Patients with ST-elevation myocardial infarction (STEMI) requiring inter-hospital transfer for primary percutaneous coronary intervention (PCI) often have delays in reperfusion. The door in-door out (DIDO) time is recommended to be less than 30 min.
ObjectivesTo assess the DIDO time of hospitals that transfer patients with STEMI to a PCI center and to assess its impact on total ischemia time and clinical outcomes in patients with STEMI.
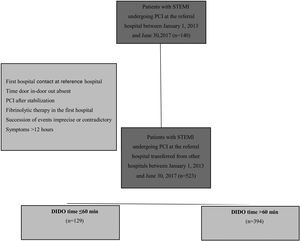
MethodsWe performed a retrospective study of 523 patients with STEMI transferred to a PCI center for primary PCI between January 1, 2013 and June 30, 2017.
ResultsMedian DIDO time was 82 min (interquartile range, 61–132 min). Only seven patients (1.3%) were transferred in ≤30 min. Patients with DIDO times over 60 min had significantly longer system delays (207.3 min vs. 112.7 min; p<0.001) and total ischemia time (344.2 min vs. 222 min; p<0.001) than patients transferred in ≤60 min. Observed in-hospital mortality was significantly higher among patients with DIDO times >60 min vs. ≤60 min (5.1% vs. 0%; p=0.006; adjusted odds ratio for in-hospital mortality, 1.27 [95% CI 1.062–1.432]). By the end of follow-up, patients belonging to the >60 min group had a higher mortality (p=0.016), and survival time was significantly shorter (p=0.011).
ConclusionA DIDO time ≤30 min was observed in only a small proportion of patients transferred for primary PCI. DIDO times of ≤60 min were associated with shorter delays in reperfusion, lower in-hospital mortality and longer survival times.
Está recomendado que os doentes com enfarte agudo do miocárdio com supra de ST (EAMcSST) que necessitam de transferência inter-hospitalar para a intervenção coronária percutânea primária (ICPP) tenham um tempo de door in-door out (DIDO) ≤30 minutos.
ObjetivosAvaliar o tempo DIDO dos hospitais que transferem pacientes com EAMcSST para ICP e o seu impacto no tempo total de isquemia e outcomes clínicos.
MétodosEstudo retrospetivo com 523 doentes com EAMcSST transferidos para a ICPP, entre 1 de janeiro de 2013 e 30 de junho de 2017.
ResultadosA mediana do tempo DIDO foi de 82 minutos (intervalo interquartílico [IQ], 61-132 minutos). Apenas 7 pacientes (1,3%) foram transferidos em ≤30 minutos. Os pacientes com um tempo DIDO >60 minutos apresentavam atrasos de sistema (207,3 min versus 112,7 min; p<0,001) e tempos totais de isquemia (344,2 min versus 222 min; p<0,001) significativamente superiores quando comparados com os doentes que eram transferidos em ≤60 minutos. A mortalidade intra-hospitalar foi significativamente superior nos pacientes com tempo DIDO >60 minutos (5,1% versus 0%; p=0,006; odds ratio ajustada para a mortalidade intra-hospitalar, 1,27 [95% IC, 1,062-1,432]). Até à data de follow-up, os doentes pertencentes ao grupo “>60 min” apresentavam uma maior proporção de eventos de morte, p=0,016 e o tempo de sobrevivência era significativamente inferior, p=0,011.
ConclusãoO tempo DIDO ≤30 minutos foi observado numa pequena proporção de doentes. DIDO ≤60 minutos associaram-se a menores atrasos na reperfusão, a menor mortalidade intra-hospitalar e a maiores tempos de sobrevivência.
Cardiovascular disease remains the most common cause of death in Europe, and is responsible for double the number of cancer-related deaths.1,2 Ischemic heart disease is the most common cause of death globally and in Europe and, although its incidence is decreasing in Europe, it is still growing worldwide.1–3 Similarly, although the incidence of ST-elevation myocardial infarction (STEMI) is falling, the incidence of non-ST-elevation myocardial infarction is rising.4,5 The diagnosis of myocardial infarction (MI) is based on evidence of necrosis of cardiac myocytes due to ischemia, specifically an increase in cardiac troponin level above the 99th percentile.6,7 Patients with STEMI can be treated using fibrinolysis or percutaneous coronary intervention (PCI), the latter being the method of choice.8,9 Regardless of reperfusion method, the main objective in STEMI is to minimize total ischemia time, which is defined as the time between symptom onset and the initiation of treatment. Current guidelines from the European Society of Cardiology (ESC) and the American College of Cardiology/American Heart Association (ACC/AHA) recommend that this time should be less than 120 min, and set targets of 90 min and 30 min for the time between first medical contact and reperfusion through PCI and fibrinolysis, respectively.7,10
The hospital network in Portugal, as in most countries, includes hospitals both with and without catheterization laboratories. Thus, patients with STEMI who are to be treated by PCI have to be screened and transported to centers with appropriate facilities.11 Several European clinical trials have shown that timely inter-hospital transfer for primary PCI is feasible and associated with lower mortality compared to fibrinolytic therapy.12,13 A critical factor in the inter-hospital transfer process is the time taken for hospitals without PCI facilities to refer patients with STEMI to centers capable of performing PCI. This parameter, called door in-door out (DIDO) time, is defined as the time from arrival to discharge at the first hospital.
DIDO time is increasingly considered an important measure in efforts to accelerate reperfusion. The ESC and ACC/AHA guidelines recommend that it should ideally be less than 30 min.7,14 Despite its importance, this parameter is still little studied. The relevance of patient characteristics and their relationship with DIDO time, as well as its effect on treatment and subsequent outcomes, are still uncertain.14 In a study of 14821 STEMI patients from several hospitals in the USA who were transferred to other centers for PCI, Wang et al. concluded that the majority did not achieve the target for DIDO time and that the shorter the time, the lower the adjusted mortality risk, thus demonstrating the importance of this parameter.15
This study aims to assess the DIDO time of hospitals transferring STEMI patients to a referral center for PCI, in addition to exploring the association of delays in reperfusion with morbidity and mortality in this population.
MethodsThis was an observational, retrospective and analytical study based on a convenience sample consisting of patients with STEMI who underwent PCI in a cardiology department between January 1, 2013 and June 30, 2017 (n=1140), thus obtaining a minimum follow-up time of one year. Data were collected from the intervention cardiology database of the reference center.
From the initial set of patients, only those who had been transferred from other hospitals were selected. Those for whom not all data of interest for the study could be obtained, particularly DIDO time, those in whom the succession of events was imprecise or contradictory, those who underwent PCI after stabilization, those who received fibrinolytic therapy in the first hospital and those who with symptoms for longer than 12 hours, were excluded. A sample of 523 patients was thus obtained from the initial set of 832 transferred patients.
Patients included in the study population were classified according to DIDO time. Wang et al.,15 investigating the same parameter, chose to divide patients into two groups: those in whom DIDO time was ≤30 min, and those in whom it was >30 min. Since Wang et al.’s study was our reference, we initially intended to adopt the same method, however, this was not feasible. After the sample was collected, it was found that if this value was used to separate the patients, the sizes of the groups would be very different, thus compromising the statistical power of this work. Specifically, the group with DIDO time ≤30 min contained only seven individuals, while the other group contained 516. It was thus decided to divide the sample using 60 min as cutoff, thus obtaining two groups: estimated DIDO ≤60 min, n=129; and estimated DIDO >60 min, n=394.
DefinitionsMI was defined as an elevation of cardiac biomarkers with a compatible curve, with at least one value above the 99th percentile of the upper reference limit, together with one or more of the following: symptoms of ischemia, changes in the ST-T segment or new left bundle branch block, imaging evidence of loss of viable myocardium, or kinetic abnormalities. ST-segment elevation was defined as an increase at the J-point in two contiguous leads of ≥0.1 mV in all leads other than V2-V3. In the latter, a cutoff of ≥0.25 mV was used in men under 40 years of age, and ≥0.20 mV over 40 years, while in women the value used was ≥0.15 mV.6 Primary PCI was defined as mechanical reperfusion in an acute context as the first therapeutic method within 12 hours of symptom onset (as opposed to rescue PCI). Patients with more than 12 hours of symptom progression, with or without cardiogenic shock, were excluded.
First medical contact was defined as the first contact with a hospital, public or private unit, basic emergency services, permanent care services, primary health care units, or emergency medical system personnel in which health professionals could perform and interpret an electrocardiogram and apply initial interventions such as defibrillation.7
DIDO time was defined as the time between the patient entering and leaving a hospital without PCI capacity and being transferred in a medical emergency vehicle to the reference hospital. The time when patients were admitted to the emergency room of the first hospital could be obtained by consulting medical records through the Health Data Platform. The departure time, however, was poorly recorded in a considerable number of patients and in some cases it was later than the time of arrival at the reference hospital. To counteract this limitation, we chose to calculate the main variable of this study indirectly. To this end, the duration of the trip was subtracted from the time between entry into the first hospital and entry into the PCI center (denoted as HB), i.e. time from door in to entry in HB, and this method was applied in all cases. The travel time was estimated using the fastest route shown in Google Maps.
Knowing the hospital provenance and the distance and travel time calculated by the application, it was possible to calculate the average speed, from which 10 km/h was subtracted to obtain an average speed appropriate for the real-life performance of medical emergency vehicles, and the approximate travel time was recalculated.
The slow-reflow phenomenon was defined as the presence of grade 2 on the Thrombolysis in Myocardial Infarction (TIMI) score after revascularization.16,17
In the follow-up, hospitalization for heart failure (HF) was defined as hospitalization with a diagnosis of decompensated HF within a year of the event. To calculate left ventricular ejection fraction after hospital discharge, only echocardiograms performed between 40 days and 18 months after the event were analyzed.18
Ethical considerationsRules of ethical conduct and good practice were followed in compliance with the Helsinki Declaration,19 the Convention on Human Rights and Biomedicine,20 the guidelines of the Council for International Organizations of Medical Sciences21 and the Guide to Good Clinical Practice guidelines.22
Statistical analysisThe statistical analysis was performed using IBM SPSS Statistics®, version 23, for Windows. A significance level of 5% was established, with p-values ≤0.05 being considered statistically significant.
For the descriptive analysis of categorical variables, the frequency distribution and percentages were calculated. Continuous variables were described as means and standard deviation (SD). Medians and interquartile range (IQR) were used for the variable DIDO time since its distribution was highly asymmetrical.
The two groups, DIDO time ≤60 min and >60 min, were compared using the t test for independent samples, and the assumption of homogeneity of variances was assessed with Levene's test.23 Cohen's D (d) was calculated as an effect size measure, and the values 0.20, 0.50 and 0.80 were considered to represent a small, medium and large difference, respectively.22–27
The chi-square test or Fisher's exact test was used for qualitative variables.28 The effect size was calculated using phi (Φ) when the variables under analysis were dichotomous and Cramer's V (V) when at least one of the variables had more than two categories, and the values 0.10, 0.30 and 0.50 were considered to represent a small, medium and large effect size, respectively.27
A binary logistic regression analysis was performed to analyze predictors of in-hospital mortality. In the first phase, 14 predictors (including the variable under study, DIDO time) were included in the model, which were selected according to a previously validated mortality model that included age, gender, body mass index (BMI), diabetes, hypertension, dyslipidemia, smoking, previous MI, previous PCI, previous coronary artery bypass grafting (CABG), previous HF, previous stroke, dialysis and peripheral arterial disease (PAD).29 Considering the number of in-hospital deaths in the sample, the number of predictors of the model was reduced and the variables BMI, diabetes, hypertension, dyslipidemia, smoking, previous MI, previous PCI, previous CABG, previous HF and previous stroke were excluded, since they did not contribute significantly to the model. Thus, the most parsimonious possible predictive model was obtained.30 The assumptions for performing this analysis were tested, specifically the absence of multilinearity and the existence of outliers that could influence the regression model.
In terms of survival, Kaplan-Meier analysis was performed for mortality according to estimated DIDO time, obtaining two survival curves.
The Z-score of proportions was calculated to compare mortality up to the end of follow-up between the two study groups (Figure 1).
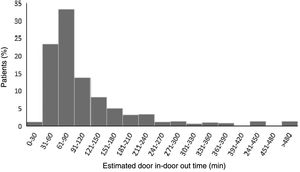
ResultsAmong the 523 patients with STEMI transferred for primary PCI, the median DIDO time was 82 min (IQR 61–132 min). As shown in Figure 2, only seven patients (1.3%) were transferred in 30 min or less, thus achieving the recommended time. By contrast, 75.3% were transferred in more than 60 min and 42.1% in more than 90 min.
The results of the descriptive study of estimated DIDO time for the two groups are summarized in Tables 1–4.
The demographic data and timings (Table 1) showed a predominance of male patients in both groups. In the ≤60 min group age ranged between 34 and 90 years (mean 59.3 years, SD 12) and in the >60 min group between 30 and 92 years (mean 61.5 years, SD 13.5). Statistically significant differences were found between the two groups in total ischemia time, with a mean of 222 min in the ≤60 min group and 344.2 min in the >60 min group [t(310.20)=−8.93, p<0.001, d=2.17], and in system delay, with shorter system delay in the ≤60 min group (mean 118.7 min, SD 31.6) compared to the >60 min group (mean 207.3 min, SD 121) [t (506.42)=−14.12, p<0.001, d=3.95].
Distribution of demographic variables and timings according to door in-door out time.
| Sociodemographic | Estimated DIDO time | p | Effect size | |
|---|---|---|---|---|
| ≤60 min (n=129) | >60 min (n=394) | |||
| BMI | 0.469 | 0.09 | ||
| Low weight, % (n) | 0.8 (1) | 0.5 (2) | ||
| Normal weight % (n) | 32.0 (41) | 30.9 (121) | ||
| Pre-obesity % (n) | 49.2 (63) | 50.5 (198) | ||
| Type I obesity % (n) | 10.2 (13) | 13.0 (51) | ||
| Type II obesity % (n) | 6.3 (8) | 4.8 (19) | ||
| Type III obesity % (n) | 1.6 (2) | 0.3 (1) | ||
| Age, years | 0.075 | 0.23 | ||
| Mean | 59.3 | 61.5 | ||
| SD (minimum–maximum) | 12 (34–90) | 13.5 (30–92) | ||
| Gender | 0.095 | 0.07 | ||
| Female | 17.1% (22) | 24.1% (95) | ||
| Male | 82.9% (107) | 75.9% (299) | ||
| System delay, mina | 0.000 | 3.95 | ||
| Mean | 112.7 | 207.3 | ||
| SD (minimum–maximum) | 31.6 (73–316) | 121 (70–1034) | ||
| Total ischemia time, minb | 0.000 | 2.17 | ||
| Mean | 222 | 344.2 | ||
| SD (minimum–maximum) | 120.5 (83–700) | 171.8 (1270–110) | ||
BMI: body mass index; DIDO: door in-door out; SD: standard deviation.
Regarding medical history (Table 2), patients with estimated DIDO time ≤60 min were more likely to have a history of smoking [chi-square (2)=6.79, p=0.034, Φc=0.12] compared to the >60 min group. In contrast, the >60 min group were more likely to have hypertension [chi-square (1)=5.08, p=0.024, Φ=0.10]. No statistically significant differences were found between the groups regarding chronic kidney disease, chronic HF, transient ischemic attack/stroke, valvular disease, PAD, coronary heart disease, CABG, previous MI, previous PCI, diabetes or hypercholesterolemia.
Medical history according to door in-door out time.
| Estimated DIDO time | p | Effect size | ||
|---|---|---|---|---|
| ≤60 min (n=129) | >60 min (n=394) | |||
| CKD, % (n) | 0.601 | −0.04 | ||
| No | 98.4 (127) | 99.2 (391) | ||
| Yes | 1.6 (2) | 0.8 (3) | ||
| CHF, % (n) | 0.573 | −0.02 | ||
| No | 99.2 (128) | 99.5 (392) | ||
| Yes | 0.8 (1) | 0.5 (2) | ||
| TIA/stroke, % (n) | 0.794 | 0.02 | ||
| No | 96.9 (125) | 95.9 (378) | ||
| Yes | 3.1 (4) | 4.1 (16) | ||
| Valvular disease, % (n) | 0.130 | 0.08 | ||
| No | 100 (129) | 97.5 (384) | ||
| Yes | 0.0 (0) | 2.5 (10) | ||
| PAD, % (n) | 0.871 | −0.01 | ||
| No | 95.3 (123) | 95.7 (377) | ||
| Yes | 4.7 (6) | 4.3 (17) | ||
| CHD, % (n) | 0.823 | 0.01 | ||
| No | 92.2 (119) | 91.6 (361) | ||
| Yes | 7.8 (10) | 8.4 (33) | ||
| CABG, % (n) | 0.573 | −0.02 | ||
| No | 99.2 (128) | 99.5 (392) | ||
| Yes | 0.8 (1) | 0.5 (2) | ||
| Previous MI, % (n) | 0.730 | −0.02 | ||
| No | 92.2 (119) | 93.1 (367) | ||
| Yes | 7.8 (10) | 6.9 (27) | ||
| Previous PCI, % (n) | 0.116 | −0.07 | ||
| No | 91.5 (118) | 95.2 (375) | ||
| Yes | 8.5 (11) | 4.8 (19) | ||
| Diabetes, % (n) | 0.098 | 0.10 | ||
| No | 83.7 (103) | 74.7 (286) | ||
| Just diagnosed | 0.0 (0) | 0.0 (0) | ||
| Yes; under diet | 0.8 (1) | 0.3 (1) | ||
| Yes; under oral treatment | 13.8 (17) | 22.5 (86) | ||
| Yes: under insulin | 1.6 (2) | 2.6 (10) | ||
| Smoking, % (n) | 0.034 | 0.12 | ||
| Never | 37.1 (46) | 48.4 (184) | ||
| Ex-smoker (>30 days) | 11.3 (14) | 13.2 (50) | ||
| Smoker | 51.6 (64) | 38.4 (146) | ||
| Hypercholesterolemia, % (n) | 0.286 | 0.05 | ||
| No | 59.7 (77) | 54.3 (214) | ||
| Yes | 40.3 (52) | 45.7 (180) | ||
| Hypertension, % (n) | 0.024 | 0.10 | ||
| No | 57.4 (74) | 45.9 (181) | ||
| Yes | 42.6 (55) | 54.1 (213) | ||
CABG: coronary artery bypass grafting; CHD: coronary heart disease; CHF: chronic heart failure; CKD: chronic kidney disease; DIDO: door in-door out; MI: myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; TIA: transient ischemic attack.
Regarding complications associated with the event and the intervention, there were no statistically significant differences in any variables (Table 3).
Event- and intervention-related complications according to door in-door out time.
| Estimated DIDO time | p | Effect size | ||
|---|---|---|---|---|
| ≤60 min (n=129) | >60 min (n=394) | |||
| Cardiogenic shock, % (n) | 0.157 | 0.06 | ||
| No | 97.7 (126) | 94.7 (373) | ||
| Yes | 2.3 (3) | 5.3 (21) | ||
| Need for hemodynamic support, % (n) | 0.430 | 0.05 | ||
| No | 97.7 (126) | 95.7 (377) | ||
| Yes | 2.3 (3) | 4.3 (17) | ||
| Tamponade, % (n) | 0.254 | −0.05 | ||
| No | 98.4 (126) | 99.5 (391) | ||
| Yes | 1.6 (2) | 0.5 (2) | ||
| Cardiac arrest, % (n) | 10.00 | 0.04 | ||
| No | 100 (128) | 99.5 (391) | ||
| Yes | 0.0 (0) | 0.5 (2) | ||
| Stroke, % (n) | – | – | ||
| No | 100 (0) | 100 (0) | ||
| Yes | 0.0 (0) | 0.0 (0) | ||
| Cardioversion, % (n) | 0.572 | −0.02 | ||
| No | 99.2 (127) | 99.5 (391) | ||
| Yes | 0.8 (1) | 0.5 (2) | ||
| Coronary perforation, % (n) | – | – | ||
| No | 100 (128) | 100 (393) | ||
| Yes | 0.0 (0) | 0.0 (0) | ||
| Slow flow, % (n) | 0.371 | −0.05 | ||
| No | 97.7 (125) | 99.0 (389) | ||
| Yes | 2.3 (3) | 1.0 (4) | ||
| MI location, % (n) | 0.706 | −0.02 | ||
| Other than anterior | 49.6 (64) | 51.5 (203) | ||
| Anterior | 50.4 (65) | 48.5 (191) | ||
DIDO: door in-door out; MI: myocardial infarction.
In-hospital mortality was 0% in the ≤60 min group and 5.1% in the >60 min group, a statistically significant difference [chi-square (1)=6.81, p=0.006, Φ=0.11]. The groups did not differ significantly in relation to the other follow-up variables (Table 4).
Information on patient follow-up according to door in-door out time.
| Estimated DIDO time | p | Effect size | ||
|---|---|---|---|---|
| ≤60 min (n=129) | >60 min (n=394) | |||
| In-hospital mortality, % (n) | 0.006 | 0.11 | ||
| No | 100 (129) | 94.9 (374) | ||
| Yes | 0.0 (0) | 5.1 (20) | ||
| LVEF at admission, % (n) | 0.971 | 0.02 | ||
| Normal | 38.8 (50) | 38.7 (149) | ||
| Mild depression | 32.6 (42) | 30.9 (119) | ||
| Moderate depression | 23.3 (30) | 25.2 (97) | ||
| Severe depression | 5.4 (7) | 5.2 (20) | ||
| LVEF at follow-upa, % (n) | 0.529 | 0.08 | ||
| Normal | 58.0 (51) | 61.9 (164) | ||
| Mild depression | 28.4 (25) | 21.1 (56) | ||
| Moderate depression | 10.2 (9) | 12.1 (32) | ||
| Severe depression | 3.4 (3) | 4.9 (13) | ||
| Readmission for HFb, % (n) | 0.530 | 0.04 | ||
| No | 98.4 (123) | 96.9 (348) | ||
| Yes | 1.6 (2) | 3.1 (11) | ||
| Time until readmission, months | 0.635 | 0.06 | ||
| Mean | 11.8 | 11.7 | ||
| SD (minimum–maximum) | 1.5 (0–12) | 1.6 (0–12) | ||
| Maximum Killip class, % (n) | 0.179 | 0.08 | ||
| 1 | 82.8 (106) | 76.0 (297) | ||
| 2 or 3 | 14.8 (19) | 18.4 (72) | ||
| 4 | 2.3 (3) | 5.6 (22) | ||
DIDO: door in-door out; LVEF: left ventricular ejection fraction.
The results of the regression model were statistically significant: chi-square (4)=19.14, p=0.001 (R2 Nagelkerke=0.13; percentage of correctly predicted cases=96.4%). Age proved to be a statistically significant predictor of in-hospital mortality (B=0.05, Wald=5.15, p=0.023), as did PAD (B=1.49, Wald=4.62, p=0.032). Older age and the presence of PAD were associated with a higher probability of in-hospital death.
Finally, DIDO time proved to be a statistically significant predictor of in-hospital mortality (B=0.00, Wald=7.96, p=0.005), and longer times were associated with a greater probability of in-hospital death (Table 5).
Logistic regression model for in-hospital mortality.
| OR | Sig | 95% CI for OR | ||
|---|---|---|---|---|
| Lower | Higher | |||
| Age | 1.05 | 0.023 | 1.01 | 1.09 |
| Gender | 1.40 | 0.526 | 0.49 | 3.99 |
| PAD | 4.44 | 0.032 | 1.14 | 17.29 |
| Estimated DIDO time, min (hour) | 1.004 (1.27) | 0.005 | 1.00 | 1.01 |
CI: confidence interval; DIDO: door in-door out; OR: odds ratio; PAD: peripheral arterial disease.
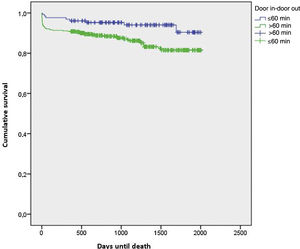
Kaplan-Meier analysis (Figure 3) produced a log-rank p-value of 0.011, so the differences in survival time between the two groups were statistically significant.
In addition to the differences in survival time, it was also observed that by the end of follow-up, the >60 min group presented higher mortality than the ≤60 min group, and these results were also statistically significant (Z=−2.41, p=0.016).
DiscussionPatients with STEMI transferred for PCI often have delayed reperfusion and only a minority meet the target of 90 min or less for the time between first medical contact and PCI.31,32 Included in this timeline is DIDO time, an important parameter that if targets are achieved can reduce delays in reperfusion, besides being an indicator of the quality of referral in the first hospital. In this study, it was found that only 1.3% of the 523 patients included met the recommended time of 30 min or less.7 Additionally, 42.1% took more than 90 min to transfer, thus making the 90 min target for first medical contact to PCI impossible to reach. It was also found that patients with DIDO times >60 min had significantly longer system delays (207.3 min vs. 112.7 min) than patients with DIDO times ≤60 min. The 2011 retrospective study by Wang et al., which included 14821 STEMI patients from several hospitals in the USA transferred for primary PCI, revealed that 11% of patients met the predicted mark of 30 min or less in relation to DIDO time, and, similarly to this study, came to the conclusion that patients with DIDO times longer than 30 min had significantly longer system delays than those with times ≤30 min (median 127 min vs. 85 min; p<0.001).15 It is important to note that the authors of the above-mentioned article used a DIDO time of 30 min to separate patients into two groups, in contrast to the 60-minute cutoff used in our study.
The main objective of these targets is to obtain the shortest possible total ischemia time. Several studies have established a direct relationship between total ischemia time and the severity of myocardial injury and associated mortality, on the principle that “time is muscle”.33–35 In our study, patients in the >60 min group had significantly longer total ischemia times (344.2 min vs. 222 min) than patients who had DIDO times ≤60 min. The study also demonstrated a significantly increased risk of in-hospital mortality in patients in the >60 min group. This risk was maintained after variables that influence mortality in MI were included in the regression model. Similarly, Wang et al. found that in-hospital mortality was significantly higher in patients with DIDO times >30 min (5.9%) than in those with times ≤30 min (2.7%; p<0.001). After construction of a predictive model of in-hospital mortality, the influence of the variable under study remained (adjusted odds ratio 1.56, 95% CI 1.15–2.12).15 In addition to DIDO time, the regression model in our study demonstrated that age and PAD also increased the probability of in-hospital death, and were thus factors constituting to a poor prognosis, results corroborated by the literature.36,37
Regarding the survival analysis, Kaplan-Meier curves showed statistically significant differences, demonstrating that patients who were transferred in >60 min had shorter survival than those who were transferred in ≤60 min. In addition to the differences in survival time, it was also observed that by the end of follow-up, the >60 min group presented higher mortality than the ≤60 min group. To the best of our knowledge, this is the first study to explore the influence of DIDO time on the survival of patients with MI.
The recommended strategy for ensuring timely reperfusion is to promote people's awareness of the common symptoms of MI and to encourage prompt use of the emergency medical system. Emergency medical vehicles play a critical role in the initial management of patients with STEMI.38 If patients go to hospitals without PCI facilities, there should be mechanisms for rapidly addressing and screening patients with chest pain and for performing an immediate evacuation protocol when a diagnosis of STEMI is confirmed. During the lifetime of the Stent for Life initiative in Portugal, there were positive changes in patient delay indicators, especially a lower proportion of patients who attended non-PCI centers, but system delay did not change significantly over this period.39 It therefore remains essential for decision-makers and public health authorities to implement public health policies to monitor and alert health professionals to the need for early identification and referral of STEMI patients, similarly to the smoking ban in 2008, the salt reduction regulation in 2010 and the coronary fast track for acute coronary syndromes in 2007.40
The current ESC guidelines for the management of STEMI recommend that if the time to PCI is greater than 120 min, fibrinolysis should be used.7
When analyzing this study, it is important to consider its limitations. Because of its retrospective nature, it was not possible to ensure that the number of omitted cases in the statistical analysis of some variables was low. Second, due to the inaccurate recording of the time patients left the first hospital, the DIDO time had to be estimated, and thus does not necessarily correspond to the actual value. It is essential that in the future, hospitals record the entry and exit of patients with STEMI methodically and accurately, and that they use these records for periodic reviews targeting DIDO time.
ConclusionDIDO time is considered an important parameter in the reperfusion of patients with STEMI who require inter-hospital transfer to undergo PCI.
This study demonstrated that DIDO times ≤60 min were associated with shorter reperfusion delays, lower adjusted in-hospital mortality, and longer survival times compared to patients who were transferred in >60 min. Additionally, it was found that most patients transferred did not meet the recommended 30-min target.
In view of these results, it is important to carry out future studies on hospitals without PCI facilities aiming to identify factors that contribute to non-compliance with targets established for DIDO time and, according to the findings of such studies, to implement appropriate interventions.
Conflicts of interestThe authors have no conflicts of interest to declare.