Coronary microvascular dysfunction (CMD) is one of the most important pathophysiological features in hypertrophic cardiomyopathy (HCM). The index of microcirculatory resistance (IMR) is an invasive method to assess the coronary microcirculation. The aim was to assess CMD in patients with HCM by IMR.
MethodsAdult patients with HCM without epicardial coronary artery disease underwent cardiac catheterization for the assessment of CMD by IMR (normal cut-off value ≤22.0) and coronary flow reserve (CFR) (normal cut-off value ≥2). Cardiovascular magnetic resonance (CMR) was performed to assess the ischemic burden by perfusion imaging during regadenoson-induced hyperemia, and the extent of myocardial fibrosis was assessed by late gadolinium enhancement (LGE), native T1 mapping and extracellular volume (ECV).
ResultsFourteen patients were enrolled with a mean age of 62.8±6.2years, 8 (57.1%) males, of whom 9 (64.3%) had obstructive HCM. Using IMR, CMD was detected in 4 (29%) patients. Among four patients with an IMR>22.0, all had non-obstructive HCM and two had angina. CFR<2 was reported in eight patients (57%). Concordance between IMR and CFR (both normal or both abnormal) was verified in 6 patients (43%). Among four patients with IMR>22.0, perfusion defects were found in two of the three patients who underwent stress CMR. Increased ECV (>28%) was documented in two of the patients with IMR>22 and in three of the patients with IMR≤22.0. LGE was >15% in 2 of the patients with IMR>22 and in 4 with IMR≤22.0.
ConclusionsIMR assessment in HCM is feasible and safe. Patients with abnormal IMR seemed to have more significant tissue abnormalities on CMR.
A disfunção microvascular coronária (DMC) constitui uma das mais importantes alterações fisiopatológicas na miocardiopatia hipertrófica (MCH). O índice de resistência microcirculatória (IRM) é um método invasivo para avaliação da microcirculação coronária. O objetivo é avaliar a DMC nos doentes com MCH utilizando o IRM.
MétodosDoentes adultos com MCH, sem doença coronária epicárdica, submetidos a cateterismo cardíaco para avaliação de DMC por IRM (valor normal≤22,0) e reserva de fluxo coronário (RFC) (valor normal≥2). Ressonância magnética cardiovascular (RMC) foi realizada para avaliação de isquémia por sequência de perfusão durante hiperemia induzida por regadenoson; a extensão de fibrose foi avaliada por realce tardio (RT), T1 mapping nativo e volume extracelular (VEC).
ResultadosForam incluídos 14 doentes com média de 62,8±6,2 anos, 8 (57,1%) do género masculino, dos quais 9 (64,3%) tinham MCH obstrutiva. Utilizando IRM, DMC foi detetada em 4 (29%) doentes. Dos 4 doentes com IRM>22,0, todos tinham MCH não obstrutiva e 2 tinham angina. RFC<2 foi verificada em 8 doentes (57%). Concordância entre IRM e RFC (ambos normais ou ambos anormais) foi verificada em 6 doentes (43%). Dos 4 doentes com IRM>22, foram encontrados defeitos de perfusão em 2 dos 3 doentes que realizaram RMC. VEC aumentado (>28%) foi documentado em 2 doentes com IRM>22 e em 3 com IRM≤22,0. RT>15% foi documentado em 2 doentes com IRM>22 e em 4 com IRM≤22,0.
ConclusãoA avaliação do IRM na MCH é segura e exequível. IRM tem potencial para se tornar uma técnica útil na avaliação da microcirculação dos doentes com MCH.
Hypertrophic cardiomyopathy (HCM) is characterized by left ventricular hypertrophy (LVH) in the absence of abnormal loading conditions capable of inducing the degree of hypertrophy observed. HCM is caused by a disease-causing sarcomere variant in up to 60% of probands.1
Coronary microvascular dysfunction (CMD) and ischemia are important pathophysiological features. The etiology of CMD is multifactorial and encompasses reduced capillary density, vascular remodeling, fibrosis, myocyte disarray, extravascular compression secondary to ventricular hypertrophy, diastolic dysfunction and LV outflow obstruction.2–5 Recurrent chronic ischemia has the potential to generate myocyte death and consequently myocardial fibrosis.6,7
As the direct visualization of coronary microcirculation in vivo is not possible, multiple invasive and non-invasive techniques have been developed.5 Coronary microcirculation may be invasively assessed by calculating the index of microcirculatory resistance (IMR). IMR provides information on the state of small vessels and is independent of the presence of epicardial stenosis.8 Three previously published studies with 20 or fewer patients point for a normal value <25.9–11 A larger study including 1096 patients assessed IMR per artery and found 22.0 as the cut-off value for left anterior descending (LAD) artery.12 Despite its well-known use and prognostic prediction in myocardial infarction,13–16 angina without coronary stenosis9,17 and cardiac allograft vasculopathy,18,19 its value in HCM has not been extensively studied. The published data are limited to a case report, reporting elevated IMR in a HCM patient with ischemic abnormalities observed on non-invasive imaging in the absence of epicardial coronary disease.20
Another parameter that may be assessed invasively is coronary flow reserve (CFR), which in the absence of epicardial coronary stenosis, reflects solely microvascular function. Coronary blood flow studies in HCM patients have detected blunted CFR.21–23
In this pilot study, we explored the potential role of IMR in the evaluation of CMD in patients with HCM. We prospectively assessed IMR and CFR in patients with HCM without epicardial coronary disease. The evaluation of the patients was complemented by the detection of perfusion defects and tissue characterization using stress cardiovascular magnetic resonance (CMR).
Materials and methodsDesign and study populationThe study prospectively included adult patients with HCM recruited from one referral center, between April 2019 and September 2020.
The diagnosis of HCM was made according to published guidelines,1 more specifically, wall thickness ≥15 mm in one or more LV myocardial segments in probands or ≥13 mm in relatives. Patients with left ventricle ejection fraction <50%, prior septal reduction therapy (myectomy or alcohol ablation) and epicardial coronary artery disease were excluded.
Patients were classified as obstructive or non-obstructive HCM according with left ventricular outflow tract gradient ≥30 mmHg or <30 mmHg, at rest or with provocation, respectively.
Patients underwent invasive coronary angiography for the assessment of microvascular dysfunction and CMR for the evaluation of ischemic burden and tissue characterization, including the evaluation of diffuse fibrosis by native T1 mapping, extracellular volume calculation (ECV), and replacement fibrosis by late gadolinium enhancement (LGE).
The research followed the principles outlined in the Declaration of Helsinki. The institutional ethics committee of the Nova Medical School and Centro Hospitalar Universitário de Lisboa Central approved the study protocol. All patients provided written informed consent.
Index of microcirculatory resistanceThe index of microcirculatory resistance is defined as the ratio between distal coronary pressure (Pd) and the inverse of the hyperemic mean transit time (TmnHyper). To determine coronary pressure and CFR (resting mean transit time (TmnRest) divided by TmnHyper), a single pressure temperature sensor-tipped coronary wire (PressureWire™ Certus™) was advanced to the distal left anterior descending artery. An interface and software (Radianalyzer X®) were used for distal coronary pressure and mean transit time records. Thermodilution-derived mean transit time was assessed after three injections of room temperature saline through the guiding catheter, based on mean transit time of the indicator to travel from the injection site to the distal sensor. Intravenous adenosine (140 μgr/kg/min) was administered to induce steady-state maximal hyperemia.
In the present study we used 22.0 as the cut-off value for normality.12 CFR≥2 was considered normal, as used in most studies demonstrating the prognostic value of thermodilution-based CFR.24,25
CMR acquisition protocol and analysisPatients underwent CMR performed on a 1.5-T system (Sola, Siemens, Erlangen, Germany). Using Compressed Sensing-based techniques cine images in 3 long-axis planes and sequential short axis slices spanning the entire left ventricle from the base to the apex were acquired. Basal, mid and apical pre- and post-contrast short axis T1 maps were generated using a Modified Look Locker Inversion (MOLLI) sequence in a 5(3)3 configuration. The same three slices were used for stress perfusion CMR, using a gradient echo sequence, 90 seconds after hyperemia induced by regadenoson (400 mcg bolus) using 0.05 mmol/kg of gadolinium (Gadovist, Bayer Schering Pharma AG, Berlin, Germany). Late gadolinium enhancement (LGE) images were acquired 10–15 minutes after intravenous administration of additional 0.10 mmol/kg of gadolinium (total of 0.15 mmol/kg).
Cardiovascular magnetic resonance interpretation was performed using commercially available software (CMR42, Circle Cardiovascular Imaging, Calgary, Alberta, Canada). For perfusion assessment, the myocardium was divided into 32 subsegments (16 American Heart Association segments subdivided into an endocardial and epicardial layer). Ischemic burden for each patient was calculated as the number of involved sub-segments, assigning 3% of myocardium to each subsegment. LGE was analyzed using a signal threshold versus reference myocardium of ≥6 standard deviation. Native T1 and post contrast T1 values of myocardium were measured from the three slices generating T1 maps and allowing the calculation of ECV.
ResultsThe study included 14 patients, mean age of 62.8±6.2 years, eight (57.1%) males, nine of whom had obstructive HCM. Angina was a common symptom, and the majority of the patients were on beta blocker (BB) and/or calcium channel blocker (CCB). Baseline characteristics are shown in Table 1.
Baseline characteristics of the 14 study patients.
| Patients characteristics | N=14 |
|---|---|
| Age (years), mean (SD) | 62.8±6.2 |
| Male, n (%) | 8 (57.1) |
| Caucasian, n (%) | 14 (100) |
| BSA (m2), mean (SD) | 1.9±0.2 |
| NYHA II/III, n (%) | 8 (57.1) |
| Angina, n (%) | 8 (57.1) |
| HCM risk SCD score (%), mean (SD) | 4.39±3.28 |
| Hypertension, n (%) | 8 (57.1) |
| Diabetes, n (%) | 1 (7.1) |
| Dyslipidemia, n (%) | 4 (28.6) |
| Smoker, n (%) | 2 (14.3) |
| BB, n (%) | 11 (78.6) |
| CCB, n (%) | 6 (42.9) |
| Sinus rhythm, n (%) | 11 (78.6) |
| Asymmetric septal hypertrophy, n (%) | 12 (85.7) |
| Obstructive HCM, n (%) | 9 (64.3) |
| MWT (mm), mean (SD) | 19.3±3.5 |
| LV mass (g/m2), mean (SD) | 93.8±23.2 |
| LV EDV (ml/m2), mean (SD) | 58.0±13.1 |
| LV ESV (ml/m2), mean (SD) | 18.0±6.1 |
| LV EF (%), mean (SD) | 70.6±10.2 |
BB: beta blocker; BSA: body surface area; CCB: calcium channel blocker; HCM: hypertrophic cardiomyopathy; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; NYHA: New York Hear Association functional class; HTN: hypertension; SCD: sudden cardiac death; SD: standard deviation.
In the overall population, Pd was 68.8±18.8 mmHg, TmnRest was 0.61±0.25 s, TmnHyper was 0.32±0.10 s, CFR was 1.93±0.69 and IMR was 21.4±6.3 U.
Among four patients with an IMR>22.0, two had angina and all had non-obstructive HCM. Two patients were on BBs or CCBs. Among 10 patients with an IMR≤22, one had non-obstructive HCM, six patients complained of chest pain, and all were on BBs or CCBs. All obstructive HCM patients had IMR≤22.0.
Blunted CFR was reported in eight patients (57%); concordance between IMR and CFR (both normal or both abnormal) was seen in six patients (43%) (Table 2).
Coronary microvascular study in hypertrophic cardiomyopathy patients without epicardial coronary artery disease.
| Patients characteristics | Cardiac catheterization | Magnetic resonance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Gender | Age | Hypertrophy pattern | MWT (mm) | LVOT obstruction | Pd (mmHg) | TmnRest (s) | TmnHyper (s) | CFR | IMR (U) | Ischemic burden (%) | Native T1 (ms) | ECV (%) | LGE (%) |
| 1 | Male | 64 | Septal | 16 | Non-obstructive HCM | 52 | 1.10 | 0.52 | 2.1 | 27.0 | 0 | 985±60 | 0.25 | 3.4 |
| 2 | Male | 63 | Septal | 24 | Obstructive HCM | 66 | 0.69 | 0.30 | 2.3 | 19.8 | 54 | 1043±81 | - | 17.1 |
| 3 | Female | 61 | Septal | 24 | Non-obstructive HCM | 80 | 0.47 | 0.48 | 1.0 | 38.4 | - | - | - | - |
| 4 | Male | 63 | Septal | 19 | Obstructive HCM | 66 | 0.95 | 0.32 | 3.0 | 20.5 | 18 | 1008±87 | 0.23 | 20.5 |
| 5 | Male | 77 | Septal | 15 | Non-obstructive HCM | 66 | 0.58 | 0.40 | 1.5 | 26.4 | 3 | 1004±56 | 0.34 | 18.7 |
| 6 | Female | 59 | Septal | 18 | Non-obstructive HCM | 123 | 0.57 | 0.19 | 3.0 | 23.4 | 9 | 1062±115 | 0.30 | 17.9 |
| 7 | Female | 74 | Apical | 16 | Non-obstructive HCM | 54 | 0.52 | 0.31 | 1.7 | 16.7 | 24 | 1031±126 | 0.28 | 8.4 |
| 8 | Female | 56 | Septal | 17 | Obstructive HCM | 88 | 0.61 | 0.25 | 2.4 | 22.0 | 3 | 1003±155 | 0.23 | 7.8 |
| 9 | Male | 70 | Septal | 23 | Obstructive HCM | 65 | 0.79 | 0.30 | 2.6 | 19.5 | 24 | 1054±66 | 0.29 | 39.8 |
| 10 | Male | 56 | Septal | 22 | Obstructive HCM | 60 | 0.45 | 0.26 | 1.7 | 15.6 | 15 | 1002±66 | 0.29 | 9.8 |
| 11 | Male | 43 | Septal | 27 | Obstructive HCM | 64 | 0.33 | 0.22 | 1.5 | 14.1 | 21 | 1072±98 | 0.30 | 18.3 |
| 12 | Female | 73 | Septal | 18 | Obstructive HCM | 47 | 0.88 | 0.46 | 1.9 | 21.6 | 21 | 1012±41 | 0.25 | 6.9 |
| 13 | Male | 61 | Septal | 18 | Obstructive HCM | 63 | 0.29 | 0.30 | 1.0 | 18.9 | 15 | 1000±29 | 0.26 | 5.8 |
| 14 | Female | 52 | Septal | 22 | Obstructive HCM | 70 | 0.27 | 0.22 | 1.2 | 15.4 | 36 | 1045±50 | 0.27 | 5.8 |
CFR: coronary flow reserve, ECV: extracelular volume; LGE: late gadolinium enhancement; LVOT: left ventricular outflow tract; MWT: maximum wall thickness; Pd: distal coronary pressure; TmnRest: resting mean transit time; TmnHyper: hyperemic mean transit time.
All procedures were uneventful regarding significant complications related to the catheterization or administration of adenosine.
Evaluation of myocardial ischemiaAll patients except one, due to claustrophobia, underwent CMR. Stress CMR demonstrated perfusion defects in 12 out of 13 patients (92%), with an ischemic burden between 3 and 54% of LV (Figure 1).
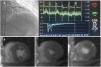
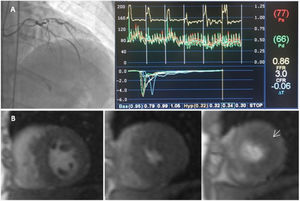
Index of microcirculatory resistance and evaluation of ischemia by coronary microvascular dysfunction in a hypertrophic cardiomyopathy patient. A-catheterization of left anterior descending artery coronary and hemodynamic parameters showed on the dedicated software: Distal coronary pressure (Pd)=66 mmHg, resting mean transit time (Bas)=95 ms, hyperemic mean transit time (Hyp)=32 ms, coronary flow reserve (CFR)=3.0. Calculated index of microcirculatory resistance (IMR) was 20.5 (not shown). B-CMR perfusion images with evidence of perfusion defect (arrow).
Among patients with IMR>22.0, perfusion defects were found in two of the three patients who underwent stress CMR (Table 2).
Evaluation of myocardial fibrosisOverall, ECV was increased in 5 patients (36%) (normal ECV values 25±3%).26 Increased ECV (>28%) was documented in two of the patients with IMR>22 and in three of the patients with IMR≤22.0. LGE was found in all patients with a range between 3.4 to 39.8% of LV mass (Table 2). LGE was >15% in two of the patients with IMR>22 and in 4 with IMR≤22.0 (Table 2).
DiscussionTo the best of our knowledge and despite our small cohort, this is the most extensive study using IMR to assess CMD in HCM patients. Based on IMR and considering the normal cut-off value for LAD of 22.0 as previously published,12 we found CMD in four patients (29%). Invasively, we also documented impaired CFR in more than half of the patients.
In our study, the evaluation was complemented by stress CMR. Imaging methods, such as CMR and positron emission tomography (PET), have been used to study ischemia due to CMD in HCM. Both methods have enabled relate the severity of ischemia to clinical manifestations and outcome. Notably, CMR studies demonstrated the link between CMD and LGE,27–29 and PET studies showed a relationship between CMD and worse outcome, including higher New York Heart Association functional class, left ventricular systolic dysfunction and death.30–32 Compared to PET, CMR has the advantage of presenting high spatial resolution and does not expose the patients to radiation.
The prevalence of CMD by IMR was significantly lower than the detection of ischemia from stress CMR, where we found perfusion defects in 92% of the patients. This finding raises the hypothesis that lower IMR values may be found in HCM patients compared with other diseases, with a possible need to recalibrate the cut-off value in HCM patients, as the pathophysiology is distinct.
We found lower values of Pd, TmnRest and TmnHyper than previously published for controls and patients with ischemic heart disease.16 Pd is proportional to coronary resistance.33 HCM patients present lower coronary resistance compared to the controls,23 leading to lower Pd and consequently lower IMR values. On the other hand, HCM patients had a greater LAD diameter and higher coronary blood flow in the LAD than the controls.23 Coronary flow has an inverse relationship with Tmn.33 If coronary flow in the LAD increases, TmnHyper consequently decreases and further contributes to the lower IMR in HCM.
These findings raise the hypothesis that the reduction in CFR presented in HCM patients may be, at least in part, secondary to near maximal baseline vasodilation of the microcirculation, instead of merely due to narrowing of small vessels or external compression leading to an increase of coronary vascular resistance.34,35
In the presence of CMD, the hemodynamic response to a non-endothelium-dependent vasodilator, such as adenosine, consists in a reduction of CFR and increase in hyperemic microcirculatory resistance. Despite CFR and IMR having been validated for microcirculation assessment, IMR has emerged as the preferential tool since it is more reproducible with a robust prognostic value.36–39 The use of CFR is associated with some limitations, including the incapability to distinguish epicardial from microvascular disease and is influenced by resting hemodynamics namely heart rate, blood pressure and contractility.36 On the other hand, IMR was shown to keep its reproducibility and less hemodynamic dependence even with significant changes in heart rate, blood pressure and contractility, when compared to CFR.37 Interestingly, IMR was demonstrated to be a stronger predictor of survival after ST-elevation myocardial infarction and to have greater value in the prediction of peri-procedural myocardial infarction, compared to CFR.38,39
For a more generalized utilization of IMR in HCM, further studies are needed. In a population where randomized trials are relatively scarce, real-world data are more relevant, and the inclusion of this technique in observational studies might be important.
In the overall study population, 57% of the patients had symptoms of angina, regardless of IMR. Chest pain affects around 25% to 50% of patients with HCM and silent ischemia may occur in up to 50% of those without symptoms.40 Despite this, chest pain is not a reliable marker of ischemia in these patients.41
Since a significant proportion of HCM patients complain of chest pain and undergo a coronary artery assessment, in the absence of epicardial coronary stenosis IMR may be an additional tool during catheterization to clarify the cause of the chest pain.
The assessment of CMD has clinical relevance since chronic and recurrent episodes of ischemia in HCM have the potential to promote adverse tissue changes, such as myocyte death and consequent myocardial fibrosis.32,42–46 In our study, patients with IMR>22.0 tended to present increased ECV and extensive LGE, although our small sample does enable a definitive association to be made.
An important limitation of our study is the small population and the absence of a control group. The main aim was to develop a pilot study for the assessment of the usefulness of IMR in HCM patients. Further research is needed to define clearly the role of this technique and the range of expected values in this population.
ConclusionCoronary microvascular dysfunction is a pathophysiological finding in HCM, and its evaluation has clinical relevance. IMR is reproducible and specific for assessing the microvasculature with further prognostic value demonstrated in other cardiac conditions. IMR assessment in the scenario of HCM is feasible and safe, with lower Pd, TmnRest and TmnHyper compared to previous reported values in ischemic heart disease. Additionally, patients with abnormal IMR seem to have more significant tissue abnormalities in CMR. Larger studies are needed to clarify the potential of IMR assessment in patients with HCM.
Conflicts of interestThe authors have no conflicts of interest to declare.
This article is dedicated to the memory of Prof. Miguel Mota Carmo.