Cardiogenic shock (CS) complicates 5–10% of cases of myocardial infarction (MI). Whether glycoprotein IIb/IIIa inhibitors (GPIs) are beneficial in these patients is controversial. Our aim is to assess the prognostic impact of GPI use on in-hospital mortality and outcomes in patients with MI and CS undergoing percutaneous coronary intervention (PCI).
MethodsBetween October 2010 and December 2019, 27578 acute coronary syndrome (ACS) patients were included in the multicenter Portuguese Registry of Acute Coronary Syndromes. Of these, 357 with an MI complicated by CS were included in the analysis and grouped based on whether they received GPI therapy (with GPI, n=107 and without GPI, n=250). The primary endpoint was in-hospital mortality. Secondary endpoints included successful PCI and in-hospital reinfarction and major bleeding.
ResultsDemographics and cardiovascular risk factors did not differ between groups. ST-elevation MI patients were more likely to receive GPIs (95% vs. 83%, p=0.002). In-hospital mortality was similar between groups (OR 1.80, 95% CI 0.96–3.37). Only age and the use of inotropes or intra-aortic balloon pump were predictors of mortality. Also, no differences between groups were noted for successful PCI (OR 0.33, 95% CI 0.62–4.06), reinfarction (OR 0.77, 95% CI 0.15–3.90), or major bleeding (OR 1.68, 95% CI 0.75–3.74).
ConclusionThe use of GPIs in the context of MI with CS did not significantly impact in-hospital outcomes.
O choque cardiogénico (CC) complica 5-10% dos enfartes agudos do miocárdio (EAM). O benefício do uso de inibidores da glicoproteína IIb/IIIa (GPI) nestes doentes é controverso. O nosso objetivo é avaliar o impacto prognóstico do uso de GPI nos outcomes intra-hospitalares em doentes com EAM e CC submetidos a intervenção coronária percutânea (ICP).
MétodosEntre outubro de 2010 e dezembro de 2019, 27 578 doentes com síndrome coronária aguda (SCA) foram incluídos no Registo Português de SCA. Destes, 357 com EAM complicado por CC foram incluídos. Dois grupos foram criados, baseados no facto de terem recebido terapêutica com GPI (GPI, N=107 e sem tratamento com GPI, N=250). O endpoint primário foi a mortalidade intra-hospitalar. Endpoints secundários incluíram sucesso de ICP, re-enfarte e hemorragia major.
ResultadosAs características demográficas e os fatores de risco cardiovasculares não diferiram entre grupos. Doentes com EAM com supra-ST receberam mais terapêutica com GPI (95% versus 83%, P=0,002). A mortalidade intra-hospitalar foi similar entre grupos (OR 1,80, 95% CI 0,96-3,37, P=0,068). Apenas a idade, uso de inotrópicos ou balão intra-aórtico foram preditores de mortalidade. Não houve diferenças para sucesso de ICP (OR 0,33, 95% CI 0,62-4,06); re-enfarte (OR 0,77, 95% CI 0,15-3,90) ou hemorragia major (OR 1,68, 95% CI 0,75-3,74).
ConclusãoO uso de GPI no contexto de EAM com CC não teve impacto significativo nos outcomes intra-hospitalares.
Cardiogenic shock (CS) complicates 5–10% of cases of ST-elevation myocardial infarction (STEMI) and 2–4% of non-ST-elevation myocardial infarction (NSTEMI).1,2 No treatment other than immediate revascularization has been shown to improve outcomes, and reported in-hospital mortality ranges from 23% to 44%.3,4
During cardiogenic shock, drug pharmacokinetics are altered due to variations in drug absorption, distribution, metabolism, and excretion secondary to acute renal and hepatic dysfunction, variable plasma protein concentrations, drug-drug interactions and underlying illness.5 In the context of myocardial infarction (MI) complicated by CS, the reduced effectiveness of antithrombotic therapy is particularly problematic, especially since most antiplatelet drugs are orally administered.
Glycoprotein IIb/IIIa inhibitors (GPIs) have been shown to reduce both mortality and ischemia in STEMI patients, albeit at the expense of an increased bleeding risk.6 Current guidelines recommend GPIs only as a bailout therapy in the event of angiographic evidence of a large thrombus, slow or no reflow, and other thrombotic complications (class of recommendation IIa; level of evidence C).7 However, there is still no consensus on the appropriate antithrombotic regimen, including GPI administration, in the particular setting of MI with CS or after cardiopulmonary resuscitation. Recommendations are based on small trials and observational studies with conflicting results, most of them conducted before the routine use of novel oral P2Y12 receptor antagonists.
ObjectiveThe aim of this registry-based real-world study was to examine the impact of adjunctive GPI therapy in patients with MI complicated by CS undergoing percutaneous coronary intervention (PCI).
MethodsStudy populationThe Portuguese Registry of Acute Coronary Syndromes (PRoACS) is a nationwide database that prospectively enrolled patients with acute coronary syndrome (ACS) from 44 Portuguese centers, beginning in October 2010. Patients were deemed eligible for inclusion if they were >18 years old, presented with angina at rest in the 48 hours prior to hospital admission and had an electrocardiogram (ECG) suggestive of ischemia (i.e. ST-segment deviation or T-wave inversion) and/or troponin or creatinine kinase myocardial band (CK-MB) elevation above the upper reference limit. Patients with atypical presentation (i.e. dyspnea or other) were also included if there was a troponin/CK-MB rise and fall consistent with MI. Patients with type 2, 4 or 5 MI according to the 2007 definition of MI were not included in the registry. Investigators from each center included data on previous medical history, clinical presentation, medication, ACS type, in-hospital treatment, and in-hospital and one-year outcomes in the overall dataset at an individual patient level.
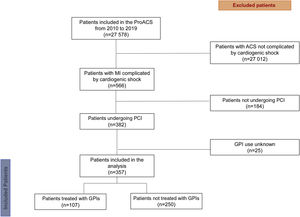
In the present study, we included patients enrolled in the PRoACS between October 2010 and December 2019 who presented with CS and underwent PCI (Figure 1).
Study analyses and endpointsThe final cohort was divided into two groups based on GPI therapy: patients who received adjunctive GPI therapy (GPI group) and those who did not (non-GPI group). The two groups were compared for baseline characteristics, treatment strategies and in-hospital outcomes. The primary endpoint was in-hospital mortality. Secondary endpoints were successful PCI, in-hospital reinfarction and major bleeding. Because clinical guidelines have evolved in the last decade, we analyzed time trends in the use of GPIs and other antithrombotic therapies.
DefinitionsCardiogenic shock was defined as hypotension (systolic blood pressure <90 mmHg for more than 30 min or the need for supportive measures to maintain systolic blood pressure of >90 mmHg) plus evidence of end-organ hypoperfusion. STEMI was defined as a persistent (i.e. >30 min duration) ST-segment elevation of >0.1 mV in at least two contiguous leads or de novo left bundle branch block plus a clinical picture compatible with an ACS; non-ST-segment elevation ACS (NST-ACS) was considered to be present in all other patients fulfilling the PRoACS inclusion criteria. Anemia was defined as admission hemoglobin <12 g/dl regardless of gender. Chronic kidney disease was defined as serum creatinine >2.0 mg/dl prior to the index admission, previous need for dialysis, or previous renal transplantation. Valvular heart disease was defined as severe valvular stenosis or regurgitation or previous valve replacement. A history of heart failure (HF) was defined as previously symptomatic HF or HF under medical treatment. Mechanical complications included free wall or interventricular septum rupture or acute mitral regurgitation due to papillary muscle involvement. Successful PCI was defined as residual stenosis <30% and post-PCI Thrombolysis in Myocardial Infarction (TIMI) 3 flow. Reinfarction was defined as recurrence of angina of >20 min duration, ischemic ECG changes, and re-elevation of cardiac biomarkers. Aborted cardiac arrest was defined as a reverted cardiac arrest during hospitalization, regardless of the cause. Major bleeding was defined according to the Global Use of Strategies to Open Occluded Arteries (GUSTO) definition.
Ethical complianceThis retrospective study complies with the Declaration of Helsinki. Only data available from existing medical records were accessed, and data were reported only in aggregate format.
Statistical analysisThe statistical analyses were performed using IBM SPSS Statistics version 20 (IBM, Armonk, NY). Categorical variables are expressed as frequencies and percentages. Continuous variables are expressed as mean±standard deviation or median and interquartile range for variables with and without a normal distribution, respectively. Comparisons between categorical variables were performed using the chi-square test and comparisons between continuous variables were performed using the Student's t test or the Wilcoxon test, according to normality of distribution. Tendencies for the use of drugs over the years was assessed using the chi-square test for trend. Logistic regression modeling was used to calculate adjusted odds ratios. Statistical significance was accepted for p-values <0.05.
ResultsStudy populationBetween October 2010 and December 2019, a total of 27 578 patients were included in the PRoACS and screened for enrollment. Of these, 566 patients had an MI complicated by CS, of whom 382 underwent PCI. Data regarding GPI therapy were missing for 25 patients, who were excluded from the analysis. Of the 357 patients enrolled, 107 received a GPI and 250 did not (Figure 1).
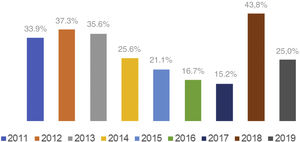
Baseline characteristics and treatment strategiesThe main baseline characteristics are summarized in Table 1. Patients treated with GPIs were younger (64±13 vs. 69±13 years, p=0.001) than patients not receiving GPI but had a similar gender distribution. The groups were comparable regarding cardiovascular risk factors and comorbidities. Patients in the GPI group more often presented with STEMI (95% vs. 83%, p=0.002) and single-vessel disease (43% vs. 31%, p=0.044). They were also more likely to receive aspirin (97% vs. 89%, p=0.01) and clopidogrel (83% vs. 72%, p=0.028) than those in the non-GPI group. Patients receiving GPIs presented with an overall more severe clinical picture, indicated by a more frequent need for inotropic support (81% vs. 61%, p<0.001), levosimendan (8% vs. 2%, p=0.018), intra-aortic balloon pump (IABP) (20% vs. 8%, p=0.003) and temporary pacemaker (25% vs. 12%, p=0.002). Also, aborted sudden cardiac death was more frequent in the GPI group (37.4% vs. 24.0%, p=0.010). Table 2 shows in-hospital treatment details. In the GPI group, the most commonly used drug was eptifibatide (45%), followed by abciximab (41%) and tirofiban (14%). In 80% of cases, GPIs were used as an adjunctive during PCI and in 20% as a bailout strategy. Although the prevalence of GPI use decreased over the years in the PRoACS population (from 19.1% in 2011 to 7.9% in 2019, p<0.001), in acute ischemic CS this tendency was not noted (p=0.158) (Figure 2). There was a trend for increased use of ticagrelor (from 0% in 2011 to 91.7% in 2019, p<0.001) and decreased use of clopidogrel (from 94.6% in 2011 to 16.7% in 2019, p<0.001) over the years.
Baseline demographic and clinical characteristics of the study population.
| Total (n=357) | No GPI use (n=250) | GPI use (n=107) | p | |
|---|---|---|---|---|
| Male gender | 243 (68.1%) | 168 (67.2%) | 75 (70.1%) | 0.591 |
| Age, years (SD) | 68±13 | 69±13 | 64±13 | 0.001 |
| Smoking history | 99 (28.4%) | 67 (27.6%) | 32 (30.5%) | 0.582 |
| Hypertension | 232 (68.6%) | 162 (68.9%) | 70 (68.0%) | 0.859 |
| Diabetes | 123 (35.8%) | 93 (38.8%) | 30 (28.8%) | 0.078 |
| BMI, kg/m2(SD) | 26 (4) | 27 (4) | 26 (4) | 0.594 |
| Dyslipidemia | 180 (55.4%) | 125 (55.1%) | 55 (56.1%) | 0.860 |
| Anemia | 103 (35.2%) | 76 (37.6%) | 27 (29.7%) | 0.187 |
| Previous MI | 50 (15.0%) | 37 (16.2%) | 13 (12.4%) | 0.369 |
| Previous PCI | 40 (11.4%) | 30 (12.2%) | 10 (9.5%) | 0.463 |
| Previous CABG | 5 (1.4%) | 3 (1.2%) | 2 (1.9%) | 0.642 |
| Valvular heart disease | 12 (3.6%) | 10 (4.3%) | 2 (1.9%) | 0.354 |
| Previous stroke | 29 (8.6%) | 17 (7.3%) | 12 (11.4%) | 0.214 |
| CKD | 31 (9.5%) | 25 (11.2%) | 6 (5.8%) | 0.126 |
| Previous bleeding | 3 (0.9%) | 2 (0.9%) | 1 (1.0%) | 1.000 |
| Previous HF | 30 (8.9%) | 23 (10.0%) | 7 (6.7%) | 0.327 |
| PAD | 19 (5.4%) | 16 (6.5%) | 3 (2.9%) | 0.165 |
| LVEF, % (SD) | 41 (13) | 42 (13) | 40 (13) | 0.413 |
| STEMI | 310 (86.8%) | 208 (83.2%) | 102 (95.3%) | 0.002 |
| Coronary anatomy | ||||
| Single-vessel disease | 88 (34.9%) | 50 (30.5%) | 38 (43.2%) | 0.044 |
| Culprit artery – left main | 31 (10.3%) | 18 (9.0%) | 13 (12.9%) | 0.290 |
| Culprit artery – left anterior descending | 111 (36.8%) | 70 (34.8%) | 41 (40.6%) | 0.327 |
| Culprit artery – left circumflex | 29 (9.6%) | 23 (11.4%) | 6 (5.9%) | 0.126 |
| Culprit artery – right coronary | 102 (33.8%) | 71 (35.3%) | 31 (30.7%) | 0.422 |
BMI: body mass index; CABG: coronary artery bypass grafting; CKD: chronic kidney disease; HF: heart failure; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-elevation myocardial infarction.
In-hospital treatment.
| Total | No GPI use | GPI use | p | |
|---|---|---|---|---|
| Aspirin | 307 (91.4%) | 204 (88.7%) | 103 (97.2%) | 0.010 |
| Clopidogrel | 269 (75.3%) | 180 (72.0%) | 88 (83%) | 0.028 |
| Ticagrelor | 78 (26.6%) | 52 (24%) | 26 (34.2%) | 0.082 |
| Enoxaparin | 177 (49.6%) | 138 (55.2%) | 39 (36.4%) | 0.001 |
| UFH | 183 (51.3%) | 129 (51.6%) | 54 (50.5%) | 0.844 |
| Fondaparinux | 11 (3.5%) | 5 (2.4%) | 6 (5.8%) | 0.190 |
| Inotropes | 239 (66.9%) | 152 (60.8%) | 87 (81.3%) | <0.001 |
| Levosimendan | 15 (4.2%) | 6 (2.4%) | 9 (8.4%) | 0.018 |
| IABP | 42 (11.8%) | 21 (8.4%) | 21 (19.6%) | 0.003 |
| LVAD | 1 (0.3%) | 0 (0.0%) | 1 (0.9%) | 0.300 |
| Temporary PM | 57 (16.0%) | 30 (12.0%) | 27 (25.2%) | 0.002 |
| Invasive ventilation | 116 (32.5%) | 75 (30.0%) | 41 (38.3%) | 0.124 |
GPI: glycoprotein IIb/IIIA inhibitor; IABP: intra-aortic balloon pump; LVAD: left ventricular assistance device; PM: pacemaker; UFH: unfractionated heparin.
Overall in-hospital mortality was 34% (120 patients). Unadjusted in-hospital mortality was similar in the GPI and non-GPI groups (35.5% vs. 32.8%; odds ratio [OR] 1.13, 95% confidence interval [CI] 0.70–1.82, p=0.619) (Table 3). In the multivariate model, GPI use was still not associated with the outcome (OR 1.80, 95% CI 0.96–3.37, p=0.068). Only age, the use of inotropes and IABP were predictors of outcome (Table 3).
Predictors of in-hospital mortality.
| Crude OR | Adjusted OR | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| GPI use | 1.13 (0.70–1.82) | 0.619 | 1.78 (0.96–3.37) | 0.068 |
| Age | 1.05 (1.03–1.08) | <0.001 | 1.05 (1.02–1.07) | <0.001 |
| STEMI | 1.77 (0.87–3.63) | 0.112 | – | – |
| Anemia | 1.53 (0.92–2.55) | 0.097 | – | – |
| Multivessel disease | 1.47 (0.86–2.50) | 0.155 | – | – |
| Inotrope use | 3.03 (1.78–5.15) | <0.001 | 4.46 (1.97–10.10) | <0.001 |
| Levosimendan | 0.48 (0.13–1.74) | 0.254 | – | – |
| IABP | 2.72 (1.42–5.23) | 0.002 | 2.32 (1.03–5.23) | 0.043 |
| Major bleeding | 0.82 (0.35–1.93) | 0.649 | – | – |
CI: confidence interval; GPI: glycoprotein IIb/IIIa inhibitors; IABP: intra-aortic balloon pump; OR: odds ratio; STEMI: ST-elevation myocardial infarction.
In-hospital outcomes are summarized in Table 4. More than 90% of patients in both groups had a successful PCI and GPI therapy had no impact on the angiographic result (OR 1.59; 95% CI 0.62–4.06, p=0.332 for successful PCI). Reinfarction (1.9% vs. 2.4%; OR 0.77, 95% CI 0.15–3.90, p=1.00) and major bleeding (OR 1.68, 95% CI 0.75–3.74, p=0.204) during hospitalization were also similar between groups.
In-hospital complications and outcomes.
| Overall population | No GPI use | GPI use | p | OR (95% CI) | |
|---|---|---|---|---|---|
| Successful PCI | 311 (92.0%) | 214 (91.1%) | 97 (94.2%) | 0.332 | 1.59 (0.62–4.06) |
| Reinfarction | 8 (2.2%) | 6 (2.4%) | 2 (1.9%) | 1.000 | 0.77 (0.15–3.90) |
| Mechanical complication | 14 (3.9%) | 11 (4.4%) | 3 (2.8%) | 0.567 | 0.63 (0.17–2.29) |
| Sustained VT | 31 (8.7%) | 14 (5.6%) | 17 (15.9%) | 0.002 | 3.18 (1.51–6.73) |
| Aborted SCD | 100 (28.0%) | 60 (24.0%) | 40 (37.4%) | 0.010 | 1.89 (1.16–3.08) |
| Major bleeding | 27 (7.6%) | 16 (6.4%) | 11 (10.3%) | 0.204 | 1.68 (0.75–3.74) |
| In-hospital mortality | 120 (33.6%) | 82 (32.8%) | 38 (35.5%) | 0.619 | 1.13 (0.70–1.82) |
CI: confidence interval; GPI: glycoprotein IIb/IIIa inhibitors; OR: odds ratio; PCI: percutaneous coronary intervention; SCD: sudden cardiac death; VT: ventricular tachycardia.
This is the largest national observational study performed to date comparing GPI use with no GPI use in MI patients admitted in CS. The main findings of this real-world multicenter study were that GPI therapy is still commonly used in patients with MI and CS and that it does not have a significant impact on in-hospital outcomes. GPI use was not associated with a significant difference in in-hospital mortality risk. Rates of reinfarction during the index admission were also comparable between the two patient groups. Curiously, GPI use was not associated with significantly higher rates of major bleeding.
Previous small studies showed a short-term survival benefit in patients treated with GPIs in the setting of MI and CS.8–12 This, however, has been challenged in more recent works. The small randomized PRAGUE-7 trial tested the routine upstream use of abciximab and showed no impact on mortality.13 One observational study, reporting the outcomes of 410 patients, also failed to show benefit of GPI therapy with abciximab on 30-day and one-year mortality.14 In a study by Kanic et al. including patients with CS and/or cardiopulmonary arrest, unadjusted short-term mortality and mortality at one year were similar with and without GPIs (46.5% vs. 54.9%, p=0.20 and 53.5% vs. 60.4%, p=0.30, respectively), but after multivariate analysis, adjunctive GPI therapy was independently associated with lower 30-day and one-year mortality.15 This was the only study, besides ours, to include patients treated with novel P2Y12 inhibitors. These results are in agreement with a previous study that also identified GPI therapy as an independent predictor of better survival at one year.16
The ADMIRAL trial showed no benefit of GPI use in CS on the combined outcome of death, reinfarction, or urgent target-vessel revascularization,17 in agreement with our results. Antoniucci et al. suggested that the clinical benefit of GPIs was not related to the patency of the infarct-related artery, as there was no benefit in events related to vessel reocclusion such as reinfarction or need for repeat revascularization. Moreover, most deaths were due to refractory ventricular failure regardless of vessel patency.11 On the other hand, it has been speculated that GPIs may have a beneficial impact on peri-MI microvascular obstruction, thereby reducing infarct area and explaining the benefits in long-term follow-up observed in some studies.18,19
Unlike most studies conducted so far,9–11,14,15 our results do not support the idea that GPIs increase the rate of successful angioplasty. This result probably reflects the fact that GPIs are more often used as a bailout strategy or when there is a perceived high thrombus burden, in accordance with current guidelines, meaning that GPIs are likely to be used more in patients with more severe disease, thus leading to a selection bias. This is supported by our finding that patients in the GPI group were more likely to be treated with inotropic support, levosimendan and intra-aortic balloon pump, reflecting more severe illness. This would also explain why these patients had a higher rate of sustained ventricular tachycardia and aborted sudden cardiac death during hospitalization.
Despite the complexity of these patients, there is consistency across observational studies that GPI use does not increase the risk of major bleeding compared to standard treatment in CS.11,13–15 These results are further corroborated by our findings. The observational basis of these studies and ours means that GPIs are probably less likely to be used in patients who have a perceived higher bleeding risk. However, this confirms that even in high-risk groups, GPIs have a good safety profile when an individual approach is applied to each patient.
The recent joint position paper from the ESC Working Group on Thrombosis, in association with the Acute Cardiovascular Care Association and the European Association of Percutaneous Cardiovascular Interventions, states that in this setting GPIs may be used as a bridge while waiting for full platelet inhibition by oral P2Y12 inhibitors, as they may improve outcomes.20 Evidence for such a recommendation is scarce and based on small studies, most of them conducted before the era of novel P2Y12 inhibitors, which are more potent and have a faster onset of action than clopidogrel. Also, most of the available studies are observational in nature, meaning that GPI use and revascularization strategy were at the discretion of the operator, varying in different timeframes or settings. The only randomized trial on this subject failed to show any benefit form routine upstream GPI administration, but the results were hampered by its small sample size.13 Only Kanic et al.15 and our group included patients medicated with novel P2Y12 inhibitors, although at lower rates than current clinical practice. A meta-analysis showed that among patients with CS complicating MI, adjunctive GPI use was both effective and safe. Overall, 30-day and one-year mortality were almost halved with the use of GPIs compared to standard treatment only. However, this reduction in short-term mortality appeared to be more important before 2000, as this benefit disappeared if only more recent studies are analyzed.21 A recently published subanalysis of the ATLANTIC trial showed that bailout GPI use in addition to ticagrelor in STEMI patients was associated with a significant increase in major bleeding, while no benefit in ischemic outcomes was noted.22
Overall, our results do not support the routine adjunctive use of GPIs in CS patients in the era of novel P2Y12 inhibitors and new revascularization strategies. However, when oral absorption is thought to be compromised there may be a rationale for intravenous (IV) antithrombotic therapy. Both GPIs and the new IV P2Y12 inhibitor cangrelor23 are appealing alternatives. The FABULOUS-FASTER trial indicates that in P2Y12-naive STEMI patients tirofiban achieves antiplatelet action more rapidly than cangrelor, and that both achieve their action faster than chewed or whole prasugrel.24 This is, however, a small study which did not assess hard endpoints, and thus needs further validation. Currently, an individualized decision on adjunctive GPI use according to thrombotic burden, gastroparesis in CS, available oral route, hypothermia, and bleeding risk, will probably be more appropriate.
LimitationsOne of the main limitations of our study is its observational nature, which inherently leads to selection bias. In the PRoACSs only 2% of the included patients had CS. We believe that selection bias may, to some extent, explain the lower-than-expected rate of CS. Patients who had a fatal outcome before admission to the coronary care unit would probably not have been included in the ProACS, while patients admitted to a general intensive care unit for other reasons (e.g. mechanical ventilation) would also probably be missing from the PRoACS. In fact, the GPI group had more need for hemodynamic support and higher rates of aborted cardiac death, which leads us to believe that GPIs were more likely to be administered in the setting of more severe illness. Mortality could therefore incorrectly appear to be higher in this group, preventing us from finding a potential benefit of GPI therapy. Also, to increase the sample size we had to include all PRoACS patients enrolled over the nine-year period of the registry, meaning that some of the patients were managed according to recommendation that are now outdated, in a clinical scenario that does not mirror current clinical practice. One consequence of this is that the use of novel P2Y12 inhibitors was low. Moreover, data on cardiac arrest prior to admission and other markers of shock such as serum lactate, urine output or kidney function were not available. We were also unable to collect data on physician-specified reasons for GPI use. Another important limitation is the small sample size, which may have hampered the detection of significant differences between groups.
Despite these limitations, our study provides valuable information about the use of GPI in a real-world population undergoing PCI for acute MI complicated by CS, for which very little data (and almost no randomized data) are available. In particular, it includes patients up until 2019, thus providing a clinical scenario that is closer to current practice than that of older studies.
ConclusionIn this retrospective analysis, a substantial proportion of patients with CS after an acute MI were treated with GPIs. GPI therapy was not associated with in-hospital survival benefit and rates of successful PCI, reinfarction and major bleeding were similar between patients who received a GPI and those who did not. Further large-scale registries and clinical trials are needed to establish the overall risk-benefit of GPI therapy in CS after an acute MI.
Conflicts of interestsThe authors have no conflicts of interests to declare.