An 83-year-old male patient with previous history of valvular rheumatic disease, permanent atrial fibrillation, and pacemaker (PM) implantation due to complete heart block was admitted to our center due to an elective replacement indicator (ERI).
The patient underwent surgery for mitral-aortic valve replacement and tricuspid annulus at the age of 68, which was complicated by conduction abnormalities resulting in permanent VVIR pacemaker implantation. Nine years later, a leadless PM (Micra™ VR; Medtronic Inc.) was implanted due to lead dysfunction, detected after a syncopal episode in which high impedance and unstable thresholds were identified. This strategy was chosen to reduce the risk of infectious and vascular complications. The procedure was uneventful.
Elective replacement indicator was detected in routine follow-up after six years. This timing is aligned with previous literature.1 Pacing threshold was 0.5 mV/0.24 ms with 100% ventricular pacing, in a PM-dependent patient. This was our first experience implanting a second leadless pacemaker (Micra™ VR; Medtronic, Inc.) in the same patient. The implant was uneventful, with easy device deployment and no vascular access complications; appropriate distancing from the first device was achieved, to avoid mechanical and electrical interference. Final pacing threshold was 0.5 mV/0.24 ms. At the eight-month follow-up, the parameters remained stable, and the patient had favorable clinical evolution. A transthoracic echocardiogram was performed, demonstrating no compromise of right ventricle or tricuspid valve function.2
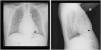
In a post-procedure chest X-ray, both leadless devices are identifiable (Figure 1).
Leadless PMs are often reserved for advanced age or in the presence of morbidities; technical and long-term clinical experience with patients with a second device is scarcely described.3 Approaches to leadless ERI are variable, including removing previous device or considering implanting a conventional lead; however, leadless abandon and new device placement is the strategy usually applied, as the first two may cause unnecessary complications in frail patients.1,3 We have described a successful implantation case, in a patient with an abandoned conventional lead and leadless pacemaker.
CRediT authorship contribution statementARB and JCP wrote the first draft of this manuscript; PGS, DC and PA gave feedback on subsequent revisions; ARB composed the figures and videos; ARB submitted the final version of this article, on behalf of all the authors.
Ethical approvalThis case report was exempt from ethics’ board approval. We obtained informed consent from the patient to publish this case report, as well as any images associated with the case. The authors of this article (A.R.B. and J.C.P.) obtained written informed consent from the patient, in accordance with COPE guidelines. The authors declare that the figures in the article do not enable the identification of the patient. Dates were omitted to comply with confidentiality.
Conflicts of interestThe authors have no conflicts of interest to declare.