Renin-angiotensin-aldosterone system inhibitors (RAASi) are the cornerstone of treatment of heart failure with reduced ejection fraction (HFrEF). RAASi optimization in real-life care is challenged by hyperkalemia, a potentially fatal adverse event, which can necessitate downtitration or discontinuation of RAASi and negatively impact survival in HFrEF. The literature on this problem is sparse. We performed a systematic review of studies on HFrEF to investigate the prevalence, incidence, and risk factors of hyperkalemia, RAASi prescription rates, frequency of RAASi downtitration or discontinuation due to hyperkalemia, and the potential negative effect of the latter on prognosis.
MethodsWe conducted a MEDLINE (PubMed) search including observational and interventional studies published between January 1987 and May 2018.
ResultsA total of 30 observational and 18 interventional studies were included in the review. The incidence of hyperkalemia reported was between 0% and 63% in observational studies and was between 0% and 30% in clinical trials. Risk factors for hyperkalemia included RAASi prescription, older age, diabetes, and chronic kidney disease. In real-life studies, RAASi were downtitrated or discontinued in 3-22% of HFrEF patients; hyperkalemia was the reported cause in 5% of cases. No reports were found on the impact on prognosis of RAASi downtitration or discontinuation due to hyperkalemia.
ConclusionsHyperkalemia and RAASi downtitration or discontinuation are frequent, particularly in real-life HFrEF studies. Further research is needed to clarify the role of RAASi downtitration or discontinuation due to hyperkalemia and to assess its long-term prognostic impact in HFrEF patients.
Os inibidores do sistema renina-angiotensina-aldosterona (iSRAA) constituem pedras basilares para o tratamento da insuficiência cardíaca com fração de ejeção reduzida (IC-FER). A otimização dos iSRAA é dificultada pela ocorrência de hipercalemia, que obriga à redução/interrupção do iSRAA, podendo impactar negativamente na sobrevivência dos doentes com IC-FER. Esta questão é raramente abordada na literatura.
MétodosRealizámos uma revisão sistemática dos estudos sobre doentes com IC-FER para investigar a prevalência, incidência e fatores de risco para hipercaliemia, frequência de utilização de iSRAA, frequência de redução/interrupção dos iSRAA por hipercaliemia e o possível impacto negativo da redução/interrupção dos iSRAA no prognóstico. A pesquisa foi feita na Medline (PubMed) incluindo estudos observacionais e interventivos publicados entre janeiro de 1987 e maio de 2018.
ResultadosForam incluídos 30 estudos observacionais e 18 interventivos. A frequência relatada de hipercalemia variou entre 0% e 63% em estudos observacionais e entre 0% e 30% em ensaios clínicos. Fatores de risco para hipercalemia incluíram uso de iSRAA, idade avançada, diabetes e doença renal crónica. Em estudos de vida real, os iSRAA foram interrompidos em 3% a 22% dos doentes com IC-FER, sendo a hipercalemia a causa em 5% destes casos. Não foram encontrados relatos do impacto prognóstico da redução/interrupção dos iSRAA devida à hipercalemia.
ConclusõesA hipercaliemia e redução/interrupção dos iSRAA são frequentes, principalmente em estudos de vida real de IC-FER. Novos estudos serão necessários para esclarecer o papel da redução/interrupção dos iSRAA devida a hipercalemia e para avaliar o seu impacto prognóstico a longo prazo em doentes com IC-FER.
Heart failure (HF) is a prevalent, fatal and costly syndrome.1–4 HF phenotypes are categorized by clinical practice guidelines according to left ventricular ejection fraction (LVEF): HF with reduced ejection fraction (HFrEF) is defined as LVEF <40%, and HF with preserved ejection fraction (HFpEF) as LVEF ≥50%.1 Depending on the definition and cohorts considered, the prevalence of HFpEF can vary from 22% to 73%.1 To date, however, there is a paucity of evidence on treatments that effectively reduce morbidity or mortality in patients with HFpEF.1,5 In contrast, the treatment of HFrEF has evolved rapidly over the past thirty years, with major clinical benefits.1,5
Renin-angiotensin-aldosterone system inhibitors (RAASi) – angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), and mineralocorticoid receptor antagonists (MRA) – are recommended for the treatment of HFrEF by all clinical practice guidelines with a class I recommendation, level of evidence A.1,5,6 Recently, the use of angiotensin receptor-neprilysin inhibitors (ARNi) has been recommended (class IB).1,5,6 In addition, the guidelines recommend that most patients with HFrEF receive triple therapy, including an ACEi or ARB or ARNi, a beta-blocker, and an MRA.1,5,6 All of these drugs will be most effective if titrated to the recommended high target doses tested in clinical trials or tolerated by the patient.1,5,6 However, in spite of the consensual benefits of high dosages of RAASi, recent clinical practice-based studies report that up to two-thirds of HFrEF patients do not receive RAASi at the guideline-recommended target doses.7–12 Moreover, clear reasons for not achieving the target doses or for discontinuing RAASi are not consistently reported.7–9,11,12 Nevertheless, when reported, one of the main reasons for RAASi downtitration or discontinuation is hyperkalemia.8,9,11
Patients with HFrEF treated with RAASi are reported to be at higher risk of developing hyperkalemia, as are patients with advanced stages of chronic kidney disease (CKD), diabetes, and resistant hypertension.5,11,13,14 Notably, these are prevalent comorbidities in patients with HF.15 It is estimated that up to 40% of patients with chronic HF develop hyperkalemia in clinical practice.11 Hyperkalemia is commonly associated with excessive intake of potassium salt substitutes and with various medications which are used to treat HFrEF, including RAASi, beta-blockers, and potassium-sparing diuretics.1,9,11,14,16 As hyperkalemia can be a serious and life-threatening condition in patients with HF and/or CKD,11,17–21 and its long-term treatment is currently limited by issues of efficacy, patient adherence, tolerability, and safety,22 clinical practice guidelines and expert consensus documents recommend close monitoring of hyperkalemia and RAASi downtitration or discontinuation according to different cut-offs of potassium levels.1,5,6,9,11,23 However, hyperkalemia management and RAASi optimization in the daily care of HFrEF patients are challenging. Several studies in real-life settings show that serum potassium levels are not properly monitored in patients receiving RAASi.11,24–27 Moreover, RAASi downtitration or discontinuation due to hyperkalemia may worsen patients’ health outcomes, especially in the HFrEF patient subgroup who would benefit most from this therapy.1,5,6,11,14,21,28–32 It has been reported that RAASi downtitration or discontinuation is associated with increased mortality in patients with HF, CKD, or diabetes.9–11
Concerns about hyperkalemia appear to be another significant factor in the clinical decision-making process in the care of HFrEF and RAASi optimization. Nevertheless, few published studies have focused on the frequency, cut-offs or risk factors of hyperkalemia and its association with adverse outcomes among patients with HFrEF in current clinical practice. Hence, a scoping systematic review of the literature is needed to examine the extent and nature of reporting of hyperkalemia in the context of RAASi use in HFrEF, to identify gaps in the most recent literature, and to make recommendations for future research. The authors accordingly performed a systematic review of observational and interventional studies on adult patients with HFrEF receiving RAASi. Specifically, reporting of the following issues was addressed: (i) prevalence or incidence of hyperkalemia, (ii) estimates of risk factors for hyperkalemia, (iii) RAASi prescription rates and hyperkalemia-related reasons for failure to achieve target RAASi doses or for discontinuation, and (iv) the effect of hyperkalemia-attributed RAASi downtitration or discontinuation on mortality or hospitalization.
MethodsCriteria for identification of studiesParticipantsStudies were identified with the following inclusion criteria for participants: (i) ≥18 years old; (ii) prescribed or receiving RAASi therapy within any period before or during the study, regardless of treatment status (ongoing, discontinued, or not treated); and (iii) diagnosis of chronic HF, stable or clinically and hemodynamically stabilized after decompensation, with confirmed or probable reduced ejection fraction, i.e. HFrEF (also known as left ventricular dysfunction, systolic dysfunction, or systolic HF). Confirmed HFrEF was defined as LVEF <50%, description of moderate to severe systolic dysfunction, or as otherwise defined by the study investigators. Probable HFrEF was defined as a report of an HF diagnosis with prescription or use of RAASi within any period before or during the study, and any of the following conditions: (i) prescription or use of beta-blocker therapy; or (ii) prescription or use of other pharmacological therapies commonly used for the treatment of HFrEF, as recommended by current clinical practice guidelines for HF.1,5,6 Data were collected only when available for patients with HFrEF as a total sample or as a subgroup of the total sample.
Types of studiesInterventional studies, observational studies and systematic reviews with or without meta-analysis in any clinical setting (e.g., inpatients at discharge, outpatients, primary care, hospital) were included.
Intervention and comparisonReports were included which had data on the prescription or use of all drug classes collectively known as RAASi, i.e. ACEi, ARB, ARNi, or MRA, describing any dosage or regimens versus any comparator or control (such as other RAASi, standard or optimal pharmacological therapy for HF, placebo, or no treatment).
Outcome measuresReports on HFrEF were included that provided data on at least one of the following outcomes, regardless of follow-up duration: (i) prevalence or incidence of hyperkalemia (number or percentage of patients); or (ii) significant risk estimates of hyperkalemia (p<0.05) by univariate or multivariate analysis (odds ratio [OR], hazard ratio [HR], or risk ratio [RR] with 95% confidence interval [CI]); or (iii) use or prescription of RAASi at baseline/randomization/study start and/or at study end/hospital discharge (number or percentage of patients), or patients at RAASi target dose (number or percentage of patients), hyperkalemia-related reasons for failure to achieve RAASi target dose or RAASi downtitration or discontinuation (reason, number or percentage of patients); or (iv) outcome estimates due to hyperkalemia or RAASi downtitration or discontinuation (percentage of patients, p-value, OR, HR or RR with 95% CI) for all-cause mortality, HF- and/or cardiovascular (CV)-related mortality, all-cause hospitalization, HF- and/or CV-related hospitalization, or related composite endpoints. Data on hyperkalemia were collected as defined by the authors of the studies, comprising abnormally high serum or plasma potassium concentrations (5.0 mmol/l or above), diagnosis code of hyperkalemia, clinically important hyperkalemia, or hyperkalemia as an investigator-reported adverse event.
Exclusion criteriaReports were excluded according to any of the following criteria: (i) patients on acute, emergency and intravenous therapy for acute HF; (ii) patients receiving dialysis (hemodialysis or peritoneal dialysis) or renal transplantation patients; (iii) publication types such as unpublished or unavailable as full-text original article, abstract, case report, comment, clinical conference, or review; (iv) not written in English; (v) hyperkalemia not a component of the reasons for RAASi downtitration or discontinuation; and (vi) no data on any of the above-mentioned outcomes of interest.
Search methodsThis systematic review followed the PRISMA guidelines for conduct and reporting.33 Studies were identified by searching the MEDLINE (PubMed) database for original full-text journal articles published between January 1, 1987 and the present. The last search was run on May 14, 2018 for ‘hyperkalemia’ or ‘potassium’ or ‘guideline adherence’ or ‘practice guidelines as topic’, crossed with ‘heart failure’ and ‘raasi’ or’mineralocorticoid receptor antagonists’ or ‘angiotensin-converting enzyme inhibitors’ or ‘angiotensin receptor antagonists’, for full-text articles published in English. The full search strategy is shown in Supplementary Material S1.
Selection of studiesOne reviewer screened titles and abstracts of all records identified in the electronic search and classified them as eligible or not eligible. The full text of the reports identified as eligible was then retrieved, screened, and identified as eligible, potentially eligible or unclear, or not eligible, and the reasons for exclusion of reports were recorded. A second reviewer screened the potentially eligible or unclear full-text reports and identified them as eligible or not eligible and recorded the reasons for exclusion. Any disagreement was solved by consensus or by consulting a third review author. Two reviewers identified and excluded duplicated and collated reports of the same study.
Data collection and managementAn electronic spreadsheet was used to record study characteristics and outcome data from the included studies. One reviewer extracted data from the included studies. Disagreements were resolved by consensus. One reviewer double-checked that data were entered correctly by comparing the data presented in the systematic review with the electronic spreadsheet. Data that were not published in a numerical format (e.g., presented graphically in the published paper) were not collected.
Data itemsData were extracted from each included study on (i) study characteristics, including study type, inclusion criteria or study population, duration of follow-up and treatment, number of participants randomized/included/analyzed, and description of analyzed subgroups; (ii) description of the intervention/exposure(s) and of comparator(s), including agent and dosage; and (iii) participant characteristics at baseline, including age and/or age subgroups, gender, serum creatinine levels, potassium levels, and HF disease characteristics, including HF diagnosis, New York Heart Association (NYHA) class, LVEF, main etiology of HF, brain natriuretic peptide (BNP) level, HF-related concomitant medication (including beta-blockers), and selected comorbidities, including CKD, diabetes, hypertension, and myocardial ischemia.
Data analysisThe main aim was to review the quantity and nature of the evidence for the management of RAASi and hyperkalemia, and its consequences and outcomes. Because of the expected heterogeneity between study populations, interventions, and outcome measures, a meta-analysis was not performed. As this was intended as a scoping review,34 no synthesis of the evidence or evaluation of the quality of the studies was carried out.
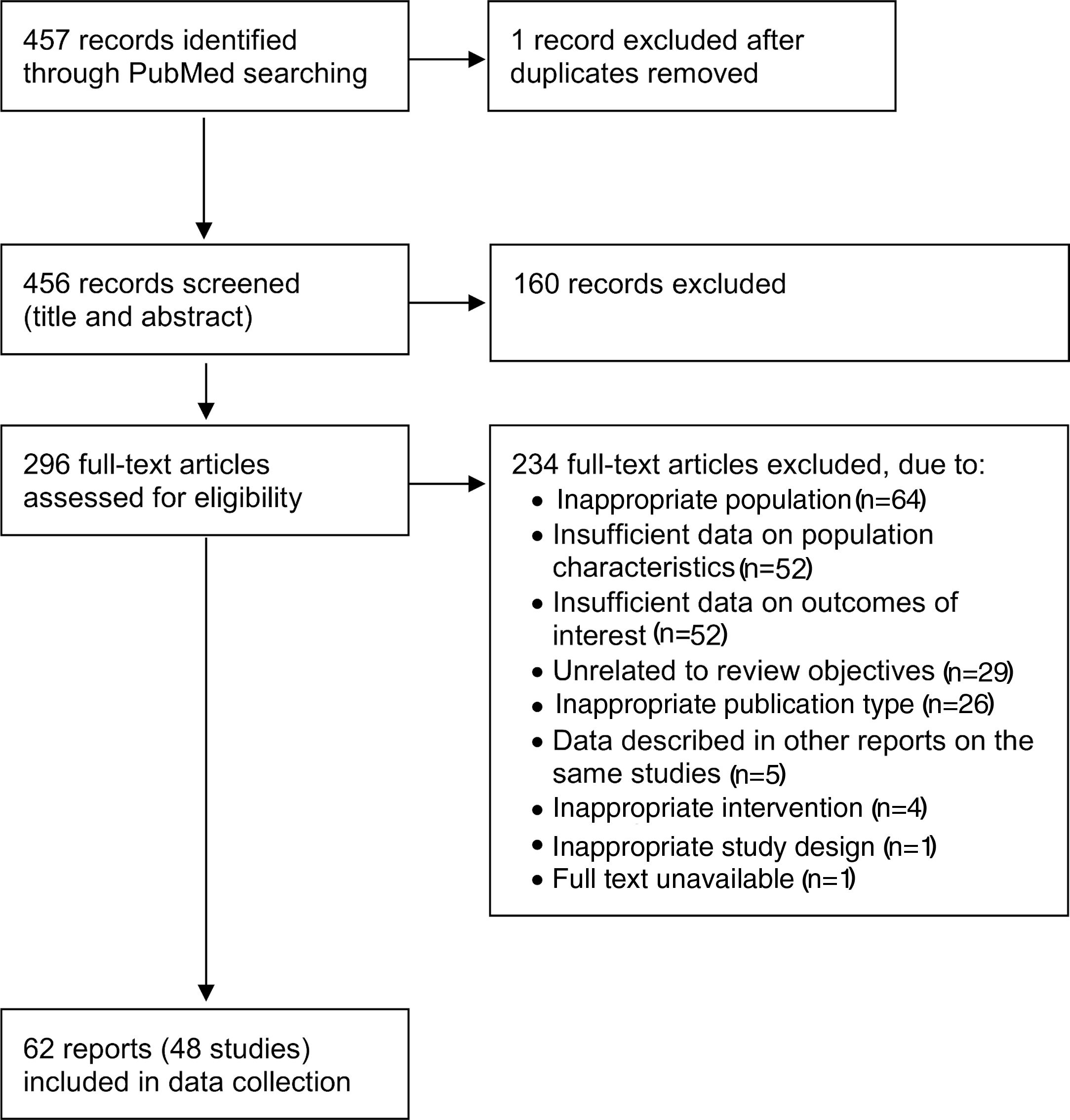
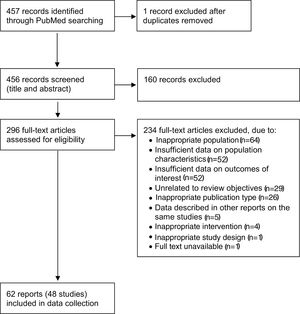
ResultsA flow diagram of the literature search and the selection process of the reports is shown in Figure 1. The MEDLINE (PubMed) search resulted in 457 reports. In total, this systematic review included 62 full-text reports7,8,12–14,16,19–21,25,27–31,35–81 from 48 studies on HFrEF populations.
Flow diagram of the selection of reports (based on Moher et al.33).
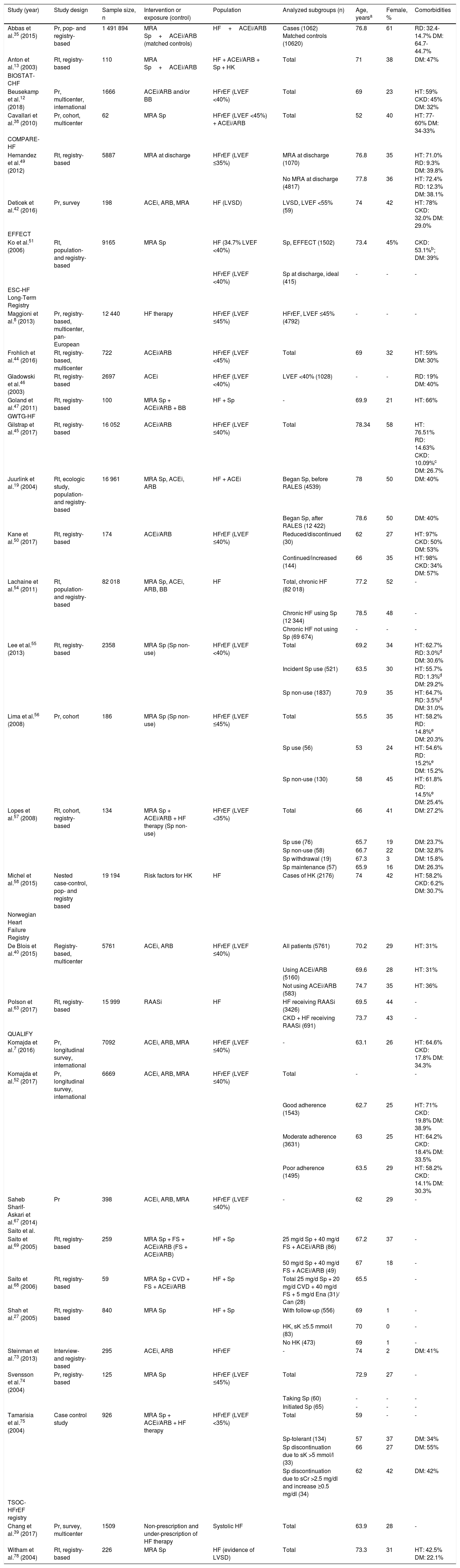
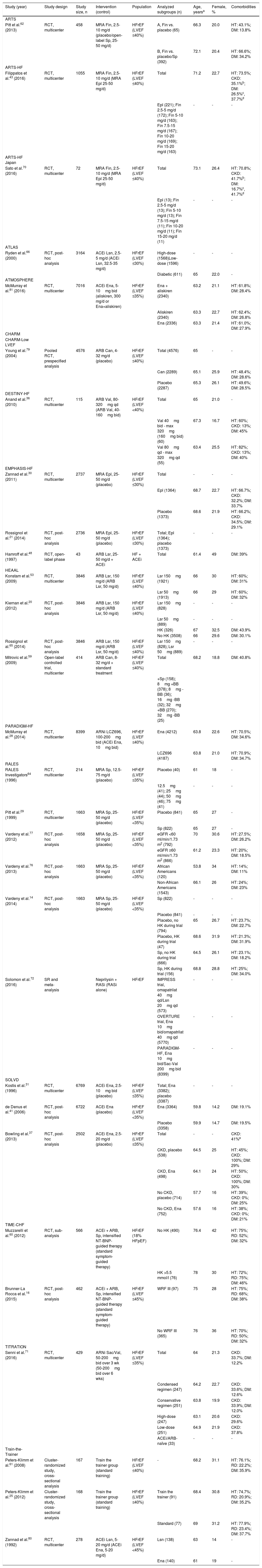
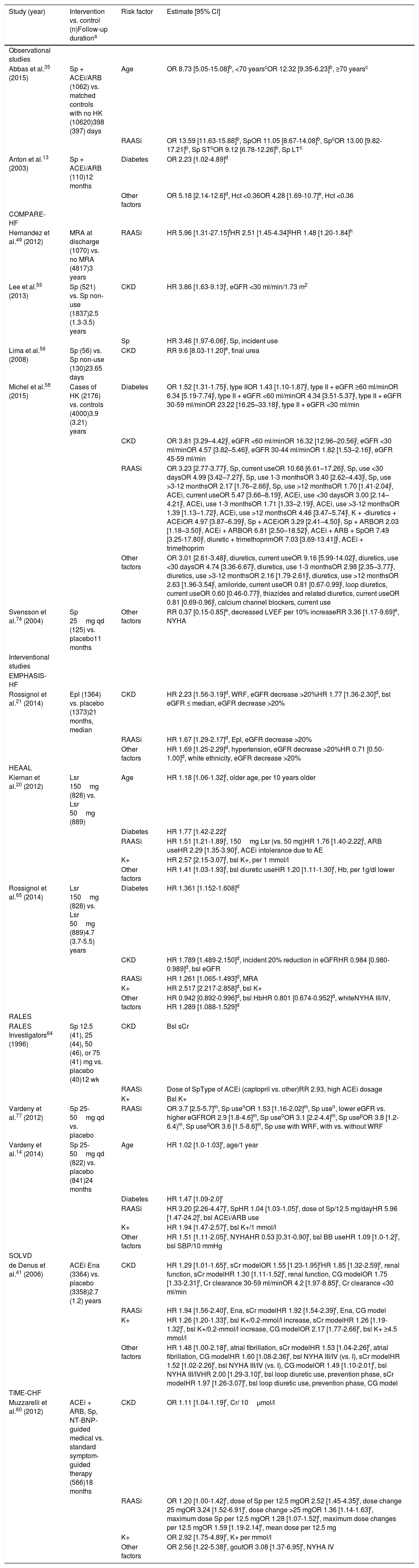
The characteristics of the studies and study populations are shown in Tables 1 and 2 and Supplementary Table ST1. A total of 32 reports7,8,12,13,19,27,35,38–40,42,44–47,49–52,54–58,63,67–69,73–75,78 of 30 observational studies involving samples ranging from 5968 to 1 491 89435 participants were included in this systematic review (Table 1). Most of these studies were registry-based or surveys.7,8,12,13,19,27,35,39,40,42,44–47,49–51,54,55,57,58,63,67,69,73,74,78 In addition, this review included 29 reports (18 studies) of randomized clinical trials (RCTs),28–31,36,43,48,53,59,62,64,70,71,80,81 sub-analyses of RCTs,14,16,20,21,37,41,60,65,66,76,77,79 or cluster-randomized studies25,61 with samples ranging from 4348 to 839928 participants, and one systematic review and meta-analysis of RCTs72 (Table 2).
Summary of main characteristics of the included observational studies.
| Study (year) | Study design | Sample size, n | Intervention or exposure (control) | Population | Analyzed subgroups (n) | Age, yearsa | Female, % | Comorbidities |
|---|---|---|---|---|---|---|---|---|
| Abbas et al.35 (2015) | Pr, pop- and registry-based | 1 491 894 | MRA Sp+ACEi/ARB (matched controls) | HF+ACEi/ARB | Cases (1062) Matched controls (10620) | 76.8 | 61 | RD: 32.4-14.7% DM: 64.7-44.7% |
| Anton et al.13 (2003) | Rt, registry-based | 110 | MRA Sp+ACEi/ARB | HF + ACEi/ARB + Sp + HK | Total | 71 | 38 | DM: 47% |
| BIOSTAT-CHF | ||||||||
| Beusekamp et al.12 (2018) | Pr, multicenter, international | 1666 | ACEi/ARB and/or BB | HFrEF (LVEF <40%) | Total | 69 | 23 | HT: 59% CKD: 45% DM: 32% |
| Cavallari et al.38 (2010) | Pr, cohort, multicenter | 62 | MRA Sp | HFrEF (LVEF <45%) + ACEi/ARB | Total | 52 | 40 | HT: 77-60% DM: 34-33% |
| COMPARE-HF | ||||||||
| Hernandez et al.49 (2012) | Rt, registry-based | 5887 | MRA at discharge | HFrEF (LVEF ≤35%) | MRA at discharge (1070) | 76.8 | 35 | HT: 71.0% RD: 9.3% DM: 39.8% |
| No MRA at discharge (4817) | 77.8 | 36 | HT: 72.4% RD: 12.3% DM: 38.1% | |||||
| Deticek et al.42 (2016) | Pr, survey | 198 | ACEi, ARB, MRA | HF (LVSD) | LVSD, LVEF <55% (59) | 74 | 42 | HT: 78% CKD: 32.0% DM: 29.0% |
| EFFECT | ||||||||
| Ko et al.51 (2006) | Rt, population- and registry-based | 9165 | MRA Sp | HF (34.7% LVEF <40%) | Sp, EFFECT (1502) | 73.4 | 45% | CKD: 53.1%b; DM: 39% |
| HFrEF (LVEF <40%) | Sp at discharge, ideal (415) | - | - | - | ||||
| ESC-HF Long-Term Registry | ||||||||
| Maggioni et al.8 (2013) | Pr, registry-based, multicenter, pan-European | 12 440 | HF therapy | HFrEF (LVEF ≤45%) | HFrEF, LVEF ≤45% (4792) | - | - | - |
| Frohlich et al.44 (2016) | Rt, registry-based, multicenter | 722 | ACEi/ARB | HFrEF (LVEF <45%) | Total | 69 | 32 | HT: 59% DM: 30% |
| Gladowski et al.46 (2003) | Rt, registry-based | 2697 | ACEi | HFrEF (LVEF <40%) | LVEF <40% (1028) | - | - | RD: 19% DM: 40% |
| Goland et al.47 (2011) | Rt, registry-based | 100 | MRA Sp + ACEi/ARB + BB | HF + Sp | - | 69.9 | 21 | HT: 66% |
| GWTG-HF | ||||||||
| Gilstrap et al.45 (2017) | Rt, registry-based | 16 052 | ACEi/ARB | HFrEF (LVEF ≤40%) | Total | 78.34 | 58 | HT: 76.51% RD: 14.63% CKD: 10.09%c DM: 26.7% |
| Juurlink et al.19 (2004) | Rt, ecologic study, population- and registry-based | 16 961 | MRA Sp, ACEi, ARB | HF + ACEi | Began Sp, before RALES (4539) | 78 | 50 | DM: 40% |
| Began Sp, after RALES (12 422) | 78.6 | 50 | DM: 40% | |||||
| Kane et al.50 (2017) | Rt, registry-based | 174 | ACEi/ARB | HFrEF (LVEF ≤40%) | Reduced/discontinued (30) | 62 | 27 | HT: 97% CKD: 50% DM: 53% |
| Continued/increased (144) | 66 | 35 | HT: 98% CKD: 34% DM: 57% | |||||
| Lachaine et al.54 (2011) | Rt, population- and registry-based | 82 018 | MRA Sp, ACEi, ARB, BB | HF | Total, chronic HF (82 018) | 77.2 | 52 | - |
| Chronic HF using Sp (12 344) | 78.5 | 48 | - | |||||
| Chronic HF not using Sp (69 674) | - | - | - | |||||
| Lee et al.55 (2013) | Rt, registry-based | 2358 | MRA Sp (Sp non-use) | HFrEF (LVEF <40%) | Total | 69.2 | 34 | HT: 62.7% RD: 3.0%d DM: 30.6% |
| Incident Sp use (521) | 63.5 | 30 | HT: 55.7% RD: 1.3%d DM: 29.2% | |||||
| Sp non-use (1837) | 70.9 | 35 | HT: 64.7% RD: 3.5%d DM: 31.0% | |||||
| Lima et al.56 (2008) | Pr, cohort | 186 | MRA Sp (Sp non-use) | HFrEF (LVEF ≤45%) | Total | 55.5 | 35 | HT: 58.2% RD: 14.8%e DM: 20.3% |
| Sp use (56) | 53 | 24 | HT: 54.6% RD: 15.2%e DM: 15.2% | |||||
| Sp non-use (130) | 58 | 45 | HT: 61.8% RD: 14.5%e DM: 25.4% | |||||
| Lopes et al.57 (2008) | Rt, cohort, registry-based | 134 | MRA Sp + ACEi/ARB + HF therapy (Sp non-use) | HFrEF (LVEF <35%) | Total | 66 | 41 | DM: 27.2% |
| Sp use (76) | 65.7 | 19 | DM: 23.7% | |||||
| Sp non-use (58) | 66.7 | 22 | DM: 32.8% | |||||
| Sp withdrawal (19) | 67.3 | 3 | DM: 15.8% | |||||
| Sp maintenance (57) | 65.9 | 16 | DM: 26.3% | |||||
| Michel et al.58 (2015) | Nested case-control, pop- and registry based | 19 194 | Risk factors for HK | HF | Cases of HK (2176) | 74 | 42 | HT: 58.2% CKD: 6.2% DM: 30.7% |
| Norwegian Heart Failure Registry | ||||||||
| De Blois et al.40 (2015) | Registry-based, multicenter | 5761 | ACEi, ARB | HFrEF (LVEF ≤40%) | All patients (5761) | 70.2 | 29 | HT: 31% |
| Using ACEi/ARB (5160) | 69.6 | 28 | HT: 31% | |||||
| Not using ACEi/ARB (583) | 74.7 | 35 | HT: 36% | |||||
| Polson et al.63 (2017) | Rt, registry-based | 15 999 | RAASi | HF | HF receiving RAASi (3426) | 69.5 | 44 | - |
| CKD + HF receiving RAASi (691) | 73.7 | 43 | - | |||||
| QUALIFY | ||||||||
| Komajda et al.7 (2016) | Pr, longitudinal survey, international | 7092 | ACEi, ARB, MRA | HFrEF (LVEF ≤40%) | - | 63.1 | 26 | HT: 64.6% CKD: 17.8% DM: 34.3% |
| Komajda et al.52 (2017) | Pr, longitudinal survey, international | 6669 | ACEi, ARB, MRA | HFrEF (LVEF ≤40%) | Total | - | - | |
| Good adherence (1543) | 62.7 | 25 | HT: 71% CKD: 19.8% DM: 38.9% | |||||
| Moderate adherence (3631) | 63 | 25 | HT: 64.2% CKD: 18.4% DM: 33.5% | |||||
| Poor adherence (1495) | 63.5 | 29 | HT: 58.2% CKD: 14.1% DM: 30.3% | |||||
| Saheb Sharif-Askari et al.67 (2014) | Pr | 398 | ACEi, ARB, MRA | HFrEF (LVEF ≤40%) | - | 62 | 29 | - |
| Saito et al. | ||||||||
| Saito et al.69 (2005) | Rt, registry-based | 259 | MRA Sp + FS + ACEi/ARB (FS + ACEi/ARB) | HF + Sp | 25 mg/d Sp + 40 mg/d FS + ACEi/ARB (86) | 67.2 | 37 | - |
| 50 mg/d Sp + 40 mg/d FS + ACEi/ARB (49) | 67 | 18 | - | |||||
| Saito et al.68 (2006) | Rt, registry-based | 59 | MRA Sp + CVD + FS + ACEi/ARB | HF + Sp | Total 25 mg/d Sp + 20 mg/d CVD + 40 mg/d FS + 5 mg/d Ena (31)/ Can (28) | 65.5 | - | |
| Shah et al.27 (2005) | Rt, registry-based | 840 | MRA Sp | HF + Sp | With follow-up (556) | 69 | 1 | - |
| HK, sK ≥5.5 mmol/l (83) | 70 | 0 | - | |||||
| No HK (473) | 69 | 1 | - | |||||
| Steinman et al.73 (2013) | Interview- and registry-based | 295 | ACEi, ARB | HFrEF | - | 74 | 2 | DM: 41% |
| Svensson et al.74 (2004) | Pr, registry-based | 125 | MRA Sp | HFrEF (LVEF ≤45%) | Total | 72.9 | 27 | - |
| Taking Sp (60) | - | - | - | |||||
| Initiated Sp (65) | - | - | - | |||||
| Tamarisia et al.75 (2004) | Case control study | 926 | MRA Sp + ACEi/ARB + HF therapy | HFrEF (LVEF <35%) | Total | 59 | - | - |
| Sp-tolerant (134) | 57 | 37 | DM: 34% | |||||
| Sp discontinuation due to sK >5 mmol/l (33) | 66 | 27 | DM: 55% | |||||
| Sp discontinuation due to sCr >2.5 mg/dl and increase ≥0.5 mg/dl (34) | 62 | 42 | DM: 42% | |||||
| TSOC-HFrEF registry | ||||||||
| Chang et al.39 (2017) | Pr, survey, multicenter | 1509 | Non-prescription and under-prescription of HF therapy | Systolic HF | Total | 63.9 | 28 | - |
| Witham et al.78 (2004) | Rt, registry-based | 226 | MRA Sp | HF (evidence of LVSD) | Total | 73.3 | 31 | HT: 42.5% DM: 22.1% |
eGFR <50 ml/min.
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BB: beta-blocker; BIOSTAT-CHF: BIOlogy Study to TAilored Treatment in Chronic Heart Failure; Can: candesartan; CKD: chronic kidney disease; COMPARE: Comparative Effectiveness of Therapies for Heart Failure; CVD: carvedilol; d: day; DM: diabetes mellitus; EFFECT: Enhanced Feedback for Effective Cardiac Treatment; eGFR: estimated glomerular filtration rate; Ena: enalapril; ESC: European Society of Cardiology; FS: furosemide; GWTG: Get With The Guidelines-Heart Failure Registry; HF: heart failure; HFrEF: heart failure with reduced ejection fraction; HK: hyperkalemia; HT: hypertension; LVEF: left ventricular ejection fraction; LVSD: left ventricular systolic dysfunction; MRA: mineralocorticoid receptor antagonist; Pop: population; Pr: prospective; QUALIFY: QUAlity of adherence to guideline recommendations for LIfe-saving treatment in heart failure: an international surveY: an observational study; RAASi: renin-angiotensin-aldosterone system inhibitors; RD: renal dysfunction; Rt: retrospective; sCr: serum creatinine; sK: serum potassium; Sp: spironolactone; TSOC: Taiwan Society of Cardiology.
Summary of main characteristics of the included interventional studies.
| Study (year) | Study design | Study size, n | Intervention (control) | Population | Analyzed subgroups (n) | Age, yearsa | Female, % | Comorbidities |
|---|---|---|---|---|---|---|---|---|
| ARTS | ||||||||
| Pitt et al.62 (2013) | RCT, multicenter | 458 | MRA Fin, 2.5-10 mg/d (placebo/open-label Sp, 25-50 mg/d) | HFrEF (LVEF ≤40%) | A, Fin vs. placebo (65) | 66.3 | 20.0 | HT: 43.1%; DM: 13.8% |
| B, Fin vs. placebo/Sp (392) | 72.1 | 20.4 | HT: 66.6%; DM: 34.2% | |||||
| ARTS-HF | ||||||||
| Filippatos et al.43 (2016) | RCT, multicenter | 1055 | MRA Fin, 2.5-10 mg/d (MRA Epl 25-50 mg/d) | HFrEF (LVEF ≤40%) | Total | 71.2 | 22.7 | HT: 73.5%; CKD: 35.1%b; DM: 26.5%c, 37.7%d |
| Epl (221); Fin 2.5-5 mg/d (172); Fin 5-10 mg/d (163); Fin 7.5-15 mg/d (167); Fin 10-20 mg/d (169); Fin 15-20 mg/d (163) | - | - | - | |||||
| ARTS-HF Japan | ||||||||
| Sato et al.70 (2016) | RCT, multicenter | 72 | MRA Fin, 2.5-10 mg/d (MRA Epl 25-50 mg/d) | HFrEF (LVEF ≤40%) | Total | 73.1 | 26.4 | HT: 70.8%; CKD: 41.7%b; DM: 16.7%c, 41.7%d |
| Epl (13); Fin 2.5-5 mg/d (13); Fin 5-10 mg/d (13); Fin 7.5-15 mg/d (11); Fin 10-20 mg/d (11); Fin 15-20 mg/d (11) | - | - | - | |||||
| ATLAS | ||||||||
| Ryden et al.66 (2000) | RCT, post-hoc analysis | 3164 | ACEi Lsn, 2.5-5 mg/d (ACEi Lsn, 32.5-35 mg/d) | HFrEF (LVEF ≤30%) | High-dose (1568)Low-dose (1596) | - | - | - |
| Diabetic (611) | 65 | 22.0 | - | |||||
| ATMOSPHERE | ||||||||
| McMurray et al.81 (2016) | RCT, multicenter | 7016 | ACEi Ena, 5-10mg bid (aliskiren, 300 mg/d or Ena+aliskiren) | HFrEF (LVEF ≤35%) | Ena + aliskiren (2340) | 63.2 | 21.1 | HT: 61.8%; DM: 28.4% |
| Aliskiren (2340) | 63.3 | 22.7 | HT: 62.4%; DM: 26.8% | |||||
| Ena (2336) | 63.3 | 21.4 | HT: 61.0%; DM: 27.9% | |||||
| CHARM | ||||||||
| CHARM-Low LVEF | ||||||||
| Young et al.79 (2004) | Pooled RCT, prespecified analysis | 4576 | ARB Can, 4-32 mg/d (placebo) | HFrEF (LVEF ≤40%) | Total (4576) | 65 | - | - |
| Can (2289) | 65.1 | 25.9 | HT: 48.4%; DM: 28.6% | |||||
| Placebo (2287) | 65.3 | 26.1 | HT: 49.6%; DM: 28.5% | |||||
| DESTINY-HF | ||||||||
| Anand et al.36 (2010) | RCT, multicenter | 115 | ARB Val, 80-320mg qd (ARB Val, 40-160mg bid) | HFrEF (LVEF <40%) | Total | 65 | 21.0 | - |
| Val 40mg bid - max 320mg (160mg bid) (60) | 67.3 | 16.7 | HT: 60%; CKD: 13%; DM: 45% | |||||
| Val 80mg qd - max 320mg qd (55) | 63.4 | 25.5 | HT: 82%; CKD: 13%; DM: 40% | |||||
| EMPHASIS-HF | ||||||||
| Zannad et al.30 (2011) | RCT, multicenter | 2737 | MRA Epl, 25-50 mg/d (placebo) | HFrEF (LVEF ≤30%) | Total | - | - | - |
| Epl (1364) | 68.7 | 22.7 | HT: 66.7%; CKD: 32.2%; DM: 33.7% | |||||
| Placebo (1373) | 68.6 | 21.9 | HT: 66.2%; CKD: 34.5%; DM: 29.1% | |||||
| Rossignol et al.21 (2014) | RCT, post-hoc analysis | 2736 | MRA Epl, 25-50 mg/d (placebo) | HFrEF (LVEF ≤30%) | Total; Epl (1364); placebo (1373) | - | - | - |
| Hamroff et al.48 (1997) | RCT, open-label phase | 43 | ARB Lsr, 25-50 mg/d + ACEi | HF + ACEi | Total | 61.4 | 49 | DM: 39% |
| HEAAL | ||||||||
| Konstam et al.53 (2009) | RCT, multicenter | 3846 | ARB Lsr, 150 mg/d (ARB Lsr, 50 mg/d) | HFrEF (LVEF ≤40%) | Lsr 150mg (1921) | 66 | 30 | HT: 60%; DM: 31% |
| Lsr 50mg (1913) | 66 | 29 | HT: 60%; DM: 32% | |||||
| Kiernan et al.20 (2012) | RCT, post-hoc analysis | 3846 | ARB Lsr, 150 mg/d (ARB Lsr, 50 mg/d) | HFrEF (LVEF ≤40%) | Lsr 150mg (828) | - | - | - |
| Lsr 50mg (889) | - | - | - | |||||
| HK (326) | 67 | 32.5 | DM: 43.9% | |||||
| No HK (3508) | 66 | 29.6 | DM: 30.1% | |||||
| Rossignol et al.65 (2014) | RCT, post-hoc analysis | 3846 | ARB Lsr, 150 mg/d (ARB Lsr, 50 mg/d) | HFrEF (LVEF ≤40%) | Lsr 150mg (828); Lsr 50mg (889) | - | - | - |
| Mitrovic et al.59 (2009) | Open-label controlled trial, multicenter | 414 | ARB Can, 8-32 mg/d + standard treatment | HFrEF (LVEF ≤40%) | Total | 68.2 | 18.8 | DM: 40.8% |
| +Sp (158); 8mg +BB (378); 8mg -BB (36); 16mg -BB (32); 32mg +BB (270); 32mg -BB (25) | ||||||||
| PARADIGM-HF | ||||||||
| McMurray et al.28 (2014) | RCT, multicenter | 8399 | ARNi LCZ696, 100-200mg bid (ACEi Ena, 10mg bid) | HFrEF (LVEF ≤40%) | Ena (4212) | 63.8 | 22.6 | HT: 70.5%; DM: 34.6% |
| LCZ696 (4187) | 63.8 | 21.0 | HT: 70.9%; DM: 34.7% | |||||
| RALES | ||||||||
| RALES Investigators64 (1996) | RCT, multicenter | 214 | MRA Sp, 12.5-75 mg/d (placebo) | HFrEF (LVEF ≤35%) | Placebo (40) | 61 | 18 | - |
| 12.5mg (41); 25mg (44); 50mg (46); 75mg (41) | - | - | - | |||||
| Pitt et al.29 (1999) | RCT, multicenter | 1663 | MRA Sp, 25-50 mg/d (placebo) | HFrEF (LVEF <35%) | Placebo (841) | 65 | 27 | - |
| Sp (822) | 65 | 27 | - | |||||
| Vardeny et al.77 (2012) | RCT, post-hoc analysis | 1658 | MRA Sp, 25-50 mg/d (placebo) | HFrEF (LVEF <35%) | eGFR <60 ml/min/1.73 m2 (792) | 70 | 30.6 | HT: 27.5%; DM: 26.2% |
| eGFR ≥60 ml/min/1.73 m2 (866) | 61.2 | 23.3 | HT: 20%; DM: 18.5% | |||||
| Vardeny et al.76 (2013) | RCT, post-hoc analysis | 1663 | MRA Sp, 25-50 mg/d (placebo) | HFrEF (LVEF <35%) | African Americans (120) | 53.8 | 34 | HT: 14%; DM: 11% |
| Non-African Americans (1543) | 66.1 | 26 | HT: 24%; DM: 23% | |||||
| Vardeny et al.14 (2014) | RCT, post-hoc analysis | 1663 | MRA Sp, 25-50 mg/d (placebo) | HFrEF (LVEF <35%) | Sp (822) | - | - | - |
| Placebo (841) | - | - | - | |||||
| Placebo, no HK during trial (794) | 65 | 26.7 | HT: 23.7%; DM: 22.7% | |||||
| Placebo, HK during trial (47) | 68.6 | 31.9 | HT: 21.3%; DM: 31.9% | |||||
| Sp, no HK during trial (666) | 64.5 | 26.1 | HT: 23.1%; DM: 18.2% | |||||
| Sp, HK during trial (156) | 68.8 | 28.8 | HT: 25%; DM: 34.0% | |||||
| Solomon et al.72 (2016) | SR and meta-analysis | Neprilysin + RASi (RASi alone) | HFrEF | IMPRESS trial, omapatrilat 40mg qd/Lsn 20mg qd (573) | - | - | - | |
| OVERTURE trial, Ena 10mg bid/omapatrilat 40mg qd (5770) | - | - | - | |||||
| PARADIGM-HF, Ena 10mg bid/Sac-Val 200mg bid (8399) | - | - | - | |||||
| SOLVD | ||||||||
| Kostis et al.31 (1996) | RCT, multicenter | 6769 | ACEi Ena, 2.5-10mg bid (placebo) | HFrEF (LVEF ≤35%) | Total; Ena (3382); placebo (3387) | - | - | - |
| de Denus et al.41 (2006) | RCT, post-hoc analysis | 6722 | ACEi Ena (placebo) | HFrEF (LVEF <35%) | Ena (3364) | 59.8 | 14.2 | DM: 19.1% |
| Placebo (3358) | 59.9 | 14.7 | DM: 19.5% | |||||
| Bowling et al.37 (2013) | RCT, post-hoc analysis | 2502 | ACEi Ena, 2.5-20 mg/d (placebo) | HFrEF (LVEF ≤35%) | Total | - | - | CKD: 41%e |
| CKD, placebo (538) | 64.5 | 25 | HT: 45%; CKD: 100%; DM: 29% | |||||
| CKD, Ena (498) | 64.1 | 24 | HT: 50%; CKD: 100%; DM: 30% | |||||
| No CKD, placebo (714) | 57.7 | 16 | HT: 39%; CKD: 0%; DM: 25% | |||||
| No CKD, Ena (752) | 57.6 | 16 | HT: 38%; CKD: 0%; DM: 21% | |||||
| TIME-CHF | ||||||||
| Muzzarelli et al.60 (2012) | RCT, sub-analysis | 566 | ACEi + ARB, Sp, intensified NT-BNP-guided therapy (standard symptom-guided therapy) | HFrEF (18% HFpEF) | No HK (490) | 76.4 | 42 | HT: 75%; RD: 52%; DM: 32% |
| HK >5.5 mmol/l (76) | 78 | 30 | HT: 72%; RD: 75%; DM: 46% | |||||
| Brunner-La Rocca et al.16 (2015) | RCT, post-hoc analysis | 462 | ACEi + ARB, Sp, intensified NT-BNP-guided therapy (standard symptom-guided therapy) | HFrEF (LVEF ≤45%) | WRF III (97) | 75 | 28 | HT: 75%; RD: 68%; DM: 38% |
| No WRF III (365) | 76 | 36 | HT: 70%; RD: 50%; DM: 32% | |||||
| TITRATION | ||||||||
| Senni et al.71 (2016) | RCT, multicenter | 429 | ARNi Sac/Val, 50-200mg bid over 3 wk (50-200mg bid over 6 wks) | HFrEF (LVEF ≤35%) | Total | 64 | 21.3 | CKD: 33.7%; DM: 12.2% |
| Condensed regimen (247) | 64.2 | 22.7 | CKD: 33.6%; DM: 12.6% | |||||
| Conservative regimen (251) | 63.8 | 19.9 | CKD: 33.9%; DM: 12.0% | |||||
| High-dose (247) | 63.1 | 20.6 | CKD: 29.6% | |||||
| Low-dose (251) | 64.9 | 21.9 | CKD: 37.8% | |||||
| ACEi/ARB-naïve (33) | - | - | - | |||||
| Train-the-Trainer | ||||||||
| Peters-Klimm et al.61 (2008) | Cluster-randomized study, cross-sectional analysis | 167 | Train the trainer group (standard training) | HFrEF (LVEF ≤40%) | - | 68.2 | 31.1 | HT: 76.1%; RD: 22.2%; DM: 35.9% |
| Peters-Klimm et al.25 (2012) | Cluster-randomized study, cross-sectional analysis | 168 | Train the trainer group (standard training) | HFrEF (LVEF ≤40%) | Train the trainer (91) | 68.4 | 30.8 | HT: 74.7%; RD: 20.9%; DM: 35.2% |
| Standard (77) | 69 | 31.2 | HT: 77.9%; RD: 23.4%; DM: 37.7% | |||||
| Zannad et al.80 (1992) | RCT, multicenter | 278 | ACEi Lsn, 5-20 mg/d (ACEi Ena, 5-20 mg/d) | HFrEF (LVEF <45%) | Lsn (138) | 63 | 14 | - |
| Ena (140) | 61 | 19 | - |
eGFR <60 ml/min/1.73 m2.
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNi: angiotensin receptor-neprilysin inhibitor; ARTS: minerAlocorticoid Receptor Antagonist Tolerability Study; ATLAS: Assessment of Treatment with Lisinopril And Survival; ATMOSPHERE: Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure; BB: beta-blocker; bid: twice a day; BNP: brain natriuretic peptide; Can: candesartan; CHARM: Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity; CKD: chronic kidney disease; COMPARE: Comparative Effectiveness of Therapies for Heart Failure; d: day; DESTINY-HF: Diovan Evaluation of Safety TwIce vs oNce dailY study in Heart Failure; DM: diabetes mellitus; EFFECT: Enhanced Feedback for Effective Cardiac Treatment; eGFR: estimated glomerular filtration rate; EMPHASIS-HF: Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; Ena: Enalapril; Epl: eplerenone; Fin: finerenone; HEAAL: Heart failure Endpoint evaluation of Angiotensin II Antagonist Losartan; HF: heart failure; HK: hyperkalemia; HT: hypertension; LVEF: left ventricular ejection fraction; Lsn: lisinopril; Lsr: losartan; MRA: mineralocorticoid receptor antagonist; NT-BNP: N-terminal brain natriuretic peptide; PARADIGM-HF: Prospective comparison of angiotensin receptor neprilysin inhibitor (ARNI) with angiotensin-converting enzyme inhibitor (ACEI) to Determine Impact on Global Mortality and morbidity in Heart Failure; HFpEF: heart failure with preserved ejection fraction; qd: once daily; RALES: Randomized Aldactone Evaluation Study; RASi: renin-angiotensin system inhibitors; RCT: randomized clinical trial; RD: renal dysfunction; Sac: sacubitril; SOLVD: Studies of Left Ventricular Dysfunction; SR: systematic review; Sp: spironolactone; TIME-CHF: Trial of Intensified versus Standard Medical Therapy in Elderly Patients with Congestive Heart Failure; wk: week; Val: valsartan; WRF: worsening renal function.
A wide range of RAASi classes and combinations were analyzed in the included studies: six reports on ACEi (four studies),31,37,41,46,66,80 six reports (four studies) on losartan,20,36,38,53,59,65 three reports on ARNi,28,71,72 20 reports (17 studies) on MRA,21,27,29,30,38,43,49,51,55–57,62,64,70,74,75,78 one report on unspecified RAASi,63 and seven reports (six studies) on ACEi, ARB, and MRA.7,8,19,42,52,54,67 The combinations of ACEi and/or ARB, ACEi/ARB and MRA, and enalapril and/or aliskiren were the subject of seven reports,12,40,44,45,50,58,73 11 reports (nine studies),13,16,25,35,39,47,60,61,68,69 and one report,81 respectively.
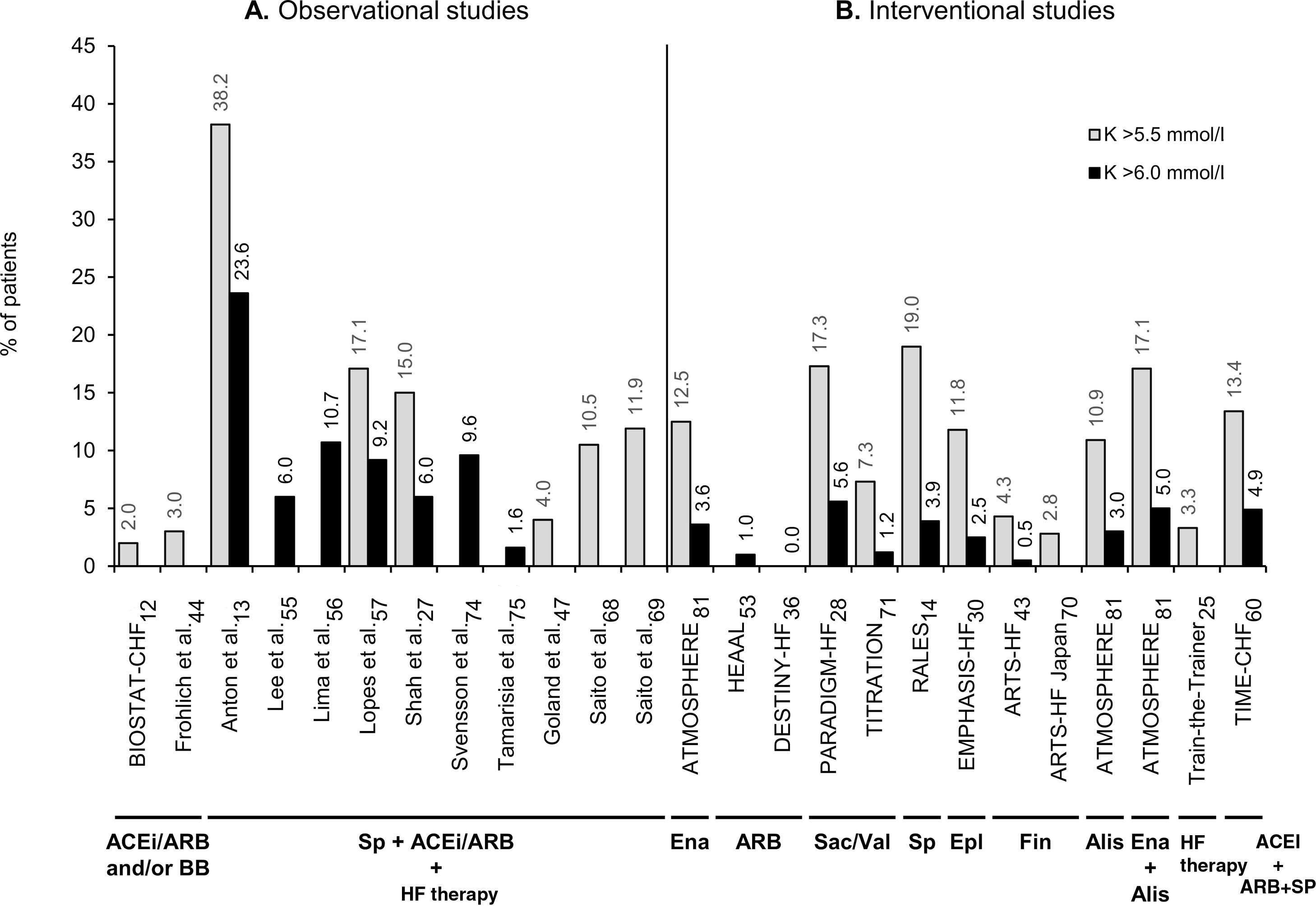
Forty-five reports had data on hyperkalemia prevalence or incidence in HFrEF patients receiving RAASi. Hyperkalemia was reported as a secondary endpoint, adverse event, or clinical outcome. Among observational studies in real-life settings,12,13,19,27,35,38,44,45,47,49,51,54–57,63,68,69,74,75,78 the proportion of patients with hyperkalemia varied between 0%51,69 and 63%27 (Supplementary Table ST2), while reports of interventional studies14,20,21,25,28–31,36,37,41,43,53,59,60,62,64,65,70–72,76,77,81 described hyperkalemia incidences between 0%36,43,70,71 and 30%77 (Supplementary Table ST3). Interestingly, observational studies that reported a 0% prevalence of hyperkalemia analyzed a highly selected population and/or a higher cut-off for hyperkalemia. This is the case of the EFFECT study by Ko et al.,51 which reported a 0% prevalence of hyperkalemia in a subgroup analysis of ideal HFrEF patients with similar characteristics to those in the RALES trial13,64 (i.e., at lower risk of developing hyperkalemia by excluding patients with baseline creatinine >2.5mg/dl, or baseline serum potassium >5 mmol/l). On the other hand, the same study reported an 18% prevalence of hyperkalemia in the total heterogeneous sample of patients with HF (HFrEF and HFpEF).51 Another observational study, by Saito et al.,52 also reported 0% prevalence in a subgroup of HF patients who were receiving only one ACEi or ARB, and were selected by exclusion criteria such as DM, CKD, serum creatinine outside the normal range, or receiving any other drugs that might affect serum potassium levels. As in clinical trials, these selected populations are not representative of the majority of HFrEF patients in daily clinical practice. By contrast, hyperkalemia levels were higher in studies on subgroups of HFrEF patients receiving combined RAASi therapy (38%),13,74 higher doses of spironolactone (24%),64 baseline creatinine ≥2.5mg/dl (63%),27 or estimated glomerular filtration rate (eGFR) <30ml/min/1.73 m2 (22%)41 or <60ml/min/1.73 m2 (26%).77
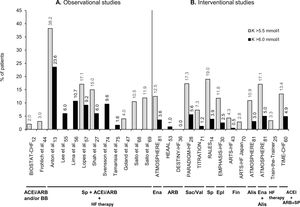
Figure 2 provides an overview of the prevalence or incidence of hyperkalemia across studies that defined hyperkalemia as serum potassium values >5.5 mmol/l or >6.0 mmol/l. The reported proportions of HFrEF patients with hyperkalemia varies depending on the study type and RAASi therapy. Overall, observational studies with combined RAASi therapy added to HF therapy presented higher hyperkalemia levels than those of interventional studies (Figure 2).
Prevalence or incidence of hyperkalemia in observational studies (A) and interventional studies (B) in patients with heart failure with reduced ejection fraction receiving renin-angiotensin-aldosterone system inhibitors (RAASi), which defined hyperkalemia as serum potassium levels >5.5 mmol/l (gray bars) or >6.0 mmol/l (black bars). The RAASi analyzed are indicated below the study names. ACEi: angiotensin-converting enzyme inhibitors; Alis: aliskiren; ARB: angiotensin receptor blockers; ARTS: minerAlocorticoid Receptor Antagonist Tolerability Study; ATMOSPHERE: Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure; BB: beta-blockers; BIOSTAT-CHF: Biology Study to Tailored Treatment in Chronic Heart Failure; DESTINY-HF: Diovan Evaluation of Safety TwIce vs oNce dailY study in Heart Failure; EMPHASIS-HF: Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; Ena: enalapril; Epl: eplerenone; Fin: finerenone; HEAAL: Heart failure Endpoint evaluation of Angiotensin II Antagonist Losartan; HF: heart failure; K: potassium; PARADIGM-HF: Prospective comparison of angiotensin receptor neprilysin inhibitor with angiotensin-converting enzyme inhibitor to Determine Impact on Global Mortality and morbidity in Heart Failure; RALES: Randomized Aldactone Evaluation Study; Sac: sacubitril; Sp: spironolactone; TIME-CHF: Trial of Intensified versus Standard Medical Therapy in Elderly Patients with Congestive Heart Failure; Val: valsartan.
Table 3 displays an overview of the 15 reports (12 studies) that estimated risk factors for hyperkalemia. Closer inspection of this table shows that the risk of hyperkalemia in HFrEF patients was significantly associated with RAASi use, as well as with age, diabetes, CKD, and elevated baseline potassium levels. In general, higher risk estimates were observed in observational studies than in clinical trials. Four-fold to 13-fold increases in the risk of hyperkalemia were associated with spironolactone or other MRA use added to background ACEi and/or ARB therapy,35,49 hematocrit <0.36,13 CKD alone41,56 or concurrent with diabetes,58 and concomitant use of potassium-sparing diuretics plus trimethoprim or ACEi58 (Table 3).
Risk factors significantly associated with hyperkalemia by univariate or multivariate analysis in observational and interventional studies on patients with heart failure with reduced ejection fraction receiving renin-angiotensin-aldosterone system inhibitors.
| Study (year) | Intervention vs. control (n)Follow-up durationa | Risk factor | Estimate [95% CI] |
|---|---|---|---|
| Observational studies | |||
| Abbas et al.35 (2015) | Sp + ACEi/ARB (1062) vs. matched controls with no HK (10620)398 (397) days | Age | OR 8.73 [5.05-15.08]b, <70 yearscOR 12.32 [9.35-6.23]b, ≥70 yearsc |
| RAASi | OR 13.59 [11.63-15.88]b, SpOR 11.05 [8.67-14.08]b, SpcOR 13.00 [9.82-17.21]b, Sp STcOR 9.12 [6.78-12.26]b, Sp LTc | ||
| Anton et al.13 (2003) | Sp + ACEi/ARB (110)12 months | Diabetes | OR 2.23 [1.02-4.89]d |
| Other factors | OR 5.18 [2.14-12.6]d, Hct <0.36OR 4.28 [1.69-10.7]e, Hct <0.36 | ||
| COMPARE-HF | |||
| Hernandez et al.49 (2012) | MRA at discharge (1070) vs. no MRA (4817)3 years | RAASi | HR 5.96 [1.31-27.15]fHR 2.51 [1.45-4.34]gHR 1.48 [1.20-1.84]h |
| Lee et al.55 (2013) | Sp (521) vs. Sp non-use (1837)2.5 (1.3-3.5) years | CKD | HR 3.86 [1.63-9.13]i, eGFR <30 ml/min/1.73 m2 |
| Sp | HR 3.46 [1.97-6.06]i, Sp, incident use | ||
| Lima et al.56 (2008) | Sp (56) vs. Sp non-use (130)23.65 days | CKD | RR 9.6 [8.03-11.20]e, final urea |
| Michel et al.58 (2015) | Cases of HK (2176) vs. controls (4000)3.9 (3.21) years | Diabetes | OR 1.52 [1.31-1.75]j, type IIOR 1.43 [1.10-1.87]j, type II + eGFR ≥60 ml/minOR 6.34 [5.19-7.74]j, type II + eGFR <60 ml/minOR 4.34 [3.51-5.37]j, type II + eGFR 30-59 ml/minOR 23.22 [16.25–33.18]j, type II + eGFR <30 ml/min |
| CKD | OR 3.81 [3.29–4.42]j, eGFR <60 ml/minOR 16.32 [12.96–20.56]j, eGFR <30 ml/minOR 4.57 [3.82–5.46]j, eGFR 30-44 ml/minOR 1.82 [1.53–2.16]j, eGFR 45-59 ml/min | ||
| RAASi | OR 3.23 [2.77-3.77]j, Sp, current useOR 10.68 [6.61–17.26]j, Sp, use <30 daysOR 4.99 [3.42–7.27]j, Sp, use 1-3 monthsOR 3.40 [2.62–4.43]j, Sp, use >3-12 monthsOR 2.17 [1.76–2.66]j, Sp, use >12 monthsOR 1.70 [1.41-2.04]j, ACEi, current useOR 5.47 [3.66–8.19]j, ACEi, use <30 daysOR 3.00 [2.14–4.21]j, ACEi, use 1-3 monthsOR 1.71 [1.33–2.19]j, ACEi, use >3-12 monthsOR 1.39 [1.13–1.72]j, ACEi, use >12 monthsOR 4.46 [3.47–5.74]j, K + -diuretics + ACEiOR 4.97 [3.87–6.39]j, Sp + ACEiOR 3.29 [2.41–4.50]j, Sp + ARBOR 2.03 [1.18–3.50]j, ACEi + ARBOR 6.81 [2.50–18.52]j, ACEi + ARB + SpOR 7.49 [3.25-17.80]j, diuretic + trimethoprimOR 7.03 [3.69-13.41]]j, ACEi + trimethoprim | ||
| Other factors | OR 3.01 [2.61-3.48]j, diuretics, current useOR 9.16 [5.99-14.02]j, diuretics, use <30 daysOR 4.74 [3.36-6.67]j, diuretics, use 1-3 monthsOR 2.98 [2.35–3.77]j, diuretics, use >3-12 monthsOR 2.16 [1.79-2.61]j, diuretics, use >12 monthsOR 2.63 [1.96-3.54]j, amiloride, current useOR 0.81 [0.67-0.99]j, loop diuretics, current useOR 0.60 [0.46-0.77]j, thiazides and related diuretics, current useOR 0.81 [0.69-0.96]j, calcium channel blockers, current use | ||
| Svensson et al.74 (2004) | Sp 25mg qd (125) vs. placebo11 months | Other factors | RR 0.37 [0.15-0.85]e, decreased LVEF per 10% increaseRR 3.36 [1.17-9.69]e, NYHA |
| Interventional studies | |||
| EMPHASIS-HF | |||
| Rossignol et al.21 (2014) | Epl (1364) vs. placebo (1373)21 months, median | CKD | HR 2.23 [1.56-3.19]d, WRF, eGFR decrease >20%HR 1.77 [1.36-2.30]d, bsl eGFR ≤ median, eGFR decrease >20% |
| RAASi | HR 1.67 [1.29-2.17]d, Epl, eGFR decrease >20% | ||
| Other factors | HR 1.69 [1.25-2.29]d, hypertension, eGFR decrease >20%HR 0.71 [0.50-1.00]d, white ethnicity, eGFR decrease >20% | ||
| HEAAL | |||
| Kiernan et al.20 (2012) | Lsr 150mg (828) vs. Lsr 50mg (889) | Age | HR 1.18 [1.06-1.32]l, older age, per 10 years older |
| Diabetes | HR 1.77 [1.42-2.22]l | ||
| RAASi | HR 1.51 [1.21-1.89]l, 150mg Lsr (vs. 50 mg)HR 1.76 [1.40-2.22]l, ARB useHR 2.29 [1.35-3.90]l, ACEi intolerance due to AE | ||
| K+ | HR 2.57 [2.15-3.07]l, bsl K+, per 1 mmol/l | ||
| Other factors | HR 1.41 [1.03-1.93]l, bsl diuretic useHR 1.20 [1.11-1.30]l, Hb, per 1g/dl lower | ||
| Rossignol et al.65 (2014) | Lsr 150mg (828) vs. Lsr 50mg (889)4.7 (3.7-5.5) years | Diabetes | HR 1.361 [1.152-1.608]d |
| CKD | HR 1.789 [1.489-2.150]d, incident 20% reduction in eGFRHR 0.984 [0.980-0.989]d, bsl eGFR | ||
| RAASi | HR 1.261 [1.065-1.493]d, MRA | ||
| K+ | HR 2.517 [2.217-2.858]d, bsl K+ | ||
| Other factors | HR 0.942 [0.892-0.996]d, bsl HbHR 0.801 [0.674-0.952]d, whiteNYHA III/IV, HR 1.289 [1.088-1.529]d | ||
| RALES | |||
| RALES Investigators64 (1996) | Sp 12.5 (41), 25 (44), 50 (46), or 75 (41) mg vs. placebo (40)12 wk | CKD | Bsl sCr |
| RAASi | Dose of SpType of ACEi (captopril vs. other)RR 2.93, high ACEi dosage | ||
| K+ | Bsl K+ | ||
| Vardeny et al.77 (2012) | Sp 25-50mg qd vs. placebo | RAASi | OR 3.7 [2.5-5.7]m, Sp usenOR 1.53 [1.16-2.02]m, Sp usen, lower eGFR vs. higher eGFROR 2.9 [1.8-4.6]m, Sp useoOR 3.1 [2.2-4.4]m, Sp usepOR 3.8 [1.2-6.4)m, Sp useqOR 3.6 [1.5-8.6]m, Sp use with WRF, with vs. without WRF |
| Vardeny et al.14 (2014) | Sp 25-50mg qd (822) vs. placebo (841)24 months | Age | HR 1.02 [1.0-1.03]r, age/1 year |
| Diabetes | HR 1.47 [1.09-2.0]r | ||
| RAASi | HR 3.20 [2.26-4.47]r, SpHR 1.04 [1.03-1.05]r, dose of Sp/12.5 mg/dayHR 5.96 [1.47-24.2]r, bsl ACEi/ARB use | ||
| K+ | HR 1.94 [1.47-2.57]r, bsl K+/1 mmol/l | ||
| Other factors | HR 1.51 [1.11-2.05]r, NYHAHR 0.53 [0.31-0.90]r, bsl BB useHR 1.09 [1.0-1.2]r, bsl SBP/10 mmHg | ||
| SOLVD | |||
| de Denus et al.41 (2006) | ACEi Ena (3364) vs. placebo (3358)2.7 (1.2) years | CKD | HR 1.29 [1.01-1.65]r, sCr modelOR 1.55 [1.23-1.95]rHR 1.85 [1.32-2.59]r, renal function, sCr modelHR 1.30 [1.11-1.52]r, renal function, CG modelOR 1.75 [1.33-2.31]r, Cr clearance 30-59 ml/minOR 4.2 [1.97-8.85]r, Cr clearance <30 ml/min |
| RAASi | HR 1.94 [1.56-2.40]r, Ena, sCr modelHR 1.92 [1.54-2.39]r, Ena, CG model | ||
| K+ | HR 1.26 [1.20-1.33]r, bsl K+/0.2-mmol/l increase, sCr modelHR 1.26 [1.19-1.32]r, bsl K+/0.2-mmol/l increase, CG modelOR 2.17 [1.77-2.66]r, bsl K+ ≥4.5 mmol/l | ||
| Other factors | HR 1.48 [1.00-2.18]r, atrial fibrillation, sCr modelHR 1.53 [1.04-2.26]r, atrial fibrillation, CG modelHR 1.60 [1.08-2.36]r, bsl NYHA III/IV (vs. I), sCr modelHR 1.52 [1.02-2.26]r, bsl NYHA III/IV (vs. I), CG modelOR 1.49 [1.10-2.01]r, bsl NYHA III/IVHR 2.00 [1.29-3.10]r, bsl loop diuretic use, prevention phase, sCr modelHR 1.97 [1.26-3.07]r, bsl loop diuretic use, prevention phase, CG model | ||
| TIME-CHF | |||
| Muzzarelli et al.60 (2012) | ACEi + ARB, Sp, NT-BNP-guided medical vs. standard symptom-guided therapy (566)18 months | CKD | OR 1.11 [1.04-1.19]r, Cr/ 10μmol/l |
| RAASi | OR 1.20 [1.00-1.42]r, dose of Sp per 12.5 mgOR 2.52 [1.45-4.35]r, dose change 25 mgOR 3.24 [1.52-6.91]r, dose change >25 mgOR 1.36 [1.14-1.63]r, maximum dose Sp per 12.5 mgOR 1.28 [1.07-1.52]r, maximum dose changes per 12.5 mgOR 1.59 [1.19-2.14]r, mean dose per 12.5 mg | ||
| K+ | OR 2.92 [1.75-4.89]r, K+ per mmol/l | ||
| Other factors | OR 2.56 [1.22-5.38]r, goutOR 3.08 [1.37-6.95]r, NYHA IV |
Hyperkalemia as serum potassium levels ≥10% upper bound of the normal range of the practice's referral laboratory (most ≥5.5 mmol/l).
Potassium level >5.4 mmol/l.
k Clinically important hyperkalemia.
ACEi: angiotensin-converting enzyme inhibitor; AE: adverse event; ARB: angiotensin receptor blocker; BB: beta blocker; bsl: baseline; CI: confidence interval; CKD: chronic kidney disease; CG: Cockcroft-Gault; COMPARE-HF: Comparative Effectiveness of Therapies for Heart Failure; Cr: creatinine; eGFR: estimated glomerular filtration rate; EMPHASIS-HF: Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; Ena: enalapril; Epl: eplerenone; Hb: hemoglobin; Hct: hematocrit; HEAAL: Heart failure Endpoint evaluation of Angiotensin II Antagonist Losartan; HFrEF: heart failure with reduced ejection fraction; HK: hyperkalemia; HR: hazard ratio; K+: potassium level; Lsr: losartan; LT: long term; MRA: mineralocorticoid receptor antagonist; NYHA: New York Heart Association class; OR: odds ratio; qd: once daily; RAASi: renin-angiotensin-aldosterone system inhibitors; RALES: Randomized Aldactone Evaluation Study; RR: risk ratio; SBP: systolic blood pressure; sCr: serum creatinine; SOLVD: Studies of Left Ventricular Dysfunction; Sp: spironolactone; ST: short term; TIME-CHF: Trial of Intensified versus Standard Medical Therapy in Elderly Patients with Congestive Heart Failure; WRF: worsening renal function.
The study selection process identified 83 reports containing data on prescription/use/discontinuation of RAASi in HFrEF patients, of which 58 were from observational studies examining real-world clinical practice (Supplementary Table ST4). Of the 83 reports, 49 (59%) did not describe any reasons for RAASi downtitration or discontinuation, while 10 (12%) reports described these reasons but did not specifically include hyperkalemia as a reason. Finally, 24 (29%) reports included hyperkalemia as a reason for RAASi downtitration or discontinuation.
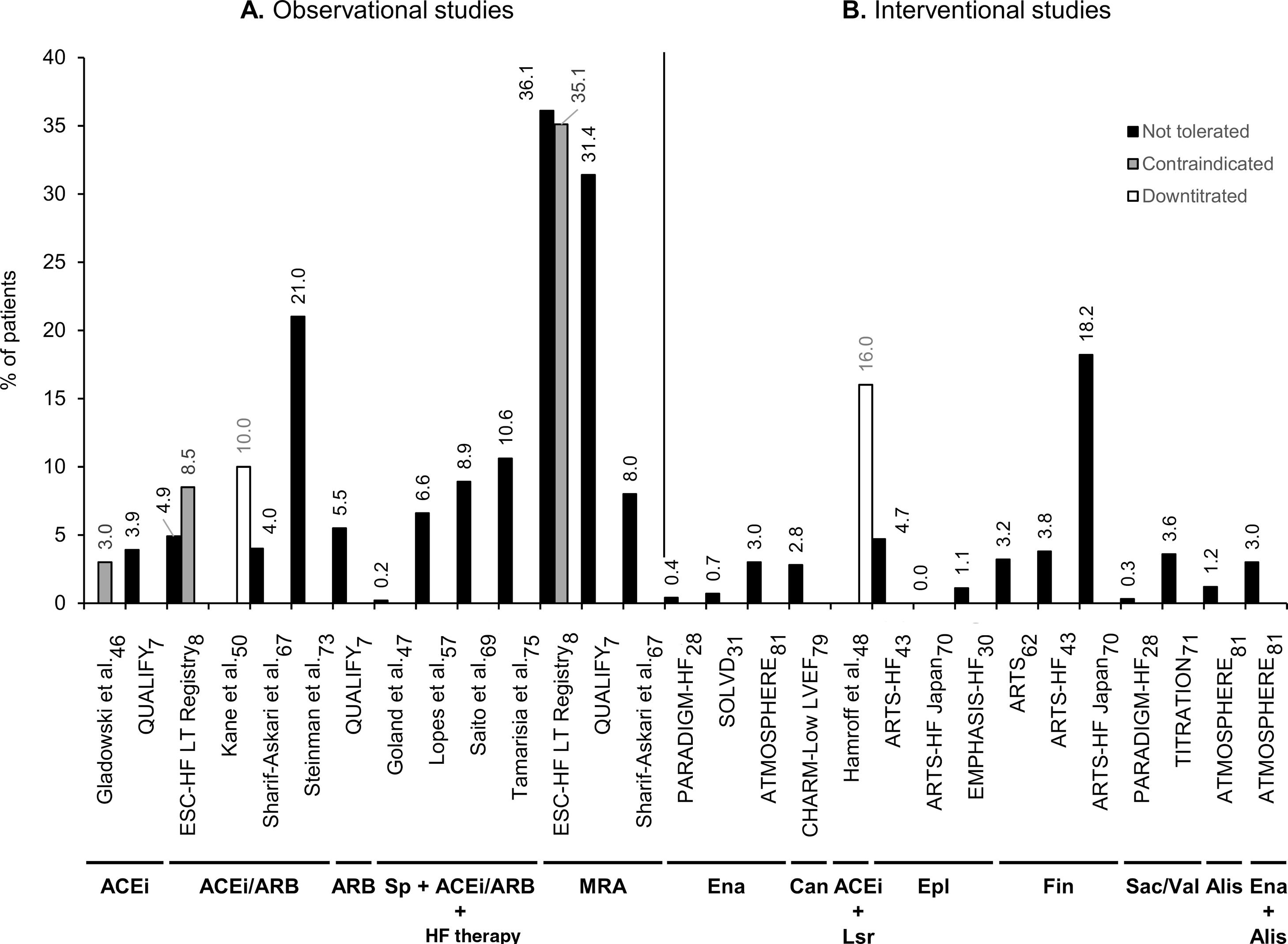
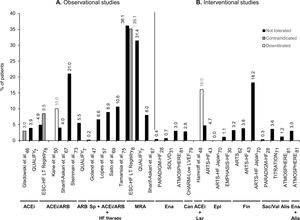
Twenty-four reports contained data on proportions of HFrEF patients in whom RAASi therapy was reduced or discontinued due to hyperkalemia. Of these, 11 reports were from observational studies7,8,46,47,50,57,67,69,73,75,78 and 13 were from clinical trials.20,28,30,31,43,48,61,62,70,71,79–81 Data on the studies that reported hyperkalemia as a reason to reduce or discontinue RAASi therapy in patients with HFrEF are summarized in Supplementary Table ST5. The ESC-HF Long-Term Registry was the first pan-European large-scale study to collect and report reasons for non-use or underuse of recommended RAASi target doses in the daily care of HF, distinguishing HFrEF patients.8 In real-life settings, prescription or use levels of RAASi were reported to be from 78%67 to 93%78 for ACEi/ARB, 66%7 to 91%57 for ACEi, 12%57 to 28%46 for ARB, and 16%46 to 69%7 for MRA in patients with HFrEF. Moreover, another large-scale real-life study (QUALIFY) reported proportions of these patients at RAASi target doses of 28% for ACEi, 40% for ARB, and 99% for MRA.7 As shown in Figure 3, regarding discontinuation or intolerance of RAASi in real-life settings, the proportion of HFrEF patients varied from 3%8 to 22%67 for ACEi/ARB, hyperkalemia being reported as a reason in 4%8,67 to 21%73 of cases. Notably, MRA use was reported as discontinued or not tolerated in 3%8 to 46%67 of patients, with hyperkalemia being reported as a reason in 8%67 to 36%8 of cases (Figure 3).
Percentage of patients with heart failure with reduced ejection fraction in observational studies (A) and interventional studies (B) that have a record of downtitration or discontinuation of renin-angiotensin-aldosterone system inhibitor (RAASi) therapy due to hyperkalemia. Not tolerated (black bars) corresponds to discontinuation of RAASi; contraindicated (gray bars) to non-use, non-prescription, or discontinuation; and downtitrated (white bars) to dose reduction, non-uptitration, or not at RAASi target doses. The RAASi analyzed are indicated below the study names. ACEi: angiotensin-converting enzyme inhibitors; Alis: aliskiren; ARB: angiotensin receptor blockers; ARTS: minerAlocorticoid Receptor Antagonist Tolerability Study; ATMOSPHERE: Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure; Can: candesartan; CHARM: Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity; EMPHASIS-HF: Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; Ena: enalapril; Epl: eplerenone; ESC: European Society of Cardiology; Fin: finerenone; HF: heart failure; Lsr: losartan; LT: long term; LVEF: left ventricular ejection fraction; MRA: mineralocorticoid receptor antagonists; PARADIGM-HF: Prospective comparison of angiotensin receptor neprilysin inhibitor with angiotensin-converting enzyme inhibitor to Determine Impact on Global Mortality and morbidity in Heart Failure; QUALIFY: QUAlity of adherence to guideline recommendations for LIfe-saving treatment in heart failure: an international surveY: an observational study; Sac: sacubitril; SOLVD: Studies of Left Ventricular Dysfunction; Sp: spironolactone; TITRATION: Safety and Tolerability of Initiating LCZ696 in Heart Failure Patients; val: valsartan.
Data were collected from 17 reports (14 studies)12,16,19–21,30,37,39,40,42,45,49,50,52,53,65,66 on the effect of hyperkalemia or of RAASi downtitration or discontinuation on the prognosis of patients with HFrEF (Supplementary Table ST6). No reports were found on the effect of RAASi downtitration or discontinuation specifically due to hyperkalemia. Nevertheless, several real-life reports, e.g. GWTG-HF,45 Kane et al.50, QUALIFY52 and the TSOC-HFrEF registry,39 showed that HFrEF patients who received lower RAASi dosages or discontinued RAASi therapy had a higher risk of mortality and/or hospitalization than those who received higher dosages or continued the therapy. In addition, a large-scale real-life study by Juurlink et al.19 and clinical trials such as HEAAL20 and EMPHASIS-HF21 indicated that hyperkalemia was associated with increased mortality and/or hospitalizations in patients with HFrEF receiving RAASi.
DiscussionThis systematic review set out to examine the reporting of hyperkalemia in patients with HFrEF receiving RAASi. As hyperkalemia is associated with worse outcomes, it has been described as a reason for RAASi downtitration or discontinuation, preventing optimized RAASi therapy in HFrEF patients who most benefit from this therapy. The first objective in this systematic review was to examine the reporting of prevalence and incidence of hyperkalemia in patients with HFrEF receiving RAASi. The most obvious findings to emerge from the analysis is that hyperkalemia is frequent in 30% to 63% of these patients, depending on the study setting (clinical trial versus real-life), background HF therapy (combination RAASi therapy), age, and/or presence of comorbidities such as CKD or diabetes. These results match those observed in recent reviews, which suggest that the incidence of hyperkalemia in clinical trials is probably underestimated.11,82 That is, clinical trials usually select samples of HFrEF patients at lower risk of developing hyperkalemia, for instance by excluding patients with baseline creatinine >2.5mg/dl or eGFR <30ml/min/1.73 m2. This might hinder the extrapolation of results of clinical trials to real-life heterogeneous populations of patients with HFrEF with diverse etiologies, comorbidities, and concomitant drugs. At the same time, several authors showed that the real-life care of patients with HFrEF can vary significantly, being influenced by factors such as patient age, physician training, or frequency of potassium monitoring,12,24–26,83,84 which in turn might influence the incidence of severe hyperkalemia in these patients.
Studies on patients with HFrEF receiving RAASi therapy identified RAASi use, age, diabetes and CKD as risk factors for hyperkalemia, as expected. Conditions such as HF, CKD, diabetes and hypertension, as well as pharmacological therapies for these conditions, may interfere with the kidney's ability to maintain a balance between potassium ingestion and excretion.82,85 Moreover, the risk of hyperkalemia associated with the combined use of ACEi/ARB with MRA or other HF therapy such as beta-blockers is greater in real-life clinical practice than is observed in clinical trials.35,49,58 This is in line with our earlier observations regarding the prevalence of hyperkalemia in unselected and heterogeneous real-life populations versus highly selected, homogeneous clinical trial populations. For instance, small studies based on clinical practice registries, such as that performed by Anton et al.,13 report very high prevalences of hyperkalemia, due to the study design and patient cohort. This study included HF patients receiving spironolactone in combination with an ACEi or an ARB. In addition, compared with clinical trial populations, such as that of RALES,13,64 the population analyzed included higher proportions of patients with older age, diabetes, CKD, and hematocrit <0.36, which are all synergistic risk factors for developing hyperkalemia. The risk of developing hyperkalemia may become increasingly important in patients with HFrEF,3,4 as CKD, diabetes, and hypertension, along with RAASi-treated HF,2,4 are increasing in prevalence in developed countries with the aging of the population.
Some studies focused on the proportions of HFrEF patients achieving RAASi target doses and reducing or discontinuing RAASi therapy due to hyperkalemia. Consistent with recent though scarce reports,11,82 data collected from real-life studies indicate that RAASi may be underused in the treatment of HFrEF patients with indication to receive them.7,8,52 In addition, hyperkalemia was reported as a reason for discontinuation or intolerance in 4-46% of patients with HFrEF receiving ACEi/ARB or MRA. However, the role and weight of hyperkalemia as a contraindication to RAASi use are not well known. Few reports distinguished between non-prescription for an undocumented reason and non-prescription due to a contraindication. Moreover, most studies reporting levels of contraindication do not distinguish between patients who have received RAASi, and discontinued due to an incident contraindication, and those who did not receive RAASi due to a prior contraindication. Nevertheless, these data are relevant to HFrEF patients who were not receiving the recommended therapy. The current review found that around 60% of reports containing data on RAASi use did not describe any reasons for their reduction or discontinuation and that less than one-third included hyperkalemia as a reason. These findings are somewhat surprising. Nowadays, there is a widespread desire to implement practice guidelines for the chronic treatment of CV and renal diseases, especially the prescription of RAASi drugs and their titration to recommended target doses.1,5,6,32,86 Nevertheless, we found a lack of reports on the incidence, clinical characteristics, and risk factors of HFrEF patients who reduced or discontinued RAASi specifically due to hyperkalemia, even though this is probably the most important clinical consequence of hyperkalemia, as it may indirectly affect the prognosis of patients with HFrEF and/or CKD, diabetes, and hypertension.9,11,12,66,82
In our review of the literature, no data were found on the association between RAASi downtitration or discontinuation specifically due to hyperkalemia and risk of mortality and/or hospitalization in patients with HFrEF. However, several studies based on real-life settings reported that poor adherence to or downtitration or discontinuation of RAASi, regardless of cause, is associated with increased risk of mortality and/or hospitalization.39,45,50,52 The benefit of these prognosis-modifying drug therapies in patients with HFrEF is now well established by a variety of studies.87 Recent developments in the treatment of hyperkalemia might change this scenario. New drugs that bind potassium in the digestive tract, such as patiromer and sodium zirconium cyclosilicate,88–90 may allow the use of higher target doses of RAASi in cardiac and renal patients, as recommended in the practice guidelines.1,5,6,32 Currently, physicians opt to reduce or discontinue RAASi in HFrEF patients at higher risk of developing hyperkalemia because there are no better therapeutic alternatives to chronically manage hyperkalemia.
Several limitations should be kept in mind when interpreting our data. First, our systematic review relied on only one database for the identification of potentially eligible studies (MEDLINE/PubMed), which increased the possibility of missing relevant references. Secondly, most studies assessing the risk of hyperkalemia in patients with HFrEF receiving RAASi therapy had low sample sizes, few events or both. Thirdly, a potential source of bias for the study is the use of different definitions of HFrEF between studies, based on the investigators’ criteria, diagnostic codes, or different LVEF cut-offs <50%. Moreover, different definitions of hyperkalemia were used between studies, such as a discharge diagnosis of hyperkalemia, an adverse event reported by an investigator, or different cut-offs of potassium levels.11 Finally, observational real-life studies were based on available medical records, which appears to have led to under-reporting of reasons for RAASi downtitration or discontinuation and hyperkalemia. This under-reporting may also apply to clinical trials, as hyperkalemia is generally regarded as a secondary outcome, and hence not a subject of comprehensive literature reporting and indexing. Further investigation and systematic reviewing of the available evidence with assessment of the quality of studies is strongly recommended.
On the other hand, one of the main strengths of this review is the inclusion of reports from real-life clinical practice. Despite its exploratory nature, this work offers some insight into the impact of hyperkalemia associated with RAASi use in unselected populations, as most clinical trials do not reflect daily practice. The findings of this scoping systematic review may help others to prioritize the chronic management of hyperkalemia in patients with HFrEF receiving RAASi, as a relevant clinical benefit should be expected.
ConclusionsHyperkalemia and RAASi downtitration or discontinuation are frequent, particularly in real-life studies. HFrEF patients at higher risk of developing hyperkalemia present comorbidities such as CKD, diabetes, and use of dual or triple therapy including RAASi and concurrent HF therapy. Although hyperkalemia has long been regarded as a reason for RAASi non-prescription, downtitration or discontinuation, it has been disregarded as a major topic in the literature.
Additional studies are needed to develop a full picture of HFrEF patients at higher risk of RAASi non-prescription, downtitration or discontinuation due to hyperkalemia in real-life settings. Moreover, further investigations will be required to assess the longer-term prognostic impact of the chronic management of hyperkalemia in real-life populations of HFrEF patients receiving RAASi. The combination of these findings will provide support for better clinical decisions to improve the prognosis of patients with HFrEF and indication to receive RAASi.
FundingThis work was supported by OM Pharma (Amadora, Portugal) for the payment of medical writing support. OM Pharma had no role in the study design, or in the collection, analysis and interpretation of data, or in the writing of the manuscript, or the decision to submit the manuscript for publication. The views expressed in the manuscript are those of the authors and not necessarily those of OM Pharma.
Conflicts of interestAuthor Cândida Fonseca has received fees for serving as a speaker, consultant and/or advisory board member from AstraZeneca Pharmaceuticals, Bayer, Novartis, Orion, Servier, and Vifor Pharma (companies that develop, market and/or distribute treatments and/or tests related to heart failure). Author Dulce Brito has received fees for serving as a speaker and/or consultant from AstraZeneca Pharmaceuticals, Boehringer Ingelheim, Novartis, Orion, Pfizer, Servier, and Vifor Pharma. Author Patrícia Branco has received fees for serving as a speaker, consultant and/or advisory board member from AstraZeneca Pharmaceuticals, Bayer, Lilly and Vifor Pharma. Author João Miguel Frazão has received fees for serving as a speaker, consultant and/or advisory board member from Amgen and Vifor Pharma. Author José Silva-Cardoso has received fees for serving as a speaker and/or consultant, or research grants from Abbott, AstraZeneca Pharmaceuticals, Bial, Boehringer Ingelheim, Menarini, Merck Serono, Merck Sharp & Dohme, Novartis, Orion, Pfizer, Sanofi, Servier, and Vifor Pharma. Author Paulo Bettencourt has received fees for serving as a speaker and/or consultant from AstraZeneca Pharmaceuticals, Merck Sharp & Dohme, Novartis, Roche Diagnostics, Servier, and Vifor Pharma.
Under the direction of the authors, Ana Morgado, Ph.D., of Scientific ToolBox Consulting (Lisbon, Portugal) provided medical writing support which was funded by OM Pharma (Amadora, Portugal).