There is a lack of consensus on the definition of response to cardiac resynchronization therapy (CRT), and it is not clear which response criteria have most influence on cardiac event-free survival.
ObjectivesTo assess the predictive value of various response criteria in patients undergoing CRT and the agreement between them.
MethodsWe performed a secondary analysis of the BETTER-HF trial. Patient response was classified at six months after CRT according to eleven criteria used in previous trials. The predictive value of response criteria for survival free from mortality, cardiac transplantation and heart failure hospitalization was assessed by Cox regression analysis. Agreement between the different response criteria was assessed using Cohen's kappa (κ).
ResultsA total of 115 patients were followed for a mean of 25 months. During follow-up, 15 deaths occurred (13%) and 29 patients had at least one adverse cardiac event (25%). Only five of the eleven response criteria were predictors of event-free survival. The most powerful isolated clinical and echocardiographic predictors were a reduction of ≥1 NYHA functional class (HR 0.39 for responders; 95% CI 0.18-0.83, p=0.014) and an increase of at least 15% in left ventricular ejection fraction (HR 0.43, 95% CI 0.20-0.90, p=0.024), respectively. Agreement between the different response criteria was poor.
ConclusionsMost currently used response criteria do not predict clinical outcomes and have poor agreement. It is essential to establish a consensus on the definition of CRT response in order to standardize studies.
Não existe consenso na definição de respondedor à ressincronização cardíaca (TRC), e desconhece-se qual o critério de resposta que poderá ter maior influência na sobrevida livre de eventos cardíacos.
ObjectivosAvaliar o valor preditivo de vários critérios de resposta em doentes submetidos à TRC, e analisar a concordância entre eles.
MétodosSubanálise do ensaio BETTER-HF. Os doentes foram classificados aos seis meses após TRC em respondedores, de acordo com onze critérios utilizados na literatura. O valor preditivo dos diferentes critérios de resposta para uma sobrevida livre de morte, transplante cardíaco e hospitalização por insuficiência cardíaca foi avaliado usando a regressão de Cox. A concordância entre os diferentes critérios foi avaliada usando o coeficiente k de Cohen.
Resultados115 doentes foram seguidos durante um período médio de 25 meses. Durante o follow-up ocorreram 15 mortes (13%) e 29 doentes tiveram, pelo menos, um evento cardíaco adverso (25%). Apenas cinco dos onze critérios de resposta foram preditores de sobrevida livre de eventos. Os preditores clínicos e ecocardiográficos isolados mais poderosos foram a redução de, pelo menos, uma classe funcional de NYHA (HR 0,39; IC 95% 0,18-0,83, p=0,014) e um aumento de, pelo menos, 15% na fração de ejeção ventricular esquerda (FEVE) (HR 0,43; IC 95% 0,20-0,90, p=0,024), respetivamente. A concordância entre os diferentes critérios de resposta foi fraca.
ConclusãoA maioria dos critérios de resposta utilizados não prevê outcomes clínicos e têm fraca concordância. É essencial criar um consenso na definição de resposta à TRC de forma a uniformizar os estudos.
Cardiac resynchronization therapy (CRT) is an approved treatment in selected heart failure (HF) patients, with beneficial effects on symptoms, cardiac remodeling and function, and survival.1–5 However, there is no consensus regarding the best definition of response to CRT. Numerous criteria, including clinical and combined endpoints, reverse remodeling, and improvement in left ventricular ejection fraction (LVEF), are currently used in the literature to define response to CRT, and this is further complicated by the poor agreement between different response criteria.6
There are several issues involved in the problem of defining response to CRT. HF is a complex and progressive disease, and despite improvements in device-based treatment, the prognosis is still very poor in patients with advanced HF. Some have questioned whether absence of change (i.e. no deterioration in function) after CRT should also be considered a response to treatment.7 Furthermore, patients and physicians may have different perceptions of the benefits of CRT.8 All these issues make the definition of response to CRT particularly challenging.
The aim of this study was to assess the impact on outcomes of different response criteria currently used in the literature in patients undergoing CRT, and the agreement between them.
MethodsStudy designWe performed a secondary analysis of the BETTER-HF trial (ClinicalTrials.gov identifier NCT02413151),9 a prospective, interventional, single-center randomized clinical trial of the effect of cardiac rehabilitation on clinical and echocardiographic response in patients with HF with reduced ejection fraction (HFrEF) treated by CRT. One hundred and twenty-one adult HFrEF patients referred for CRT were prospectively enrolled between April 2011 and February 2015. Patients underwent baseline assessment before CRT, including clinical parameters, laboratory tests, transthoracic echocardiogram and cardiopulmonary exercise test (CPET), and were reassessed at six months after CRT.
All patients provided written informed consent before any procedure. The study was approved by the institutional review board and ethics committee.
Study populationThe BETTER-HF trial9 included consecutive patients referred for CRT with or without indication for defibrillator implantation.
The inclusion criteria were stable HF patients receiving optimal HF pharmacologic therapy for at least three months, age ≥18 years, LVEF <35%, QRS duration ≥120 ms, referral for CRT at Santa Marta Hospital according to European Society of Cardiology (ESC) recommendations,10–12 and stable condition for >1 month (no hospitalization for congestive HF, no change in medication, and no change in New York Heart Association [NYHA] functional class).
Exclusion criteria were residence far from the hospital making frequent hospital visits difficult or impossible; incapacitating orthopedic, neurologic or other conditions precluding exercise testing; refusal to participate in the study; inability to sign informed consent; previous treatment with an intravenous inotropic agent within the 30 days prior to implantation; or death or CRT device removal during the first six months.
Data collectionBaseline and follow-up data up to November 2015 were obtained from hospital files, systematic follow-up phone calls, and the national health database, which holds information from national health care providers.
AssessmentPatient assessment included NYHA functional class at baseline and six months after CRT implantation, the HeartQoL quality-of-life score,13 transthoracic echocardiography (LVEF, left ventricular end-systolic volume [LVESV], left ventricular end-diastolic volume [LVEDV] and stroke volume), CPET (peak oxygen consumption [peak VO2]), and blood samples (serum creatinine and B-type natriuretic peptide [BNP]) for a more objective quantification of the physiologic and clinical benefits of CRT.
Baseline demographic data (age and gender), HF etiology, risk factors (hypertension, hyperlipidemia, diabetes, smoking and obesity), medication and electrocardiogram were also recorded.
The primary endpoint was a composite of all-cause death, heart transplantation and hospitalization for HF (unplanned hospitalization for more than 24 hours caused by HF decompensation) during follow-up.
Cardiac resynchronization therapy response criteriaTaking into account the seventeen different primary response criteria identified by Fornwalt et al.6 in a review of the 26 publications with most citations addressing response to CRT, and adapting them to our routine assessment of CRT patients, we included in the analysis eleven different response criteria: four clinical, six echocardiographic, and one combined criterion, as listed in Table 1.
Analyzed response criteria.
| Clinical |
| 1. ↓≥1 NYHA class14–17 |
| 2. ↑QoL ≥0.512 |
| 3. ↑peak VO2 >10%18 |
| 4. ↓≥1 NYHA class or ↑peak VO2 >10% and alive, no hospitalization for HF17 |
| Echocardiographic |
| 5. ↑LVEF ≥5% (absolute)13,19 |
| 6. ↑LVEF ≥15%20,21 |
| 7. LVESV <15% of baseline22 |
| 8. ↓LVESV >15%14,23–28 |
| 9. ↓LVEDV >15%14 |
| 10. ↑Stroke volume ≥15%21,29,30 |
| Combined |
| 11. ↑LVEF ≥5% (absolute) and ↓≥1 NYHA class or ↑QoL ≥0.531 |
↑: higher: ↓: lower; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; NYHA: New York Heart Association functional class; peak VO2: oxygen consumption at peak exercise; QoL: HeartQoL quality-of-life score.
Data analysis was performed using IBM SPSS® version 20. Continuous data were expressed as mean ± standard deviation and compared using the non-parametric Wilcoxon rank test. Categorical data were displayed as frequencies and percentages, and compared using the chi-square test or Fisher's exact test as appropriate.
Response criteria that were potential predictors of survival free from major adverse cardiac events (MACE) were assessed by univariate Cox regression analysis. The results are expressed as hazard ratios (HR) with 95% confidence intervals (CI). The best predictive criteria (clinical and echocardiographic) were subsequently combined (a patient who had fulfilled both response criteria was classified as a responder) to determine whether the predictive value improved with each isolated criterion. The two-sided level of significance was set at p<0.05.
Agreement between the different response criteria was assessed using Cohen's kappa (κ), ranging from -1 (perfect disagreement) to +1 (perfect agreement), with 0 indicating that agreement is exactly that expected by chance.32 κ values ≥0.75 were considered as strong agreement, 0.4-0.75 as moderate agreement, and ≤0.4 as poor agreement.33
ResultsBaseline characteristics and major adverse cardiac events at follow-upOf the 121 consecutive patients included in the BETTER-HF trial,9 six were excluded from this analysis (in two, the system was removed in the first six months due to infection, two died before the six-month reassessment, and two did not undergo the six-month exams).
A total of 115 patients with advanced HF were included (73.9% in NYHA class III-IV), mean age 68 years, 68% male. Ischemic HF was present in 37% of the patients and atrial fibrillation in 24%. Mean baseline QRS duration was 146 ms and mean LVEF was 27%. A CRT defibrillator (CRT-D) was implanted in 95 patients (82.6%) and a CRT pacemaker (CRT-P) in the remaining.
During a mean follow-up of 25±13 months, at least one MACE occurred in 29 patients (25%). Fifteen patients (13%) died (two from sudden cardiac death [SCD], 10 from congestive HF, and three from systemic infection), 25 were hospitalized for HF (21.7%), and one (0.8%) underwent heart transplantation. The incidence of SCD (two patients with CRT-D) was 1% per year. There were no significant differences between patients with and without MACE in most of the baseline characteristics analyzed, except for serum creatinine and BNP levels, both of which were higher in patients with MACE (serum creatinine 1.28±0.52 mg/dl vs. 1.04±0.39 mg/dl, p=0.007; BNP 734±683 pg/ml vs. 392±390 pg/ml, p=0.005).
The characteristics of the overall population at baseline and according to occurrence of MACE are presented in Table 2.
Characteristics of the overall population at baseline and according to the occurrence of major adverse cardiac events.
| Total (n=115) | With MACE (n=29) | Without MACE (n=86) | p | |
|---|---|---|---|---|
| Male, n (%) | 78 (67.8) | 23 (79.3) | 55 (64.0) | 0.126 |
| Age, years | 68.4±10.1 | 68.4±9.1 | 68.4±10.4 | 0.602 |
| Ischemic HF, n (%) | 42 (36.5) | 12 (41.4) | 30 (34.9) | 0.530 |
| Cardiovascular risk factors | ||||
| Hypertension, n (%) | 102 (88.7) | 23 (79.3) | 79 (91.9) | 0.065 |
| Hyperlipidemia, n (%) | 80 (69.6) | 20 (69.0) | 60 (69.8) | 0.935 |
| Diabetes, n (%) | 47 (40.9) | 10 (34.5) | 37 (43.0) | 0.418 |
| Smoking, n (%) | 24 (20.9) | 9 (31.0) | 15 (17.4) | 0.119 |
| Obesity (BMI ≥30), n (%) | 30 (26.1) | 8 (27.6) | 22 (25.6) | 0.832 |
| Cardiovascular medication | ||||
| BB, n (%) | 100 (87.0) | 27 (93.1) | 73 (84.9) | 0.349 |
| ACEI/ARB, n (%) | 103 (89.6) | 26 (89.7) | 77 (89.5) | 1.000 |
| Diuretics, n (%) | 106 (92.2) | 28 (96.6) | 78 (90.7) | 0.445 |
| NYHA ≥III, n (%) | 85 (73.9) | 21 (72.4) | 64 (74.4) | 0.812 |
| QoL | 0.95±0.69 | 1.03±0.80 | 0.93±0.66 | 0.831 |
| Baseline electrocardiogram | ||||
| QRS, ms | 146±22 | 143±23 | 147±21 | 0.487 |
| LBBB, n (%) (n=85) | 78 (91.8) | 21 (91.3) | 57 (91.9) | 1.000 |
| AF, n (%) | 28 (24.3) | 9 (31.0) | 19 (22.1) | 0.622 |
| Baseline echocardiogram | ||||
| LVEF, % | 27±7 | 25±7 | 27±7 | 0.243 |
| LVESV, ml | 154±58 | 165±60 | 150±57 | 0.116 |
| LVEDV, ml | 208±71 | 222±69 | 202±72 | 0.109 |
| Baseline CPET | ||||
| Peak VO2, ml/kg/min | 14.5±5.3 | 15.0±5.7 | 13.4±4.1 | 0.299 |
| Baseline blood tests | ||||
| BNP (pg/ml) | 493±515 | 734±683 | 392±390 | 0.005 |
| Creatinine (mg/dl) | 1.10±0.44 | 1.28±0.52 | 1.04±0.39 | 0.007 |
| Follow-up, months | 25±13 | 22±12 | 26±13 | 0.189 |
ACEI: angiotensin-converting enzyme inhibitor; AF: atrial fibrillation; ARB: angiotensin receptor blocker; BB: beta-blocker; BMI: body mass index; BNP: B-type natriuretic peptide; CPET: cardiopulmonary exercise testing; HF: heart failure; LBBB: left bundle branch block; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; MACE: major adverse cardiac events; NYHA: New York Heart Association functional class; Peak VO2: oxygen consumption at peak exercise; QoL: HeartQoL quality-of-life score.
Patients without MACE during the follow-up period were more frequently responders by all the response criteria, achieving significantly higher response rates on the NYHA, LVEF and LVESV criteria (Table 3).
Response rates in patients with and without major adverse cardiac events.
| Response criteria | Response rates | ||
|---|---|---|---|
| With MACE (n=29) | Without MACE (n=86) | p | |
| Clinical, n (%) | |||
| 1. ↓NYHA ≥1 | 17 (58.6) | 71 (82.6) | 0.017 |
| 2. ↑QoL ≥0.5 | 18 (62.1) | 66 (76.7) | 0.230 |
| 3. ↑peak VO2 >10% | 15 (51.7) | 45 (52.3) | 0.839 |
| 4. ↓NYHA ≥1 or ↑peak VO2 >10% and alive, no hospitalization for HF | 18 (62.1) | 78 (90.7) | 0.000 |
| Echocardiographic, n (%) | |||
| 5. ↑LVEF ≥5% (absolute) | 15 (51.7) | 66 (76.7) | 0.010 |
| 6. ↑LVEF ≥15% | 15 (51.7) | 67 (77.9) | 0.006 |
| 7. LVESV <15% of baseline | 24 (82.8) | 77 (89.5) | 0.253 |
| 8. ↓LVESV >15% | 10 (34.5) | 51 (59.3) | 0.037 |
| 9. ↓LVEDV >15% | 6 (20.7) | 35 (40.7) | 0.067 |
| 10. ↑Stroke volume ≥15% | 13 (44.8) | 45 (52.3) | 0.458 |
| Combined, n (%) | |||
| 11. ↑LVEF ≥5% (absolute) and ↓NYHA ≥1 or ↑QoL ≥0.5 | 12 (41.4) | 59 (68.6) | 0.015 |
↑: higher; ↓: lower; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; NYHA: New York Heart Association functional class; peak VO2: oxygen consumption at peak exercise; QoL: HeartQoL quality-of-life score.
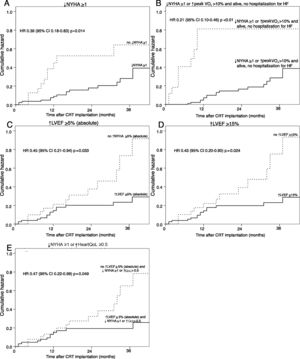
Of all the response criteria analyzed, only five were predictors of MACE. The strongest predictor was the combined clinical criterion including reduction of at least one NYHA class or >10% improvement in peak VO2 and being alive, with no hospitalization for HF in the first six months after CRT implantation (HR 0.21; 95% CI 0.10-0.46, p<0.001). The most strongly predictive isolated clinical response criterion was reduction of at least one NYHA class, which was associated with 61% lower risk for future MACE (HR 0.39; 95% CI 0.18-0.83, p=0.014). The best echocardiographic response criterion was a relative increase in LVEF of at least 15%, which was associated with 57% lower risk for future MACE (HR 0.43, 95% CI 0.20-0.90, p=0.024) (Table 4 and Figure 1).
Predictors of major adverse cardiac events.
| Response criteria | Univariate analysis | |
|---|---|---|
| HR (95% CI) | p | |
| Clinical | ||
| 1. ↓NYHA ≥1 | 0.39 (0.18-0.83) | 0.014 |
| 2. ↑QoL ≥0.5 | 0.57 (0.22-1.44) | 0.233 |
| 3. ↑peak VO2 >10% | 0.70 (0.35-2.03) | 0.695 |
| 4. ↓NYHA ≥1 or ↑peak VO2 >10% and alive, no hospitalization for HF | 0.21 (0.10-0.46) | <0.001 |
| Echocardiographic | ||
| 5. ↑LVEF ≥5% (absolute) | 0.45 (0.21-0.94) | 0.033 |
| 6. ↑LVEF ≥15% | 0.43 (0.20-0.90) | 0.024 |
| 7. LVESV <15% of baseline | 0.44 (0.17-1.18) | 0.103 |
| 8. ↓LVESV >15% | 0.59 (0.27-1.30) | 0.190 |
| 9. ↓LVEDV >15% | 0.54 (0.22-1.33) | 0.179 |
| 10. ↑Stroke volume ≥15% | 0.91 (0.42-1.97) | 0.816 |
| Combined | ||
| 11. ↑LVE F≥5% (absolute) and ↓NYHA ≥1 or ↑QoL≥0.5 | 0.47 (0.22-0.99) | 0.049 |
LVEF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; NYHA: New York Heart Association functional class; peak VO2: oxygen consumption at peak exercise; QoL: HeartQoL quality-of-life score.
Survival curves of cumulative occurrence of major adverse cardiac events in responders or non-responders to cardiac resynchronization therapy according to the most strongly predictive response criteria. (A) Criterion of ↓NYHA ≥1; (B) criterion of ↓NYHA ≥1 or ↑peak VO2>10% and alive, no hospitalization for heart failure; (C) criterion of ↑LVEF ≥5% (absolute); (D) criterion of ↑LVEF ≥15%; (E) criterion of ↑LVEF ≥5% (absolute) and ↓NYHA ≥1 or ↑HeartQoL ≥0.5. ↑: higher: ↓: lower; CI: confidence interval; CRT: cardiac resynchronization therapy; HF: heart failure; HR: hazard ratio; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association functional class; QoL: HeartQoL quality-of-life score.
The combination of the best clinical and echocardiographic criteria (reduction of at least one NYHA class or >10% improvement in peak VO2 and being alive, with no hospitalization for HF in the first six months after CRT implantation, and increase in LVEF of at least 15%) was more strongly predictive than the echocardiographic criterion but not more so than the clinical criterion alone (HR 0.256, 95% CI 0.118-0.554, p=0.001). The combination of reduction of at least one NYHA class and increase in LVEF of at least 15% was more predictive of no MACE than either of the two criteria alone (HR 0.292, 95% CI 0.132-0.646, p=0.002).
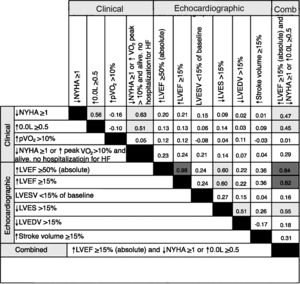
Agreement between response criteriaAgreement between the different response criteria as a group (κ=0.24±0.25), and also between echocardiographic response criteria and clinical criteria (κ=0.19±0.21 and 0.25±0.36, respectively) was poor. Of the total of 55 pairs analyzed, the majority (78.2%) had poor agreement, and only three (5.5%) had κ values in the range of strong agreement. Excluding response criteria whose definition included multiple variables, and assessing the isolated clinical criteria, there was a moderate agreement between improvement in NYHA functional class and quality of life (κ=0.56), but poor agreement between these and increase in peak VO2 (κ=-0.16 and κ=-0.10, respectively). Between the six echocardiographic criteria, only three pairs achieved moderate agreement, higher LVEF/lower LVESV (κ=0.60) and lower LVEDV/higher LVESV (κ=0.51) (Figure 2).
Agreement between response criteria was strong in only 7.6% of response criteria pairs. Cohen's κ values are color-coded according to the following ranges: dark gray=strong agreement (κ ≥0.75), light gray=moderate agreement (κ 0.4-0.75), none=poor agreement (κ ≤0.4). ↑: higher: ↓: lower; Comb: combined; HF: heart failure; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; NYHA: New York Heart Association functional class; peak VO2: oxygen consumption at peak exercise; QoL: HeartQoL quality-of-life score.
While the beneficial effects of CRT on symptoms, cardiac remodeling and function, and survival have been well established in multiple trials, there is still no consensus regarding the best definition of response to CRT. The primary result of the present analysis is that in our sample of HF patients treated by CRT, only a minority (five out of 11) of commonly used criteria of response to CRT in the literature predicted MACE-free survival 25 months after CRT implantation. A previous study by Boidol et al.,34 including 97 patients followed for one year, showed similar findings, with only eight of 15 analyzed criteria differentiating patients with MACE from those who remained event-free. These results highlight the lack of uniformity and predictive ability of the multiple criteria used in the literature.
The most powerful isolated predictors of MACE identified were NYHA functional class and LVEF. Patients who fulfilled these separately had a lower risk of major events at follow-up of 61% and 57%, respectively. Similarly, Boidol et al.34 concluded that improvement in NYHA functional class was the most strongly predictive clinical criterion (adjusted relative risk [RR] 4.4; p=0.002), while the most strongly predictive echocardiographic response criterion was a decrease in LVESV index (adjusted RR 3.49; p=0.002).
A recent study by Rickard et al.35 assessed echocardiographic predictors of long-term survival following CRT in 436 patients followed for 5.4±2.3 years. Various changes in LVESV, LVEDV and LVEF were tested. The authors concluded that LVEF improvement and LVESV reduction were similar in terms of prognosis and were both superior to definitions using LVEDV, as was shown in our study, in which LVEDV reduction was also not a predictor of event-free survival.
On the other hand, we found that combining clinical and echocardiographic parameters adds predictive power. Although the combination of criteria may hamper their application in daily clinical practice, this could be a way to improve identification of patients who will have a better prognosis.
Our population included patients with CRT devices with or without defibrillator. We do not consider this a confounding factor, as the incidence of SCD was low (1% per year), and only occurred in two ischemic patients with CRT-D.
Another finding of our study was the poor agreement between the response criteria analyzed as a group (κ=0.25), and between clinical and echocardiographic criteria. This was also found by Fornwalt et al.,6 who concluded that agreement between different methods to define CRT response was poor 75% of the time and pointed out that this severely limits the ability to generalize and compare results between studies. This discrepancy between different criteria could in part be explained by the different aspects of HF status that they assess.36
To our knowledge, the current study is the largest comparing the impact on long-term cardiac events of commonly used echocardiographic and clinical CRT response criteria. We demonstrate that the criteria have poor agreement with each other and do not have the same impact on prognosis. It is essential to establish a consensus on the definition of CRT response in order to standardize studies. More importantly, if there is better understanding of which patients will achieve the best response to CRT, this may lead to changes in selection for treatment.
LimitationsThe current study has the limitations inherent to any single-center study, which may include selection bias. Also, patients who died within six months of CRT implantation were excluded from our analysis, and so no conclusions can be drawn for this specific population. Response was only assessed at six months after CRT implantation, and therefore we cannot know if the same results would be found if response had been assessed before or after this time.
Moreover, echocardiographic responses to CRT is inherently limited by inter- and intraobserver variability, which is a limitation in any study involving these parameters.
A relative limitation is the follow-up duration. Although this was one of the longest studies comparing different clinical and echocardiographic response criteria, the predictive value of different criteria is still unknown in terms of their impact on longer-term cardiac events.
ConclusionIn advanced HF patients treated by CRT, not all of the commonly used response criteria predicted outcomes, and there was poor agreement between them. Improvement in NYHA functional class and in LVEF were the isolated criteria with the most powerful predictive value for MACE-free survival. The association of these two criteria improved their predictive value. These findings may mean that these criteria are to be preferred to others when defining CRT response in future studies. It is essential to establish a consensus on the definition of CRT response in order to standardize studies.
Conflicts of interestThe authors have no conflicts of interest to declare.
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
The authors wish to thank all the staff of the Santa Marta Electrophysiology and Pacing Department and Echocardiographic Laboratory for their assistance with this project.