Up to one-third of patients indicated for transcatheter aortic valve implantation (TAVI) may be unsuitable for transfemoral TAVI (TF-TAVI) according to manufacturers’ recommendations and numerous professional societies.
ObjectiveThis study aimed to investigate the predictive value of manufacturers’ guidelines for major vascular access site complications using the Perclose ProGlide device.
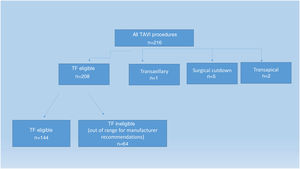
MethodsAmong 208 patients undergoing TF-TAVI, 144 patients (69.2%) were deemed eligible for TF-TAVI according to the manufacturer's instructions. A minimal lumen diameter (MLD) of the femoral artery below the manufacturer's specified limits and/or the presence of circumferential calcification were the reasons for ineligibility. Calcium score (CS), sheath-to-femoral artery ratio (SFAR) and MLD were estimated from computed tomography imaging. Vascular complications (VCs) (defined according to VARC-2 criteria) were retrospectively compared.
ResultsPatients in the ineligible group had higher SFAR (1.13±0.15 vs. 0.88±0.107, p<0.001) and CS (1.66±0.99 vs. 1.24±0.73; p=0.003), and significantly lower MLD (7.72±1.03 vs. 6.31±0.96 mm; p<0.001) compared to the eligible group. Major (6.3% vs. 12.3%, p=0.13) and minor VCs (10.4% vs. 15.6%, p=0.29) were similar in the eligible and ineligible groups. The ineligible group had higher rates of rupture (0.7% vs. 6.3%; p=0.03). SFAR was the only independent predictor of major VCs (OR 469.1, 95% CI 4.95-44466.57, p=0.008).
ConclusionThe TAVI team should not decide whether the patient is suitable for a femoral approach based solely on the manufacturer's criteria, and should incorporate additional factors that could be predictive of major VCs.
Até um terço dos doentes pode não ser elegível para implantação de válvula aórtica percutânea transfemoral (TAVI-TF) devido a recomendações do fabricante e de múltiplas sociedades profissionais.
ObjetivoInvestigar o valor preditivo das recomendações do fabricante nas complicações major no local de acesso vascular utilizando o dispositivo Perclose Proglide.
MétodosDe 208 doentes submetidos a TAVI-TF, 144 (69,2%) foram considerados elegíveis para TAVI-TF de acordo com as instruções do fabricante. Um diâmetro mínimo do lúmen iliofemoral (DMLI) abaixo dos limites especificados pelos fabricantes e/ou a presença de calcificação concêntrica constituíram as razões de não indiciação/elegibilidade. O score de calcificação (SC), a relação bainha-artéria femoral (RBAF) e o DMLI foram avaliados através de tomografia computorizada. As complicações vasculares (CV) (definidas segundo os critérios VARC-II) foram comparadas retrospetivamente.
ResultadosOs doentes do grupo não elegível apresentaram valores mais elevados de RBAF (1,13±0,15 versus 0,88±0,107, p<0,001), SC (1,66±0,99 versus 1,24±0,73; p=0,003) e valores significativamente mais baixos de DMLI (7,72±1,03 versus 6,31±0,96; p<0,001) quando comparados com o grupo elegível. As CV major (6,3% versus 12,3%, p=0,13) e minor (10,4% versus 15,6%, p=0,29) foram semelhantes nos dois grupos. O grupo não elegível apresentou taxa de rotura mais elevada (0,7% versus 6,3%; p=0,03). A RBAF foi o único fator preditivo independente de CV major (OR 469,1 [IC] 95% 4,95-44466,57, p=0,008).
ConclusõesA equipa responsável pela TAVI não deve decidir a elegibilidade para a abordagem femoral com base apenas nos critérios do fabricante, devendo incorporar na avaliação fatores adicionais que possam ser preditivos de complicações vasculares major.
Up to one third of transcatheter aortic valve implantation (TAVI) procedures may not be suitable for a transfemoral approach. Manufacturers’ size charts for determining whether transfemoral TAVI is feasible are not evidence-based. Current manufacturers’ recommendations for transfemoral TAVI cannot predict the possibility of major vascular complications. The sheath-to-femoral artery ratio seems to be the only predictor of major vascular events.
Transcatheter aortic valve implantation (TAVI) has become the generally accepted treatment option for inoperable or high- and intermediate-risk patients with severe aortic stenosis.1,2 Compared to surgical aortic valve replacement, TAVI is associated with fewer major bleeding events and similar survival rates, but has a higher incidence of vascular complications (VCs) and conduction abnormalities.3 Transfemoral access is the most commonly used pathway for the vast majority of TAVI procedures, although the proportion of patients undergoing transfemoral access varies widely, and it is projected that 89% of patients will undergo transfemoral access with the use of new-generation TAVI systems.4,5
Despite advances in device technology, including contemporary lower-profile delivery systems, in conjunction with improved vascular screening and operator experience, VCs remain the most frequent adverse events associated with transfemoral (TF) TAVI (TF-TAVI) procedures.6
Risk factors such as small femoral artery diameter and arterial calcification have been defined to help identify high-risk patients particularly vulnerable to VCs.7 In addition, valve manufacturers provide guidelines recommending minimal artery diameters for their device sizes, but these are not evidence-based. However, a standardized methodology to predict complications at the vascular access site is still lacking. This study sought to clarify whether manufacturers’ recommendations could predict VCs in TF-TAVI.
MethodsBetween April 2015 and December 2019, 208 patients undergoing TF-TAVI treated with the Perclose ProGlide suture-mediated closure system were analyzed in this single-center study. All the study procedures were approved by the local research ethics committee and patients provided written informed consent for the procedure and inclusion in the registry. Screening included baseline characteristics, iliac and femoral artery characteristics (minimal lumen diameter [MLD], calcification and tortuosity), procedural data including sheath type and size, and VCs. Contrast-enhanced multislice computed tomography (MSCT) of the aorta including the iliofemoral vessels was performed in all patients. All cases were discussed, and the indications were confirmed, with the institutional heart team.
Only patients with a TF approach (n=208) were included in the final analysis. During the study period, TF-TAVI was performed using the following valve types: balloon-expandable Edwards SAPIEN S3TM and Edwards SAPIEN XTTM valves (Edwards Lifesciences Inc., Irvine, CA); self-expanding Medtronic CoreValve® EvolutTM R System valves (Medtronic, Minneapolis, MN); and the PorticoTM valve (St. Jude Medical, Minneapolis, MN).
Patients were divided into two subgroups, classified as eligible or ineligible based on the MLD recommendations given by the valve manufacturers and presence of circumferential arterial calcification. Current recommendations require an MLD of 6.0, 6.5 and 7.0 mm for the 23, 26, and 29 mm SAPIEN XTTM (Edwards), respectively. The recommended MLD for the SAPIEN 3TM is 5.5 mm for the 14F eSheath and 6 mm for the 16F eSheath. A 14F-equivalent system with InLineTM (MLD 5.0 mm) is used with the EvolutTM R (Medtronic) TAVI system. Whereas 23- and 25-mm valves are loaded onto an 18F delivery system, 27- and 29-mm valves are loaded onto a 19F delivery system with a minimum MLD requirement of 6 mm for the PorticoTM valve (St. Jude Medical, Inc.).
Vessel access and definitionsAssessment and measurement of peripheral access before TAVI was accomplished with preprocedural MSCT. Vessel tortuosity and calcifications were also assessed by MSCT.8,9 Tortuosity was graded as no tortuosity, mild (30°-60°), moderate (60°-90°), and severe (>90°). Arterial calcification was graded as no calcification, mild (90° of total circumferential arc), moderate (90°-180° of total circumferential arc), marked (180°-270° of total circumferential arc), and severe (>270° of total circumferential arc). The sheath-to-femoral artery ratio (SFAR) was defined as the ratio between the outer sheath diameter and the MLD of the femoral artery10 and was calculated for all patients. Major clinical endpoints were assessed according to the updated Valve Academic Research Consortium (VARC-2) criteria.11
Close attention was paid to procedure-related VCs such as rupture, dissection, perforation, access site hematoma and pseudoaneurysm formation, as well as to management of peripheral interventions including balloon angioplasty, stenting, and need for unplanned surgical intervention.
Statistical analysisThe statistical analysis was performed using IBM SPSS Statistics for Windows, version 25 (IBM SPSS, Armonk, NY). Continuous variables are expressed as mean (standard deviation). Categorical variables are reported as frequencies (%). Normality of distribution of continuous variables was tested by the Kolmogorov-Smirnov goodness-of-fit test. Continuous variables were compared with the Student's t test or the Mann-Whitney U test, as appropriate. Categorical variables were compared with the chi-square test. Multivariate logistic regression was used to identify independent predictors of VCs. Receiver operating characteristic (ROC) curve analysis was used to assess the SFAR threshold with the best sum of sensitivity and specificity. Variables with a p-value ≤0.1 were entered in the regression model, and variables known to be associated with an increased risk of VCs (gender, vascular calcification, ineligibility, anticoagulation, and SFAR) were also included a priori into the analysis. The level of significance was set at p<0.05.
ResultsDuring the study period, 208 of 216 patients underwent TF-TAVI (Figure 1). Table 1 represents the baseline characteristics of the overall study population. After appropriate screening, 144 patients fulfilled the manufacturer's recommendations for a transfemoral approach (TF eligible group). In addition, 64 patients with either circumferential calcifications or less than the recommended iliofemoral MLD underwent TF-TAVI (TF ineligible group).
Baseline patient and procedural characteristics.
| Total (n=208) | TF eligible (n=144) | TF ineligible (n=64) | p | |
|---|---|---|---|---|
| Age, years | 79.15±7.62 | 79.3±7.0 | 78.9±8.8 | 0.73 |
| Male, n (%) | 109 (52.4%) | 79 (54.9%) | 30 (46.9%) | 0.28 |
| log EuroSCORE | 25.9±4 | 26.07±4.2 | 25.58±3.5 | 0.42 |
| NYHA III-IV, n (%) | 109 (52.4%) | 78 (54.2%) | 31 (48.4%) | 0.44 |
| LVEF, % | 51.3±12 | 50.5±12.7 | 52.9±10.1 | 0.15 |
| BMI, kg/m2 | 25.9±3.38 | 26.07±4.2 | 25.6±3.5 | 0.32 |
| Hypertension, n (%) | 187 (89.9%) | 130 (90.3%) | 57 (89.1%) | 0.79 |
| Diabetes, n (%) | 46 (22.1%) | 36 (25%) | 10 (15.6%) | 0.13 |
| CAD, n (%) | 120 (57.7%) | 77 (53.5%) | 43 (67.2%) | 0.07 |
| PAD, n (%) | 45 (21.6%) | 26 (18.1%) | 19 (29.7%) | 0.06 |
| GFR, ml/min/1.73 m2 | 70.4±25.2 | 70.4±25.4 | 70.7±24.8 | 0.93 |
| Previous MI, n (%) | 55 (26.4%) | 37 (25.7%) | 188 (28.1%) | 0.71 |
| Previous CVO, n (%) | 12 (5.8%) | 8 (5.6%) | 4 (6.3%) | 0.72 |
| Previous PCI, n (%) | 56 (26.9%) | 43 (29.9%) | 13 (20.3%) | 0.15 |
| CABG, n (%) | 39 (18.8%) | 26 (18.1%) | 13 (20.3%) | 0.7 |
| AF, n (%) | 50 (24%) | 38 (26.6%) | 12 (18.8%) | 0.22 |
| Anticoagulation, n (%) | 60 (28.8%) | 48 (33.3%) | 12 (18.8%) | 0.03 |
| Iliofemoral CSa | 1.37±0.84 | 1.24±0.73 | 1.66±0.99 | 0.003 |
| Tortuosity scorea | 1.39±0.96 | 1.44±0.96 | 1.39±0.98 | 0.97 |
| IFMLD, mm | 7.29±1.2 | 7.72±1.03 | 6.31±0.96 | <0.001 |
| CFMLD, mm | 7.6±1.24 | 8.05±1.11 | 6.59±0.89 | <0.001 |
| SFAR | 0.96±0.17 | 0.88±0.107 | 1.13±0.15 | <0.001 |
| Median SFAR (IQR) | 0.94 (0.85-1.06) | 0.90 (0.8-0.96) | 1.08 (1.05-1.16) | <0.001 |
| Sheath ≥18F, n (%) | 108 (51.9%) | 41 (38%) | 67 (64%) | 0.019 |
| Sheath outer diameter, mm | 6.83±0.70 | 6.74±0.69 | 7.03±0.72 | 0.005 |
| Number of PP per patient | 1.91±0.46 | 1.88±0.48 | 2±0.39 | 0.07 |
| Adjunctive Angio-seal after ≥2 PP failures, n (%) | 46 (39.1) | 26 (18.1) | 20 (31.3) | 0.03 |
| 1 PP+1 AS, n (%) | 29 (13.9) | 24 (16.7) | 5 (7.8) | 0.09 |
AF: atrial fibrillation; AS: Angio-seal; BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CFMLD: common femoral artery minimal lumen diameter; CS: calcium score; CVO: cerebrovascular occlusion; IFMLD: iliofemoral artery minimal lumen diameter; MI: myocardial infarction; NYHA: New York Heart Association functional class; PAD: peripheral artery disease; PP: Perclose ProGlide; SFAR: sheath-to-femoral artery ratio; TF: transfemoral approach; VC: vascular complication.
The groups were similar with regard to gender, age, and log EuroSCORE. The ineligible group had a slightly higher rate of peripheral artery disease (29.7% vs. 18.1%, p=0.06) and coronary artery disease (67.2% vs. 53.5%, p=0.07). Anticoagulant therapy was more frequent in eligible patients (33.3% vs.18.8%, p=0.03). The ineligible cohort had smaller MLD (7.72±1.03 vs. 6.31±0.96 mm; p<0.001) and higher calcification scores (1.24±0.73 vs. 1.66±0.99; p=0.003), however no difference was observed in vessel tortuosity score between eligible and ineligible patients (1.44±0.96 vs. 1.39±0.98; p=0.96, respectively). Double Perclose ProGlide failure was more frequent in ineligible patients and adjunctive Angio-seal was used to achieve hemostasis in this cohort (18.1% vs. 31.3%; p=0.03).
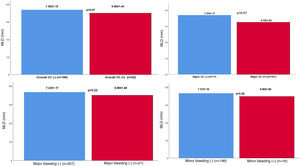
Vascular complications and in-hospital mortalityThe characteristics and outcomes of VCs are reported in Table 2. Forty-two patients (20.2%) experienced VCs. There were no significant differences in the incidence of major vascular events (eligible group 6.3% vs. ineligible group 12.5%, p=0.13) or minor vascular events (eligible group 10.4% vs. ineligible group 15.6%, p=0.29). Overall, the in-hospital mortality rate was 7.7%, with no difference between eligible and ineligible patients (n=11, 7.6% vs. n=5, 7.8%, p=0.96). Minor bleeding tended to occur more frequently in the ineligible group (5.6% vs. 12.5%, p=0.08). MLD did not differ significantly according to complication types, although MLD was lower in the major and overall complication groups (p=0.070) (Figure 2). In terms of complication type, peripheral arterial rupture was significantly more frequent in the ineligible group (n=1, 0.7% vs. n=4 6.3%, p=0.03). A detailed list of major VCs is presented in Table 3. Most (13/17; 80%) of the complications occurred with the SAPIEN XT, suggesting that newer-generation devices with lower profiles may have reduced the incidence of VCs over time.
Vascular access site complications according to group.
| Total (n=208) | TF eligible (n=144) | TF ineligible (n=64) | p | |
|---|---|---|---|---|
| Major VARC-2 complication, n (%) | 17 (8.2%) | 9 (6.3%) | 8 (12.5%) | 0.13 |
| Minor VARC-2 complication, n (%) | 25 (12%) | 15 (10.4%) | 10 (15.6%) | 0.29 |
| Overall VARC-2 complication, n (%) | 42 (20.2) | 24 (16.7%) | 18 (28.2%) | 0.057 |
| Major bleeding, n (%) | 21 (10.1%) | 12 (8.3%) | 9 (14.1%) | 0.21 |
| Minor bleeding, n (%) | 16 (7.7%) | 8 (5.6%) | 8 (12.5%) | 0.08 |
| Hematoma, n (%) | 12 (5.8%) | 8 (5.6%) | 4 (6.3%) | 1 |
| Surgical treatment, n (%) | 10 (4.8%) | 5 (3.5%) | 5 (7.8%) | 0.29 |
| Aortic dissection, n (%) | 1 (0.5%) | 1 (0.7%) | - | - |
| Rupture, n (%) | 5 (2.4%) | 1 (0.7%) | 4 (6.3%) | 0.03 |
| Stenosis/occlusion, n (%) | 16 (7.7%) | 10 (6.9%) | 6 (9.4%) | 0.58 |
| Pseudoaneurysm, n (%) | 2 (1%) | 1 (0.7) | 1 (1.6%) | 0.52 |
| Closure device failure, n (%) | 4 (1.9%) | 2 (1.4%) | 2 (3.1%) | 0.58 |
| Annular rupture, n (%) | 1 (0.5%) | 1 (0.7%) | - | - |
| Length of hospital stay, days (IQR) | 4 (3-6) | 4 (3-7) | 4 (3-5) | 0.9 |
| In-hospital mortality, n (%) | 16 (7.7%) | 11 (7.6%) | 5 (7.8%) | 0.96 |
| Stroke, n (%) | 10 (4.8%) | 8 (5.6%) | 2 (3.1%) | 0.72 |
IQR: interquartile range; TF: transfemoral approach; VARC-2: Valve Academic Research Consortium-2.
Overview of Valve Academic Research Consortium-2 major vascular complications.
| Patient | Group | Year | Sheath type | Type of vascular complication | Treatment strategy | Treatment success |
|---|---|---|---|---|---|---|
| 1 | Ineligible | 2015 | 16F SAPIEN XT | VCD failure | Surgical repair | Yes |
| 2 | Ineligible | 2016 | 16F SAPIEN XT | Iliac rupture | Surgery | No, death |
| 3 | Ineligible | 2016 | 20F SAPIEN XT | Hematoma, transfusion of 2 units of RBC | Manual compression | Yes |
| 4 | Ineligible | 2016 | 18F SAPIEN XT | CFA rupture | Stent graft | Yes |
| 5 | Eligible | 2017 | 20F SAPIEN XT | Hematoma, transfusion of 3 units of RBC | Manual compression | Yes |
| 6 | Eligible | 2017 | 20F SAPIEN XT | Aortic dissection | Surgery | No, death |
| 7 | Ineligible | 2017 | 16F SAPIEN XT | VCD failure, transfusion of 3 units of RBC | Surgical repair | Yes |
| 8 | Ineligible | 2017 | 14F SAPIEN 3 | Iliac rupture | Stent graft | Yes |
| 9 | Eligible | 2018 | 18F SAPIEN XT | Annulus rupture | Surgery | No, death |
| 10 | Eligible | 2018 | 16F SAPIEN XT | Right CFA occlusive dissection | Failed Stenting | No, death |
| 11 | Eligible | 2018 | 18F Sapen XT | Hematoma, transfusion of 3 units of RBC | Manuel Compression | Yes |
| 12 | Eligible | 2018 | 18F SAPIEN XT | Right CFA nonocclusive dissection | Stenting | No, acute renal failure and death |
| 13 | Ineligible | 2018 | 18F SAPIEN XT | Sheath fracture leading to failure of sheath removal | Surgery | Yes |
| 14 | Eligible | 2019 | 18F SAPIEN XT | Right CFA rupture, retroperitoneal hemorrhage | Surgery | Yes |
| 15 | Eligible | 2019 | 19F Portico | Pseudoaneurysm requiring transfusion of 3 units of RBC | Thrombin injection | Yes |
| 16 | Eligible | 2019 | 14F Evolute R | Hematoma requiring 2 units of RBC | Manuel compression | Yes |
| 17 | Ineligible | 2019 | 16F Evolute R | Iliac rupture | Stent graft | Yes |
CFA: common femoral artery; RBCs: red blood cells; VCD: vascular closure device.
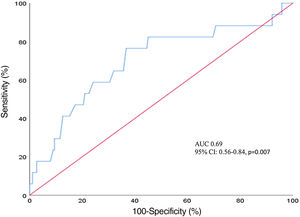
The multivariate logistic regression analysis showed that SFAR (odds ratio [OR] 469.1, 95% confidence interval [CI] 4.95-44466.57, p=0.008), but not iliofemoral calcification (OR 0.67, 95% CI: 0.33-1.37, p=0.27) or manufacturer's recommendations (OR: 0.39, 95% CI: 0.09-1.77, p=0.22) predicted major VCs (Table 4). As shown in Figure 3, ROC analysis identified a cutoff value of SFAR=0.99 for major vascular complication after TAVR with a sensitivity of 69% and specificity of 77% (area under the curve [AUC]=0.69, 95% CI: 0.56-0.84, p=0.007).
Predictors of major vascular complications.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 0.969 (0.91-1.03) | 0.33 | ||
| Female gender | 2.146 (0.76-6.03) | 0.15 | 2.49 (0.78-7.98) | 0.12 |
| EuroSCORE | 1.03 (0.92-1.16) | 0.59 | ||
| BMI | 1 (0.86-1.16) | 0.99 | ||
| Diabetes | 0.738 (0.20-2.67) | 0.64 | ||
| PAD | 2.82 (1.01-7.89) | 0.05 | 2.91 (0.92-9.24) | 0.07 |
| GFR<60 ml/min/1.73 m2 | 1.08 (0.98-1.03) | 0.47 | ||
| SFAR | 54.66 (3.72-802.48) | 0.004 | 469.1 (4.95-44466.57) | 0.008 |
| Iliofemoral CS | 1.07 (0.60-1.90) | 0.81 | 0.67 (0.33-1.37) | 0.27 |
| Tortuosity score | 0.953 (0.57-1.59) | 0.85 | ||
| CAD | 0.95 (0.34-2.60) | 0.92 | ||
| Late experience group | 0.81 (0.29-2.19) | 0.67 | ||
| Anticoagulation | 0.31 (0.06-1.38) | 0.12 | 3.37 (0.68-16.7) | 0.14 |
| Ineligibility | 2.14 (0.78-5.84) | 0.13 | 0.39 (0.09-1.77) | 0.22 |
| LVEF | 0.99 (0.97-1.03) | 0.69 | ||
| Sheath ≥18F | 1.33 (0.48-3.6) | 0.58 | ||
BMI: body mass index; CAD: coronary artery disease; CI: confidence interval; CS: calcium score; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction; PAD: peripheral artery disease; SFAR: sheath-to-femoral artery ratio.
Our study shows that vascular access site complications are still common among patients undergoing TF-TAVI using the ProGlide device. The major access site complication rate was 8.2%, which is comparable to that described by other centers, ranging between 5.0% and 7%.1,12 Moreover, this study emphasizes another important point, that manufacturers’ guidelines do not provide an individualized risk prediction of major VCs. However, SFAR (>0.99) provided prior knowledge of which patients were most likely to have major vascular adverse events.
The incidence of major VCs in the TF-eligible and TF-ineligible groups was 6.3% and 12.5%, respectively, while minor VCs occurred in 10.4% and 15.6% of patients in the respective groups. Barbash et al. reported major and minor complication rates of 1.9% and 18%, respectively, using the Perclose ProGlide device.13 Dimitridas et al.14 demonstrated major and minor complication rates of 3.8% and 12.6%, respectively; however, in the present study the higher rates of major and minor access site complications in the overall cohort compared to previous studies could plausibly be explained by the presence of the ineligible TF-TAVI group.
Several variables have been described as predictive factors of access site complications, including center and surgeon experience, female gender, femoral artery calcification (especially when circumferential), MLD, and SFAR≥1.05.15,16 Since previously defined SFAR measures were mainly based on the use of 22F and 24F delivery sheaths, lower SFAR values in our study could be due to smaller, flexible delivery systems with hydrophilic coatings. Insertion of a large-caliber delivery sheath through an extensively calcified artery causes compression and displacement of large amount of plaque burden, and can lead to dissection or rupture. Barbant et al. found that the risk of vascular trauma increases with larger sheath sizes (complication rates 0.5% vs. 10.5% in 14-18F vs. 19-24F sheaths) and showed a downward trend with the new-generation TAVR delivery systems.17 However, Kodali et al. and Manoharan et al. reported the rate of major VCs as 6.1% and 8.3%, respectively, which was associated with the use of 14F and 16F sheaths.18,19 These conflicting results mean that larger-scale randomized trials are needed to ascertain whether sheath diameter and/or SFAR can affect the incidence of VCs.
Access site-related VCs were mainly driven by bleeding and/or hematoma. Iliofemoral MLD and circumferential calcifications were defined as determinants of appropriateness for TF-TAVI. According to these recommendations, 30.8% (64/208) of our cohort were classified as ineligible for TF-TAVI. Although alternative pathways could be preferred for patients who had small iliofemoral vessel diameters and/or iliofemoral circumferential calcifications, major VCs did not differ between the TF-TAVI eligible and ineligible groups. However, overall VCs tended to occur more frequently in the ineligible group, as did rupture. In addition, even though not statistically significant (p=0.08), the ineligible group had more minor bleeding events.
Similar to our study, Reinthaler et al.20 showed that patients unsuitable for TF-TAVI experienced slightly more major VCs during TF-TAVI (10.7% vs. 2.6%, p=0.07). Circumferential calcifications and SFAR were the predictors of major vascular events. Interestingly, we did not find circumferential iliofemoral calcification as a predictor. The sheath diameters used during TF-TAVI in the present study (≥18F, 51.9%) were smaller than in the study by Reinthaler et al. using either ProGlide or Prostar vascular closure devices (≥18F, 89%). In our opinion, the impact of iliofemoral calcifications, rather than SFAR or MLD, on access site complications could have been mitigated by use of lower-profile delivery systems and the ProGlide compared to the Prostar XL.13
LimitationsThis study has some limitations. First, due to its retrospective, non-randomized nature, selection bias cannot be excluded. A randomized prospective study or propensity score matching would provide a more reliable analysis. Second, our study was performed as a single-center analysis and the size of the study population was limited. Third, the physical properties of four sheaths with different manufacturers and models could have affected outcomes in our study.
ConclusionsManufacturers’ recommendations may not predict the incidence of major VCs during TF-TAVI. Of note, only SFAR was associated with major VCs in patients undergoing TF-TAVI in the multivariate regression analysis. Smaller peripheral anatomy and circumferential calcifications should not be the only criteria for unsuitability for TF-TAVI.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of interestThe authors have no conflicts of interest to declare.