly – in terms of anti-inflammatory agents – given the need for further data from randomized comparisons for CV event reduction).3,32,47 Thus, although the influence of inflammation in CVD has been extensively reported, its relative impact compared to other CV risk factors and their treatment is still not fully elucidated, as expressed in the current guidelines. Nevertheless, as discussed below, recent data on secondary prevention continue to provide new perspectives on this unresolved issue and could lead to further reappraisal of the subject.
Inflammation in patients with ischemic heart diseaseAs discussed above, there is ample evidence supporting the role of inflammation at different stages of atherosclerosis, interacting with other factors to promote damage signaling and thus modulate this process.5,12,39 It should be borne in mind that inflammatory stimuli can be associated with several CV risk factors, leading to atherogenesis and thus IHD.5,26,47 Another factor that further influences disease expression is the intense interplay between inflammation and hemostasis, which can lead to a prothrombotic state.12,57,58
Several reports suggest that after an acute coronary event, inflammation may provide a link to a higher residual risk.18,19,41,47 Although the beneficial impact of optimized standard secondary prevention strategies is undisputed, the significant risk of further events in this patient population has led to marked interest in strategies to reduce this residual risk.3,6,59–61 Given this background and the wealth of data supporting the increased risk of CV events among IHD patients with elevated hs-CRP,41,47 the putative role of residual inflammatory risk has increasingly attracted attention. While the potential of inflammatory modulation in CV risk has been hypothesized for over twenty years,44 data exploring the role of specific blocking of inflammatory pathways in IHD are still scarce.18 Insights from randomized controlled trials published in recent years have highlighted the link between inflammation and residual risk in IHD, providing novel paradigms in risk mitigation.14,18,19,21,22,62,63
Modulating inflammation to reduce cardiovascular risk: therapeutic targetsGeneral aspectsAs inflammation is a general description for several distinct mechanistic pathways, involving different interlocutors and many ubiquitous processes, its has proved challenging to modulate.7,14,47,63 Unsurprisingly, given their scope and breadth, multi-layered non-pharmacological interventions such as exercise and nutritional changes can have a significant impact on inflammation.64,65 It should be noted that while atherosclerosis development and plaque rupture are currently viewed as an active and dynamic process, their resolution is also active in nature.8,25,65 Exercise can thus play an important role in these proceedings, due to its direct effect not only on the heart, but also on other targets such as the vasculature and skeletal muscle.65,66 Beyond these interventions, the ability of several drugs to modulate inflammation in IHD has also been assessed in various studies.14,18,19,21,22,62,63
Evidence from clinical trialsStatinsStatins are among the most extensively studied classes of pharmacological agents.13,32,67–69 Although the role of LDL-C in atherogenesis and the importance of reducing LDL-C levels are now indisputable,11,32 observations from studies on statin therapy have led to the hypothesis that at least some of the effect of these agents are due to so-called pleiotropic effects.67,69 In their mechanism of action, statins affect the mevalonate pathway and can thus have important effects on inflammation and immune expression (and particularly on CRP levels, whose secretion by hepatocytes is reduced).31,67,69,70
An important breakthrough in this area was reported over twenty years ago, in a study assessing the possible impact of pravastatin on CRP levels among a randomly selected subset of participants in the CARE trial.71 In this study, CRP levels were significantly reduced in patients randomized to pravastatin, but not placebo, and this effect was not related to the magnitude of lipid lowering. The subsequent randomized, placebo-controlled PRINCE trial prospectively assessed the effect of pravastatin on hs-CRP levels in individuals with no prior CVD (primary prevention group, with LDL-C of at least 130 mg/dl), with an additional open-label study in patients with a history of MI, stroke or arterial revascularization (secondary prevention group).68 Pravastatin significantly reduced hs-CRP levels in both cohorts of patients in a largely LDL-C-independent manner.68 Though these results were of interest, underscoring the interplay between statin therapy and CRP reduction, it should be noted that the PRINCE trial was designed to assess changes in hs-CRP levels, and not clinical events. Following these observations, the potential role of CRP reduction was the subject of increasing study.67 The seminal JUPITER trial reported that in individuals without CVD who had LDL-C levels <130 mg/dl and elevated hs-CRP levels, rosuvastatin reduced the incidence of major adverse cardiovascular events (MACE) and mortality (Table 1).13 As mentioned above, however, in this study rosuvastatin led to significant reductions in both LDL-C and hs-CRP.13 While this point should be taken into consideration, as should questions concerning the study's design and subjects’ background risk, the JUPITER trial provided important data on this issue.72
Currently available data attest to the potential role of statins as inflammatory modulators.13,67,68,70,73 Despite this effect, given their pivotal role in reducing LDL-C, the overall absolute clinical impact of their potential anti-inflammatory properties remains elusive.32,67 Nevertheless, these reports, when taken together with data supporting the added benefit of reductions in both LDL-C and hs-CRP and emphasizing the potential importance of inflammatory modulation, were instrumental in paving the way for further studies specifically addressing anti-inflammatory targeting in CV prevention.41,50,67,73
Interleukin-1 inhibitorsDue to its prominent role in inflammation, the potential of IL-1 inhibition to reduce the activity of this pathway and ultimately ischemic events has been the focus of attention.17,18,25,74 Two pharmacological agents, anakinra and canakinumab, have attracted particular interest.18,74,75
Anakinra, a recombinant IL-1 receptor antagonist, has been assessed in several IHD studies.16,74,75 In the MRC-ILA Heart Study, patients with non-ST-elevation acute coronary syndrome (ACS) who presented <48 hours from onset of chest pain were randomized to anakinra or placebo for 14 days.75 There was a significant decrease in both hs-CRP and IL-6 in the treatment arm at 14 days. After day 14, however, hs-CRP levels increased significantly in the anakinra group, and at 30 days hs-CRP levels were significantly higher in this group, whereas no differences were reported in IL-6. In this study, the rates of MACE were similar at one and three months. Although the study was not powered for clinical outcomes, at one year there was a significant increase in MACE in the active treatment group (driven mainly by a non-significant increase in recurrent MI).75 The recently reported VCUART3, which randomized 99 ST-elevation MI (STEMI) patients to anakinra or placebo, although also insufficiently powered to assess clinical events, showed an interesting signal.76 Although there was no reduction in ischemic events, a reduction in heart failure (HF) outcomes provided a promising new avenue for further investigation.74,76 These results, taken together with prior data on STEMI (also showing a reduction in HF), provided valuable knowledge in terms of IL-1 modulation in IHD.74,76
Major progress in this field came from CANTOS, which assessed the effect of canakinumab (a human monoclonal antibody targeting IL-1β) in patients with prior MI and elevated hs-CRP, defined as ≥2 mg/l (Table 1).18 In this landmark trial, there was a significant reduction in the primary composite outcome of MI, stroke, or CV death, but no impact on total mortality. Importantly, canakinumab reduced hs-CRP levels, with no significant effect on LDL-C.18,77 In a pre-specified secondary analysis of CANTOS, patients who achieved on-treatment hs-CRP levels <2 mg/l had a significant reduction in both MACE and mortality, whereas this effect was not seen among those with levels above 2 mg/l.77 Of note, this response was seen after a single dose of canakinumab, therefore early in the trial protocol. These findings offer an additional view of the possible utility of hs-CRP in IHD, particularly in tailoring therapies in this entity, as assessing the hs-CRP response to canakinumab could allow the selection of patients most likely to benefit from this therapy.47,59,77 Two additional points should also be considered concerning the CANTOS trial. Firstly, there was an increase in fatal infections or sepsis, as well as in the incidence of leukopenia.18 This should be carefully addressed, given the complexity of the immune system and the need to maintain homeostasis in terms of the overall immune response.7,12,23,30 Secondly, although the overall incidence of cancer did not differ between groups, cancer mortality in CANTOS was significantly lower in the treatment arm.18,78 Interestingly, compared to those who did not develop cancer during the study period, those who developed lung cancer had significantly higher baseline levels of hs-CRP and IL-6.78 These results are of great interest given the known link and the many common pathways between overall inflammation and cancer, especially of the lung, in which inflammation is particularly important.14,30,74,78,79 Ongoing studies are investigating the potential role of canakinumab in cancer.47 In this regard, the CANOPY program will assess canakinumab in lung cancer patients.47,80 Finally, although these results are encouraging, the role of canakinumab in the contemporary management of IHD is still evolving, and the question of its cost-effectiveness should also be considered.61,77,81
ColchicineColchicine is a potent oral anti-inflammatory agent that has been used for centuries in the treatment of various rheumatological conditions.17,19 Over the years, it has been thoroughly researched in terms of both its mechanisms of action and possible applications in CVD.20,62,82 Although much research has focused on pericardial disease,82 colchicine's potential use in IHD has also been hypothesized for over twenty years.83 After administration, colchicine is widely distributed and is taken up by various cells, mainly leukocytes and endothelial cells, inhibiting the polymerization of tubulin and subsequent microtubule assembly, thereby affecting numerous processes.19,62 Its role as a non-specific inhibitor of the NLRP3 inflammasome and hence its overall impact on the inflammatory response has gained increasing recognition as a new target to further reduce the risk of ischemic events.7,16,17,62,63,84
In this context, the prospective randomized LoDoCo trial assessed the addition of low-dose colchicine (0.5 mg/day) to standard treatment in stable coronary artery disease (CAD).20 In this trial, colchicine significantly reduced the composite primary endpoint of ACS, out-of-hospital cardiac arrest (CA) or noncardioembolic ischemic stroke. Interestingly, this was mainly due to a reduction in the incidence of ACS, and specifically non-stent-related ACS. Although the trial was significant, the lack of a placebo control and the small number of patients enrolled should be noted.19,20
More recently, COLCOT provided further data on the potential role of colchicine in IHD.19,61,85 In this study a total of 4745 MI patients, 93% treated by percutaneous coronary intervention (PCI) and 99% under statins, were randomized to colchicine (0.5 mg/day) or placebo (Table 1). Patients treated with colchicine had a significantly lower incidence of the primary composite endpoint of MI, stroke, CV death, resuscitated CA or urgent hospitalization for angina leading to coronary revascularization.19 In this study, however, this was mainly driven by reductions in stroke (an effect also reported in smaller studies) and hospitalizations for angina.19,86 The robust results shown in COLCOT highlight the potential of this intervention, with an analysis of time-to-treatment initiation suggesting an increased benefit of early initiation after MI.19,85 Several issues, however, are worthy of mention. Firstly, although the safety profile of colchicine has been widely studied, there was an increase in the incidence of pneumonia in COLCOT.19 Secondly, the median follow-up was 22.6 months, and therefore the long-term impact of this strategy is still not fully explored.19,62,63 Thirdly, the mechanisms by which colchicine exerted its effects are still not fully known.19,84 In COLCOT, a subgroup of individuals underwent assessment of hs-CRP and white cell counts. In this subgroup no differences were noted in these markers.19 The latter result is in agreement with the LoDoCo-MI study, in which low-dose colchicine (0.5 mg/day), although well tolerated, did not result in a significant reduction in hs-CRP levels at 30 days among 237 MI survivors, compared to placebo,87 and with the smaller COLIN study in which colchicine (1 mg/day) did not lead to a lower hs-CRP peak value during hospitalization for STEMI.88 Intriguingly, these findings contrast with those reported by Deftereos et al., in which STEMI patients randomized to colchicine (2 mg and then 0.5 mg twice daily for five days, or once daily if <60 kg body weight) presented lower peak hs-CRP levels and neutrophil counts compared to the control group.89 In addition, the COLCHICINE-PCI trial reported on a subgroup of patients in whom IL-6 and hs-CRP had been assessed.90 Briefly, in this study colchicine (1.8 mg total) was administered prior to PCI. Colchicine did not reduce the primary outcome of PCI-related myocardial injury.90 In the substudy (comprising 280 of the total of 400 individuals who underwent PCI), there was a smaller increase in both IL-6 and hs-CRP at 22-24 hours in the colchicine arm.90 These discordant results in terms of inflammatory biomarkers, even given the different study designs and timing of sampling, should be further reviewed to allow clarification of the pathways by which colchicine exerts its CV effect. In this regard, an animal study has shed some light on colchicine's effect at the level of the atherosclerotic plaque, by reducing inflammation and plaque burden.91 As recently demonstrated in patients with SARS-CoV-2 infection (COVID-19), colchicine can have a potent effect on the immune response and on the interaction between the immune and cardiovascular systems, thus reinforcing the value of further studies with this agent.92,93
Following COLCOT, the recently reported LoDoCo2 trial provided further data on colchicine in individuals with chronic CAD (Table 1).21 In this study, 5522 patients with chronic CAD were randomized to colchicine (0.5 mg/day) or placebo. The primary composite endpoint of MI, stroke, CV death or ischemia-driven revascularization was significantly less frequent (31% relative risk reduction) with colchicine.21 Notwithstanding these encouraging results, some caveats should be addressed. Firstly, the study design included a one-month run-in period. Importantly, 15.4% of patients in this stage did not proceed to randomization, mostly due to gastrointestinal side effects. Secondly, while an exploratory proteomic substudy conducted during the run-in phase in 174 patients provided elegant mechanistic data, illustrating a reduction in hs-CRP levels and in the NLRP3 inflammasome pathway as well as in other proteins and showcasing the effects of colchicine beyond this pathway, hs-CRP and other inflammatory markers were not routinely assessed.21,84 Finally, this study showed a numerical increase in non-CV deaths, which did not reach statistical significance but needs to be further explored.21 In this regard, the recently reported Australian COPS randomized trial assessing colchicine in 795 ACS survivors should also be noted.22 Although a reduction in revascularization was reported as expected, in contrast to COLCOT there was a significant increase in mortality, driven by non-CV mortality with an important contribution from sepsis19,22 (Table 1). While, as acknowledged by the authors, several issues should be considered when analyzing the present data, when taken together with the signal from the LoDoCo2 study these findings warrant further consideration and clarification prior to the possible generalization of colchicine treatment in this setting.21,22
In summary, the current evidence on colchicine reinforces its broad potential to reduce the risk of ischemic events in patients with IHD, with two large randomized trials showing a similar effect.19–21,94 Nevertheless, the findings from the LoDoCo2 and the Australian COPS trials in terms of non-CV mortality highlight the complexity of inflammatory modulation.21,22 More data are needed to understand the specific mechanisms underlying colchicine's effects and to optimize patient selection.19–22,47,59
Other anti-inflammatory agentsOther anti-inflammatory agents have also been studied in IHD.14,39,95 Among these, methotrexate (at a low dose of 15 or 20 mg/week) was assessed in CIRT.14 In this trial, patients with prior MI or multivessel CAD (in addition to type 2 diabetes or metabolic syndrome) were randomized to methotrexate or placebo. Methotrexate failed to reduce the primary composite endpoint of MI, stroke, or CV death.14 Also, levels of hs-CRP, IL-6 and IL-1β did not differ between groups. Unlike previous observations with other agents, methotrexate was associated with an increased risk of cancer (mostly non-basal cell skin cancer). Several caveats should be acknowledged when interpreting the CIRT data. Unlike in CANTOS, patients in CIRT were not screened for hs-CRP (median level of which was 1.6 mg/l at baseline). When reviewing data from CANTOS on the impact of hs-CRP variation in terms of outcomes, this factor can be postulated as being of importance.14,77 In addition, differences in the pathway affected (and magnitude of immune modulation) could provide an additional explanation for the discrepancies observed between studies.14,15,47 Indeed, as has been extensively reported, the unfavorable effect of non-steroidal anti-inflammatory agents in terms of ischemic events should be recalled, as (at least partially) demonstrative of the significance of the type and setting of inflammatory modulation.96
Current paradigms and future perspectivesThough specific anti-inflammatory therapy is still not a major topic in the current guidelines,3,4,11,32,97 the promising results of CANTOS, COLCOT and LoDoCo2 have highlighted the potential scope of such therapy.18,19,21 While various questions regarding these interventions remain, particularly in terms of therapeutic tailoring, adherence and long-term safety, as well as cost-effectiveness, current evidence unquestionably points contemporary practice toward this translational view, while attesting to the value of the residual risk hypothesis.22,39,41,47,61,94,98,99
As previously proposed, the latter concept should also be refined, given that individuals may present distinct patterns of residual risk such as ‘inflammatory’, ‘lipid’ or ‘thrombotic’, and differentiating between these could enable appropriate tailoring for personalized secondary prevention measures.47,59 In this regard, the use of hs-CRP could be of interest for both screening and assessing treatment response.18,47,77 Nevertheless, the positive results in CANTOS and the LoDoCo2 trial, in which elevated hs-CRP was not an inclusion criterion, should be kept in mind as reflecting both the need for further tools with which to assess residual inflammatory risk and the broad range of individuals who could benefit from this approach.9,19,21
Future and ongoing trials, such as CLEAR SYNERGY with colchicine and ASSAIL-MI with tocilizumab (an IL-6 receptor antagonist), will provide further data regarding the significance of these interventions and their role in the therapeutic armamentarium for IHD.47,62,100
ConclusionRecent decades have provided extensive and mounting evidence concerning the pivotal interplay between inflammation and IHD, reinforcing the concept of inflammation not only as a risk marker but also as a risk factor for the development and progression of atherosclerotic disease. Recently, studies on anti-inflammatory agents such as canakinumab and colchicine have shown highly promising results demonstrating the significance of inflammatory modulation in the reduction of ischemic events. Although additional assessment is needed to fully ascertain the full scope of this intervention, particularly in terms of patient selection and optimal therapeutic tailoring in light of contemporary CV prevention strategies, these findings have the potential to challenge the current status quo of ischemic risk reduction.
As the holistic approach to IHD continues to be further refined, based on a robust translational background and benefiting from the input of greatly improved ancillary diagnostic methods and an expanding therapeutic armamentarium, investigation of the specific role of inflammatory modulation is set to take center stage, in the current age of precision-based medicine.
Conflicts of interestThe authors have no conflicts of interest to declare.