Atrial fibrillation (AF) is related to a higher risk of thromboembolic events and mortality. Some studies have demonstrated that the inflammatory biomarker interleukin-6 (IL-6) is associated with a higher risk of higher thrombosis in AF patients, but the real effect of IL-6 remains a controversy.
MethodsWe conducted a systematic review and meta-analysis to investigate the association between IL-6 and thromboembolic events, as well as bleeding events, acute coronary syndrome (ACS) events and all-cause mortality in AF.
ResultsA total of five studies involving 22 928 patients met our inclusion criteria for the systematic review. The higher level of IL-6 in AF patients is related to long-term thromboembolic events including stroke (RR 1.44, CI 95% 1.09-1.90, p=0.01). IL-6 meant a higher risk of long-term bleeding risk (RR 1.36, CI 95% 1.06-1.74, p=0.02), ACS risk (RR 1.81, CI 95% 1.43-2.30, p<0.001) and all-cause mortality (RR 2.35, CI 95% 2.09-2.65, p<0.001).
ConclusionA higher level of IL-6 may predict a greater number of long-term thromboembolic events and bleeding events, ACS events and mortality in AF patients. Further studies such as the cut-off point of IL-6 need to be conducted in the future.
A fibrilhação auricular (AF) está relacionada com maior risco de eventos tromboembólicos e de mortalidade. Alguns estudos mostraram que o biomarcador inflamatório interleucina-6 (IL-6) estava associado a maior risco trombótico em doentes com AF, mas o efeito real da IL-6 continua controverso.
MétodosRealizámos uma revisão sistemática e meta-análise para investigar a associação de IL-6 com eventos tromboembólicos assim como eventos hemorrágicos, eventos síndrome coronária aguda e mortalidade total na AF.
ResultadosCinco estudos que envolveram 22 928 doentes preencheram os nossos critérios de inclusão para revisão sistemática. Maior nível de IL-6 em doentes com AF relaciona-se com eventos tromboembólicos em longo prazo, incluindo acidente vascular cerebral (RR 1,44, 95% CI 1,09-1,90, P 0,01), risco de ACS (RR 1,81, 95% CI 1,43-2,30, P<0,001) e mortalidade total (RR 2,35, 95% CI 2,09-2,65, P<0,001).
ConclusãoUm nível mais elevado de IL-6 pode prever um maior número de eventos tromboembólicos assim como de eventos hemorrágicos, eventos ACS e mortalidade em doentes com AF. Mais estudos sobre o ponto de corte de IL-6 necessitam ser realizados.
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice which can cause thromboembolic events such as stroke.1–3 So far, anticoagulation therapy is the major strategy for AF treatment.4 We applied CHA2DS2-VASc (Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female)) score to assess the risk of stroke in AF patients. The patients with a high score (male ≥2, female ≥3) require anti-coagulation therapy.5 We used HAS-BLED (Hypertension, Abnormal renal and liver function, Stroke, Bleeding, Labile INRs, Elderly, Drugs or alcohol) to estimate the bleeding risk in AF patients. A score ≥3 means there is a high risk of bleeding.
However, the area under curve (AUC) for CHA2DS2-VASc is approximately 0.660.5 In our previous investigation, less than 30% patients received anticoagulation therapy before stroke. One of the important reasons was that the score before stroke was <2. There must be other factors such as biomarkers which lead to stroke and thromboembolism. Similarly, the AUC for HAS-BLED is approximately 0.60.6 We also are unable to estimate the risk of other adverse events, such as cerebral infarction and mortality in AF patients by using these scores. It is necessary to discover other methods to assess the prognosis of AF patients.
Inflammation can cause AF and thrombosis. Several biomarkers have been studied in AF patients such as C-reactive protein (CRP)7,8 and interleukin (IL),9 while the role of IL-6 in AF and adverse events, especially thrombotic risk, remains controversial.10,11 We therefore conducted a systematic review and meta-analysis to provide an overview of the association between IL-6 with thromboembolic and other adverse events in AF.
MethodsSearch strategyWe used electronic databases to identify relevant studies. The PubMed, EMBASE, Web of Science, and Cochrane databases were systematically searched for studies published up to January 2020. We used mesh terms including “atrial fibrillation” and “interleukin 6” and “inflammation”.
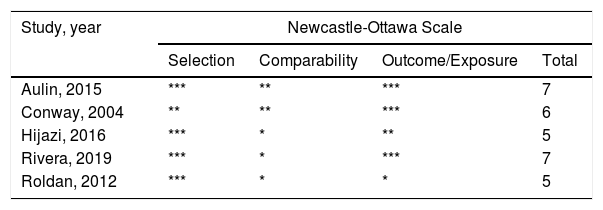
Study selectionWe enrolled the studies according to the the following criteria: (1) cohort studies (both retrospective and prospective) about stroke or thromboembolic risk in AF patients and (2) studies which focused on IL-6. In case of multiple publications, the most recent studies or studies of a larger population were used. Citations were selected by initially screening the title and abstract of the studies. We assessed the study quality using the Newcastle-Ottawa scale. The total scores ranged from 0 (worst) to 9 (best) for case-control or cohort studies.
Data extractionUsing standard data extraction forms, two reviewers (Zhou and Zhao) extracted data from the relevant studies. The following data were independently extracted: author's name, year of publication, type of study design, study population, sample size, mean duration of follow-up, study end points, risk ratio (RR) with 95% confidence interval (CI), cut-off value and reported adjustment for potential confounders. If the study provided the IL-6 level, we extracted the means and standard deviations in each group. If the study provided the medians and ranges instead of the means and standard deviations, we imputed the means and standard deviations using the method developed by Hozo et al.12
Data analysisFor cohort studies, we used pooled hazard ratio (HR) as a summary statistic to estimate the prognostic effect of IL-6 level on thromboembolic events in AF patients. An HR>1 indicated the correlation between elevated IL-6 and stroke and thromboembolic events.
Statistical heterogeneity across studies was assessed using the I2 statistic. An I2≥50% suggests that there is significant heterogeneity between studies. The HR was pooled using a fixed-effects model to manage heterogeneity if it was insignificant (I2<50%); otherwise, a random-effects model was applied. A p value <0.05 was considered to be statistically significant. Statistical analyses were performed using the RevMan 5.3 software.
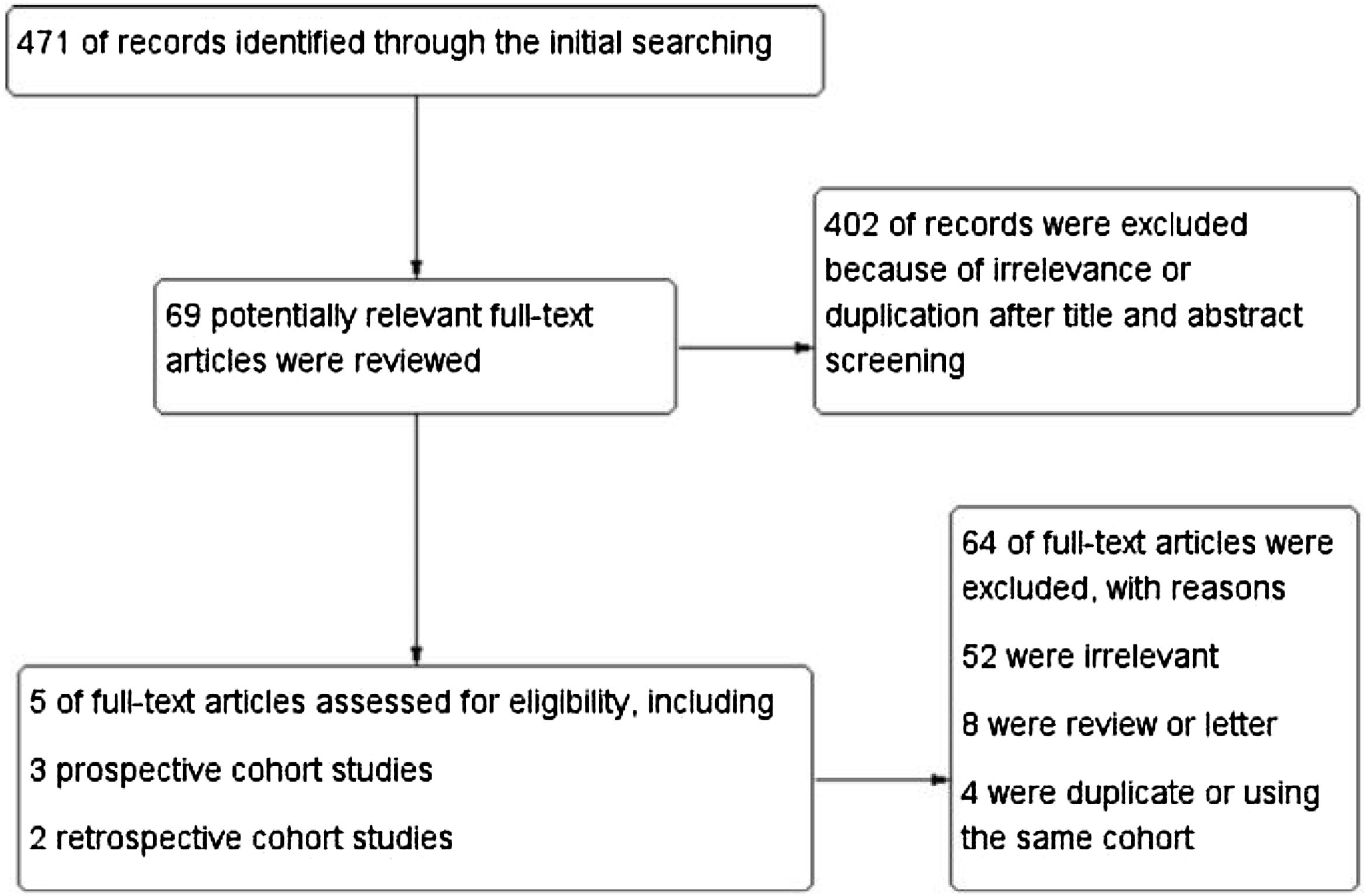
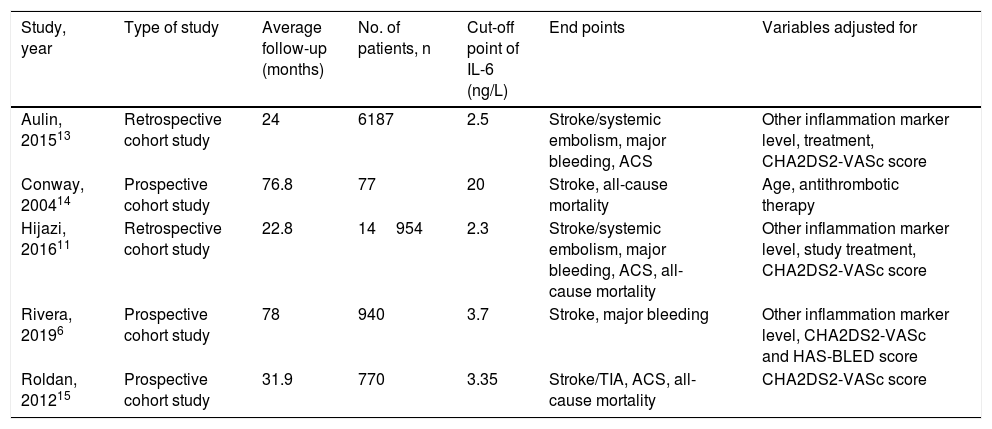
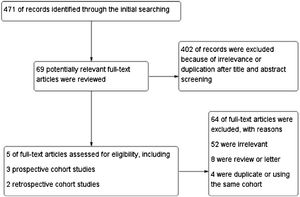
ResultsStudy characteristicsIn total, 471 records were retrieved in our primary literature search by using the mesh term atrial fibrillation and interleukin-6. 402 records were excluded after screening the titles and abstracts. 69 potentially relevant full-text articles were reviewed, and five studies with a total of 22 928 participants were included in the final analysis (Figure 1), including two retrospective cohort studies and three prospective cohort studies (Table 1). Quality assessment is reported in Table 2.
Studies on association between IL-6 and thromboembolic events in AF.
| Study, year | Type of study | Average follow-up (months) | No. of patients, n | Cut-off point of IL-6 (ng/L) | End points | Variables adjusted for |
|---|---|---|---|---|---|---|
| Aulin, 201513 | Retrospective cohort study | 24 | 6187 | 2.5 | Stroke/systemic embolism, major bleeding, ACS | Other inflammation marker level, treatment, CHA2DS2-VASc score |
| Conway, 200414 | Prospective cohort study | 76.8 | 77 | 20 | Stroke, all-cause mortality | Age, antithrombotic therapy |
| Hijazi, 201611 | Retrospective cohort study | 22.8 | 14954 | 2.3 | Stroke/systemic embolism, major bleeding, ACS, all-cause mortality | Other inflammation marker level, study treatment, CHA2DS2-VASc score |
| Rivera, 20196 | Prospective cohort study | 78 | 940 | 3.7 | Stroke, major bleeding | Other inflammation marker level, CHA2DS2-VASc and HAS-BLED score |
| Roldan, 201215 | Prospective cohort study | 31.9 | 770 | 3.35 | Stroke/TIA, ACS, all-cause mortality | CHA2DS2-VASc score |
AF: atrial fibrillation; TIA: transient ischemia attack; ACS: acute coronary syndrome; CHA2DS2-VASc: Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female).
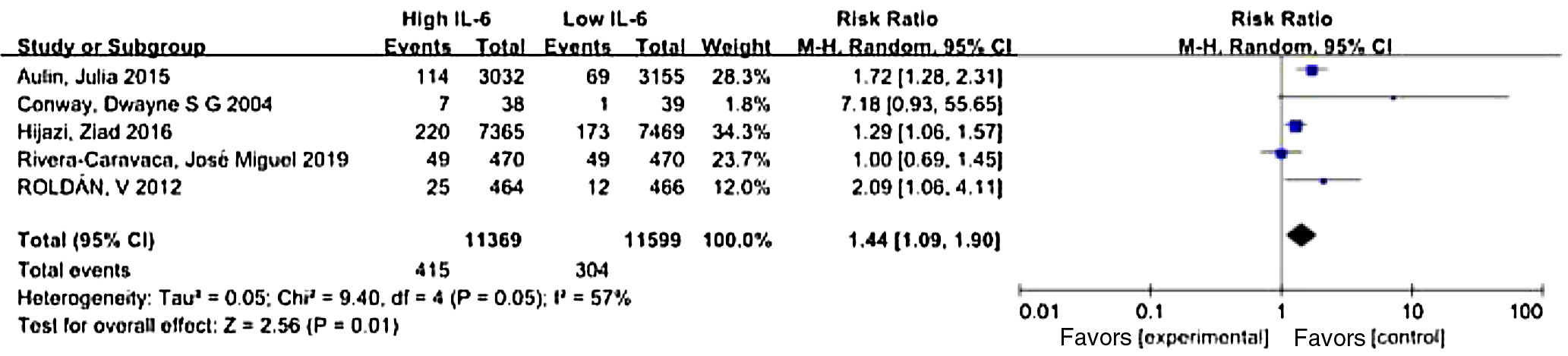
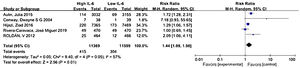
Where IL-6 was affected by other factors, such as antithrombotic therapy or comorbidity, the researchers adjusted the outcomes to determine whether IL-6 was an independent risk factor for stroke and thromboembolic events. We used the data after adjustment. Meta-analysis showed that elevated IL-6 meant a higher risk of long-term stroke and thromboembolic risk in AF patients with a pooled RR 1.44 (CI 95% 1.09-1.90, p=0.01) (Figure 2).
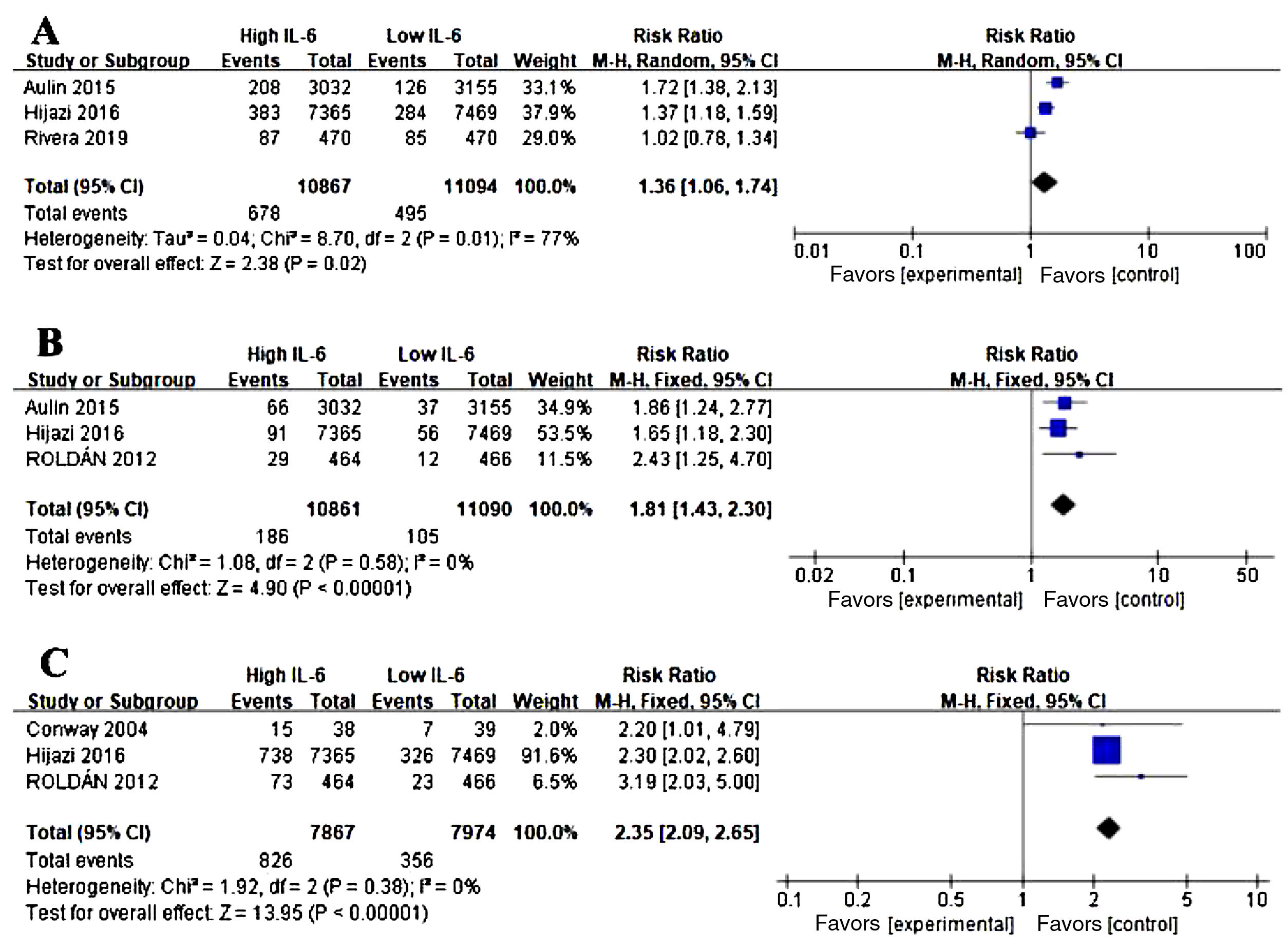
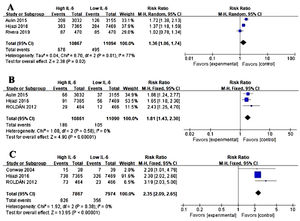
Association between IL-6 and other adverse events in AFWe conducted the analysis of major bleeding events (Figure 3A), acute coronary syndrome (ACS) (Figure 3B), all-cause mortality (Figure 3C) in AF and the level of IL-6. Three studies were eligible for the analysis. Meta-analysis showed that elevated IL-6 meant a higher risk of long-term bleeding risk (RR 1.36, CI 95% 1.06-1.74, p=0.02), ACS risk (RR 1.81, CI 95% 1.43-2.30, P<0.001) and all-cause death (RR 2.35, CI 95% 2.09-2.65, p<0.001).
Association of IL-6 and other adverse events in atrial fibrillation (AF). (A) Forest plots of risk ratio (RR) and CI 95% for the association between IL-6 and major bleeding events in AF. (B) Forest plots of RR and CI 95% for the association between IL-6 and acute coronary syndrome events in AF. (C) Forest plots of RR and CI 95% for the association between IL-6 and all-cause mortality in AF.
IL-6 is one of the inflammatory cytokines with a pleiotropic effect on immune response and inflammation. It is synthesized in immune cells such as macrophages and monocytes.16 When pattern recognition receptors of immune cells are activated, a range of signaling pathways including factor kappa B are initiated to enhance the transcription of the message RNA of inflammatory cytokines such as IL-6, tumor necrosis factor (TNF), and IL-1β.17 IL-6 can also be produced in endothelial cells as a result of the stimulation of IL-1, TNF and interferon-γ.18
IL-6 synthesized in the inflammatory lesion is released to the blood and moves to the liver, which means IL-6 is the major regulator of acute phase response in human hepatocytes.19 A series of acute phase proteins were then induced such as CRP, fibrinogen and plasminogen activator inhibitor-1.19 IL-6 promotes megakaryocyte maturation after reaching the bone marrow, thus leading to the release of platelets and thrombocytosis.20 IL-6 may induce the expression of tissue factor,21,22 factor VIII23 and von Willebrand factor.24 In addition, an increased interleukin-6 level decreases the natural inhibitors of hemostasis such as antithrombin, protein S25 and thrombomodulin.26 Elevated IL-6 is related to enhanced coagulation function and weakened anticoagulation function to establish a prothrombotic state, which in turn is linked to thrombotic risk and ACS. IL-6 also induces excess production of vascular endothelial growth factor, leading to enhanced angiogenesis and increased risk of bleeding.16
Emerging studies have proved that AF is a kind of inflammation, containing inflammatory cells and inflammatory cytokines. Thrombogenesis of AF is also associated with inflammation. CRP level is significantly associated with stroke risk in AF patients.7 In AF patients, platelet P-selectin levels and adenosine diphosphate -induced platelet aggregation are significantly higher in the left atrium compared to the right atrium,27 which may be related to the different inflammation level. IL-6 level is positively related to the CHA2DS2 score, while anticoagulation therapy can decrease the IL-6 level.28,29 Inflammation can cause endothelial dysfunction, platelet and endothelial cell activation, and coagulation cascade activation.9
In our meta-analysis, a higher IL-6 level may predict a higher thrombotic risk as well as a higher bleeding risk, ACS risk and mortality risk in AF patients. It is possible to assess the thromboembolic risk and bleeding risk by adding IL-6 into theCHA2DS2-VASc score and HAS-BLED score. Anti-inflammation or anti-IL-6 therapy may be administered in AF patients. Further research needs to be conducted such as the cut-off point of IL-6.
There are several limitations in our study. The participants’ baseline characteristics, such as age and complications were various and the mean follow-up time and cut-off point were all different among the studies. Publication bias was not detected because of the limited number of studies. Publication bias might exist in this study.
ConclusionOur meta-analysis showed that higher level of IL-6 may predict a greater number of long-term thromboembolic events, as well as bleeding events, ACS events and mortality in AF patients. Further studies such as the cut-off point of IL-6 need to be conducted in the future.
Funding sourcesThis work was supported by the Science and Technology Research and Development Program of Shanghai (No. 17ZR1403700).
Conflicts of interestThe authors have no conflicts of interest to declare.