To identify the relationship between red blood cell distribution width (RDW, %), interleukin-6 (IL-6) (pg/ml), high sensitivity-c-reactive protein (hs-CRP) (mg/l), in-hospital mortality and disease severity among patients with heart failure (HF).
MethodsProspective cohort. We included adults diagnosed with acute non-ischemic HF in 2015. The dependent variables were in-hospital mortality (yes or no) and disease severity. The latter was assessed with the Get With The Guidelines-HF score. We used hierarchical regression models to describe the pattern of association between biomarkers, mortality, and severity. We used the Youden index to identify the best cut-off for mortality prediction.
ResultsWe included 167 patients; the mean age was 72.61 (SD: 11.06). The majority of patients presented with New York Heart Association classification II (40.12%) or III (43.11%). After adjusting for age and gender, all biomarkers were associated with mortality. After adding comorbidities, only IL-6 was associated. The final model with all clinical variables showed no effect from any biomarker. The best cut-off for RDW, hs-CRP and IL-6 for mortality were 14.8, 68.7 and 52.9, respectively. IL-6 presented the highest sensitivity (100%), specificity (75.35%) and area under the curve (0.91).
ConclusionsNo biomarker is independent from the most important clinical variables; therefore it should not be used for management modifications.
Identificar a relação entre a amplitude de distribuição dos eritrócitos (ADE, %), IL-6 (pg/nl), hs-PCR (mg/l), mortalidade intra-hospitalar e gravidade da doença em doentes com insuficiência cardíaca (IC).
MétodosCoorte prospetiva. Incluímos adultos com IC aguda de etiologia não isquémica durante o ano de 2015. As variáveis dependentes foram a mortalidade intra-hospitalar (sim ou não) e a gravidade da doença. A última foi avaliada com o score de Get With Guidelines-HF (GWTG-HF). Utilizámos modelos de regressão hierarquizados para descrever o padrão de associação entre os biomarcadores, mortalidade e gravidade. Utilizámos o índice Youden para identificar o melhor cut-off na previsão da mortalidade.
ResultadosForam incluidos 167 doentes. A idade média foi de 72,61 (MS: 11,6). A maioria dos doentes estava em Classe II (40,12%) ou III NYHA (43,11%). Após ajuste para idade e género, todos os biomarcadores se associaram à mortalidade. Após adicionar as comorbilidades, só a IL-6 se associou com mortalidade. O modelo final com todas as variáveis clínicas incluidas não mostrou qualquer efeito de qualquer dos biomarcadores estudados. Os melhor cut-off de ADE, hs-PCR e IL-6 para mortalidade foi de 14,8, 68,7 e 52,9 respetivamente. A IL-6 apresentou a sensibilidade mais elevada (100%), especificidade (75,35%) e área sob a curva (0,91).
ConclusõesNenhum dos biomarcadores analisados se revelou independente das variáveis clínicas mais importantes, pelo que os biomarcadores estudados não devem ser utilizados como orientação clínica.
Heart failure (HF) is a complex clinical syndrome characterized by the inability of the heart to pump enough blood to meet the body's demand. HF prevalence increases with age1,2; indeed, a cohort showed that very old adults present a quite higher prevalence in comparison to non-older adults (21% vs. 0.04%).2 Moreover, in-hospital mortality can rise up to 50%.3 Consequently, HF prognosis is a challenge in clinical practice.
Numerous parameters and scores predict mortality in HF patients. The European Society of Cardiology has stated that the clinical applicability of reported prognostic markers, such as severity of HF, clinical status variables, among others, is limited4; moreover, a systematic review of risk prediction tools concluded that models include few common markers and several novel markers that are not accessible.5 Indeed, the real problem is not the accessibility or cost, but the lack of effect on management modification guided by prognostic tools. More reliable tools must be validated in order to generate a real effect on management.
Red blood cell distribution width (RDW) is a component of routine complete blood count. It measures the size variability of erythrocytes, which may play a role in HF prognosis. A high RDW is a product of decreased oxygen-carrier capacity, consequently it contributes to a reduced oxygenation.6 Eventually, the latter will cause myocardial injury7; worsen coronary disease severity8; and increase the risk of cardiovascular events, such as cardiac death, angina, and myocardial infarction.9
Another routine test is C-reactive protein (CRP). Previous studies have reported higher levels of CRP in patients with severe HF.10 A large cohort demonstrated that increased high-sensitivity CRP (hs-CRP) raises in-hospital mortality among patients with cardiovascular (CV) diseases.11 Since it is an inflammation marker, hs-CRP may play a role in HF worsening.
Although interleukin-6 (IL-6) is not a routine test, several papers have tried to justify its routine use in CV patients. Aulin et al.12 showed that it is related to a higher risk of stroke, thromboembolic events, and cardiovascular death in atrial fibrillation patients. Animal studies have shown that IL-6 administration results in ventricular dilation and a negative inotropic effect on the myocardium.13
Taking these premises, we aimed to identify the relationship between RDW, IL-6, high-sensitivity-CRP (hs-CRP), mortality and disease severity among patients with HF.
Material and methodsStudy designProspective cohort.
SettingThis is study was conducted at Hospital Nacional Edgardo Rebagliati Martins, a national hospital in Lima, Peru.
ParticipantsWe included adults diagnosed with acute non-ischemic HF during 2015. HF was defined upon when suggestive symptom criteria were met, NT-proBNP ≥125 pg/ml, and cardiac functional/structural alterations.4 We calculated a sample size of 167 cases in order to ensure a statistical power of 0.80.
Our exclusion criteria were pregnancy, hematologic diseases, blood transfusion, drugs that induce morphological changes in erythrocytes, cirrhosis, alcoholism, chemotherapy, major bleeding, and active infection. HF ischemic etiologies and other pro-inflammatory states, such myocarditis, and other inflammatory cardiomyopathies, are associated with increased inflammatory markers (RDW, IL-6, hs-CRP) and disease severity,14 therefore they are confounders of this association. We thus decided to exclude presenting a fine analysis with specific external validity.
Study variablesThe dependent variables were in-hospital mortality (yes or no) and disease severity. The latter was assessed with the Get With The Guidelines-HF (GWTG-HF),15 which is a severity score composed of systolic blood pressure (SBP), blood urea nitrogen (BUN), sodium, age, heart rate, race, and chronic obstructive pulmonary disease (COPD). The independent variables were RDW (%), hs-CRP (mg/l) and IL-6 (pg/ml). The covariables were collected at admission. They were age (years), gender (female or male), prior diseases (HF, diabetes, hyperlipidemia, chronic kidney disease, hypertension, COPD, and arrhythmia), type of HF (HF with preserved, mid-range or reduced ejection fraction)4 and number of hospital admissions. We also collected the vital signs (SBP, heart rate and respiratory rate), glucose, urea, BUN, and hemoglobin. We assessed the New York Heart Association (NYHA) Functional Classification (I-IV), the N-terminal pro-B-type natriuretic peptide, left ventricular ejection fraction (LVEF), and hospitalization duration (days).
Blood samples were used to determine a complete blood count, processed by the Sysmex XE-2100L brand blood cell, which carries out a daily and weekly calibration. We used the sequential immunometric method of enzyme solid-phase chemiluminescent immunoassay and the IMMULITE 2000 immunoassay analyzer (Siemens) to quantify IL-6 and hs-CRP.
Data analysisWe presented the qualitative variables in frequencies (absolute and relative), and the quantitative variables in mean (standard deviation, SD) or median (interquartile range (IQR)) according to its distribution, which was assessed with the histogram. To detect differences among patients who were dead or alive, we used the Chi-squared test, Fisher's exact test, Student's t-test, or Mann-Whitney U test according to distribution. Then, we used the Poisson regression model to determine the association between biomarkers and mortality. It was adjusted for significant factors according to the bivariate analysis and published literature. A p-value ≤0.05 indicated statistical significance. We excluded from the multivariate model the variables that presented collinearity. To assess the pattern of association between biomarkers and outcomes, we performed three hierarchical regression models per outcome. The first model was adjusted for age and gender; we added comorbidity confounders in the second model; and the third model was adjusted for all confounders. We reported the adjusted R2 of each model. We used the Youden index to identify the best mortality prognostic cut-off, additionally, we reported the receiver operating characteristic curves, area under the curve (AUC), sensitivity and specificity. Analyses were carried out in Stata v.14.0 (College Station, TX: StataCorp LP).
Ethical considerationsThe Institutional Review Board of the Hospital Nacional Edgardo Rebagliati Martins approved this study protocol. The patients were identified with numeric codes in the database, and we did not collect any personal information. The costs of the tests were fully borne by the authors. We respected the ethical principles of the Declaration of Helsinki.
ResultsSociodemographic variablesWe assessed 167 patients with acute non-ischemic HF. Mean age was 72.61 (SD: 11.06), and male gender accounted for 59.28%. Median hospitalization duration was four days (IQR: 2-12). More than half reported an HF history (56.89%), moreover, hypertension was the most frequent comorbidity (60.48%). Regarding symptom classification, the majority of patients presented with NYHA II (40.12%) or III (43.11%), and less than half of patients had reduced ejection fraction (41.32%). RDW and IL-6 medians were 15% (IQR: 14.2-16.1) and 29 pg/ml (7.97-120.9), respectively. Hs-CRP mean was 48.60 mg/l (SD: 38.81) (Table 1).
Clinical and sociodemographic variables according to the vital status (n=167).
| Variables | Total |
|---|---|
| Age in years, mean (SD) | 72.61 (11.06) |
| Gender | |
| Female | 68 (40.72%) |
| Male | 99 (59.28%) |
| HF history | |
| No | 72 (43.11%) |
| Yes | 95 (56.89%) |
| Diabetes history | |
| No | 114 (68.26%) |
| Yes | 53 (31.74%) |
| Hyperlipidemia history | |
| No | 138 (82.63%) |
| Yes | 29 (17.37%) |
| CKD history | |
| No | 116 (69.46%) |
| Yes | 51 (30.54%) |
| Hypertension history | |
| No | 66 (39.52%) |
| Yes | 101 (60.48%) |
| COPD history | |
| No | 136 (81.44%) |
| Yes | 31 (18.56%) |
| Arrhythmia history | |
| No | 105 (63.25%) |
| Yes | 61 (36.75%) |
| NYHA classification | |
| I | 5 (2.99%) |
| II | 67 (40.12%) |
| III | 72 (43.11%) |
| IV | 23 (13.77%) |
| Type of HF | |
| Reduced ejection fraction | 69 (41.32%) |
| Mid-range ejection fraction | 15 (8.98%) |
| Preserved ejection fraction | 83 (49.70%) |
| SBP (mmHg), median (IQR) | 125 (105-130) |
| Heart rate (beats per minute), mean (SD) | 101.61 (21.69) |
| Respiratory rate (breaths per minute), mean (SD) | 23.89 (4.96) |
| Blood glucose (mg/ml), mean (SD) | 145.29 (56.70) |
| Urea (mg/ml), median (IQR) | 46 (34-72) |
| BUN (mg/ml), median (IQR) | 21.5 (15.9-33.6) |
| NT Pro-BNP (pg/ml), median (IQR) | 5820 (980-28 766) |
| Hemoglobin (g/dl), median (IQR) | 12.1 (11.3-13.1) |
| RDW (%), median (IQR) | 15 (14.2-16.1) |
| LVEF (%), median (IQR) | 48 (30-58) |
| hs-CRP (mg/l), mean (SD) | 48.60 (38.81) |
| IL-6 (pg/ml), median (IQR) | 29 (7.97-120.9) |
| Number of previous admissions, median (IQR) | 1 (0-3) |
| Hospitalization duration (days), median (IQR) | 4 (2-12) |
BNP: brain natriuretic peptide; BUN: blood, urea, nitrogen; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; HF: heart failure; hs-CRP: high-sensitivity C-reactive protein; IL-6: interleukin-6; IQR: interquartile range; LVEF: left ventricular ejection fraction; NT Pro-BNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; RDW: red blood cell distribution width; SBP: systolic blood pressure; SD: standard deviation.
Twenty-five patients (14.97%) died in-hospital. Compared to living patients, those who died presented significantly higher frequency or mean/median of hypertension (84% vs. 56.34%), COPD (72% vs. 9.15%), NYHA IV (36% vs. 9.86%), HF with reduced ejection fraction (100% vs. 30.99%), median urea (69 vs. 43.5), median NT Pro-BNP (32 444 vs. 2429.5), median RDW (17% vs. 14.5%), hs-CRP (96.62 vs. 40.14), and median IL-6 (386 vs. 19) (Table 2).
Bivariate analysis according to vital state (n=167).
| Variables | Living | Deceased | p value |
|---|---|---|---|
| Age in years, mean (SD) | 72.57 (10.95) | 72.80 (11.84) | 0.924a |
| Gender | |||
| Female | 58 (40.85%) | 10 (40%) | 0.937b |
| Male | 84 (59.15%) | 15 (60%) | |
| HF history | |||
| No | 62 (43.66%) | 10 (40%) | 0.733b |
| Yes | 80 (56.34%) | 15 (60%) | |
| Diabetes history | |||
| No | 95 (66.90%) | 19 (76%) | 0.367b |
| Yes | 47 (33.10%) | 6 (24%) | |
| Hyperlipidemia history | |||
| No | 116 (81.69%) | 22 (88%) | 0.328c |
| Yes | 26 (18.31%) | 3 (12%) | |
| CKD history | |||
| No | 102 (71.83%) | 14 (56%) | 0.113b |
| Yes | 40 (28.17%) | 11 (44%) | |
| Hypertension history | |||
| No | 62 (43.66%) | 4 (16%) | 0.009b |
| Yes | 80 (56.34%) | 21 (84%) | |
| COPD history | |||
| No | 129 (90.85%) | 7 (28%) | <0.001c |
| Yes | 13 (9.15%) | 18 (72%) | |
| Arrhythmia history | |||
| No | 93 (65.96%) | 12 (48%) | 0.086b |
| Yes | 48 (34.04%) | 13 (52%) | |
| NYHA classification | |||
| I | 5 (3.52%) | 0 (0%) | 0.005c |
| II | 62 (43.66%) | 5 (20%) | |
| III | 61 (42.96%) | 11 (44%) | |
| IV | 14 (9.86%) | 9 (36%) | |
| Type of HF | |||
| Reduced ejection fraction | 44 (30.99%) | 25 (100%) | <0.001c |
| Mid-range ejection fraction | 15 (10.56%) | 0 (0%) | |
| Preserved ejection fraction | 83 (58.45%) | 0 (0%) | |
| SBP (mmHg), median (IQR) | 128 (118-131) | 88 (85-91) | <0.001d |
| Heart rate (beats per minute), mean (SD) | 98.58 (20.74) | 118.84 (19.00) | <0.001a |
| Respiratory rate (breaths per minute), mean (SD) | 23 (4.91) | 26.08 (4.77) | 0.016a |
| Blood glucose (mg/ml), mean (SD) | 144.38 (57.95) | 150.44 (49.69) | 0.623a |
| Urea (mg/ml), median (IQR) | 43.5 (34-65) | 69 (42-99) | 0.013d |
| BUN (mg/ml), median (IQR) | 20.3 (15.9-30.3) | 32.2 (19.6-46.2) | 0.013d |
| NT Pro-BNP (pg/ml), median (IQR) | 2429.5 (914-25 501) | 32 444 (20 358-35 000) | <0.001d |
| Hemoglobin (g/dl), median (IQR) | 12.15 (11.4-13.1) | 11.9 (11-12.4) | 0.025d |
| RDW (%), median (IQR) | 14.5 (14.1-15.9) | 17 (16.1-18.5) | <0.001d |
| LVEF (%), median (IQR) | 52 (35-59) | 25 (20-30) | <0.001d |
| hs-CRP (mg/l), mean (SD) | 40.14 (35.80) | 96.62 (7.77) | <0.001a |
| IL-6 (pg/ml), median (IQR) | 19 (7.3-52.9) | 386 (133.6-521.9) | <0.001d |
| Number of previous admissions, median (IQR) | 0 (0-3) | 4 (4-5) | <0.001d |
| Hospitalization duration (days), median (IQR) | 3 (2-11) | 12 (9-15) | <0.001d |
BUN: blood urea nitrogen; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; HF: heart failure; hs-CRP: high-sensitivity C-reactive protein; IQR: interquartile range; IL-6: interleukin-6; LVEF: left ventricular ejection fraction; NT Pro-BNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; RDW: red blood cell distribution width; SBP: systolic blood pressure; SD: standard deviation.
In univariate analysis, SBP, hemoglobin, and LVEF were associated with decreased risk of mortality, in contrast, hypertension, COPD, NYHA III-IV, reduced ejection fraction, heart rate, respiratory rate, urea, BUN, NT Pro-BNP, RDW, hs-CRP, IL-6, and number of admissions were associated with increased risk of mortality. After controlling for confounders, only SBP (RR: 0.90, 95 CI%: 0.81-0.99) was associated with decreased risk of mortality (Table 3).
Regression between study variables and mortality (n=167).
| Variables | Crude model | Multivariate modela | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | p value | RR | 95% CI | p value | |
| Age | 1.002 | 0.968-1.037 | 0.927 | 1.06 | 0.97-1.15 | 0.184 |
| Gender | ||||||
| Female | Reference | Reference | ||||
| Male | 1.03 | 0.49-2.16 | 0.937 | 2.80 | 0.41-18.911 | 0.292 |
| HF history | ||||||
| No | Reference | Reference | ||||
| Yes | 1.14 | 0.54-2.39 | 0.735 | 1.16 | 0.21-6.60 | 0.863 |
| Diabetes history | ||||||
| No | Reference | Reference | ||||
| Yes | 0.68 | 0.29-1.61 | 0.378 | 0.37 | 0.06-2.32 | 0.290 |
| Hyperlipidemia history | ||||||
| No | Reference | Reference | ||||
| Yes | 0.65 | 0.21-2.03 | 0.458 | 0.54 | 0.04-7.53 | 0.645 |
| CKD history | ||||||
| No | Reference | Reference | ||||
| Yes | 1.79 | 0.87-3.67 | 0.114 | 0.55 | 0.08-4.09 | 0.563 |
| Hypertension history | ||||||
| No | Reference | Reference | ||||
| Yes | 3.43 | 1.23-9.57 | 0.019 | 2.18 | 0.27-17.26 | 0.461 |
| COPD history | ||||||
| No | Reference | Reference | ||||
| Yes | 11.28 | 5.15-24.69 | <0.001 | 2.52 | 0.59-10.79 | 0.212 |
| Arrhythmia history | ||||||
| No | Reference | Reference | ||||
| Yes | 1.86 | 0.91-3.83 | 0.090 | 0.60 | 0.11-3.31 | 0.555 |
| NYHA classification | ||||||
| I-II | Reference | Reference | ||||
| III-IV | 3.03 | 1.19-7.71 | 0.020 | 2.98 | 0.38-23.15 | 0.297 |
| Type of HF | ||||||
| Preserved ejection fraction | Reference | - | - | - | ||
| Mid-range ejection fraction | 1.00 | 0.61-1.65 | 1.000 | - | - | - |
| Reduced ejection fraction | 7.09 | 5.18-9.71 | <0.001 | - | - | - |
| SBP | 0.88 | 0.86-0.90 | <0.001 | 0.90 | 0.81-0.99 | 0.041 |
| Heart rate | 1.03 | 1.02-1.04 | <0.001 | 0.98 | 0.93-1.04 | 0.466 |
| Respiratory rate | 1.08 | 1.02-1.14 | 0.006 | 1.06 | 0.89-1.27 | 0.515 |
| Blood glucose | 1.001 | 0.997-1.006 | 0.556 | 0.99 | 0.97-1.02 | 0.556 |
| Urea | 1.012 | 1.003-1.021 | 0.010 | 1.006 | 0.98-1.03 | 0.572 |
| BUN | 1.026 | 1.006-1.046 | 0.010 | - | - | - |
| NT Pro-BNP | 1.00008 | 1.00005-1.0001 | <0.001 | 1.00001 | 0.99986-1.02825 | 0.881 |
| Hemoglobin | 0.74 | 0.57-0.97 | 0.032 | 0.83 | 0.27-2.49 | 0.743 |
| RDW | 1.49 | 1.32-1.68 | <0.001 | 1.05 | 0.70-1.56 | 0.822 |
| LVEF | 0.88 | 0.85-0.91 | <0.001 | 0.90 | 0.76-1.08 | 0.259 |
| hs-CRP | 1.07 | 1.04-1.09 | <0.001 | 1.04 | 0.85-1.13 | 0.381 |
| IL-6 | 1.0026 | 1.0019-1.0033 | <0.001 | 1.001 | 0.999-1.005 | 0.461 |
| Number of previous admissions | 1.58 | 1.35-1.85 | <0.001 | 1.58 | 0.92-2.72 | 0.097 |
95% CI: 95% confidence interval; BUN: blood urea nitrogen; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; HF: heart failure; hs-CRP: high-sensitivity C-reactive protein; IL-6: interleukin-6; LVEF: left ventricular ejection fraction; NT Pro-BNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; RDW: red blood cell distribution width; RR: relative risk; SBP: systolic blood pressure.
Regarding the hierarchical regressions, in the first model all biomarkers were associated with increased mortality. This model explained the response variable at 32%. The second model explained the response variable at 48%. In this model, only IL-6 was associated with mortality (β: 0.0003, p-value: 0.054). In the third model, biomarkers did not show a significant association (Table 4). Similarly, we assessed a hierarchical regression between markers and severity. RDW and hs-CRP only had a significant effect in the first and second models (Table 5).
Hierarchical regression between study variables and mortality (n=167).
| Variables | Model 1, β | Model 2, β | Model 3, β |
|---|---|---|---|
| Intercept | -0.83 | -0.74 | 1.36 |
| Adjusted R2 | 0.32 | 0.48 | 0.63 |
| RDW | 0.04a | 0.036 | 0.01 |
| hs-CRP | 0.002a | 0.0014 | -0.001 |
| IL-6 | 0.0004a | 0.0003a | 0.00002 |
| Age | 0.002 | 0.003 | 0.0002 |
| Gender | -0.002 | -0.01 | -0.006 |
| Heart failure history | - | -0.07 | -0.05 |
| Diabetes history | - | -0.10a | -0.09 |
| Hyperlipidemia history | - | -0.007 | -0.032 |
| CKD history | - | 0.045 | 0.039 |
| Hypertension history | - | 0.004 | 0.011 |
| COPD history | - | 0.39b | 0.24 |
| Arrhythmia history | - | 0.028 | 0.055 |
| NYHA classification | - | - | 0.040 |
| SBP | - | - | -0.01b |
| Heart rate | - | - | 0.002 |
| Respiratory rate | - | - | 0.002 |
| Blood glucose | - | - | -0.0004 |
| Urea | - | - | -0.0004 |
| NT Pro-BNP | - | - | -8.01a |
| Hemoglobin | - | - | 0.002 |
| LVEF | - | - | -0.003 |
| Number of previous admissions | - | - | 0.04a |
CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; hs-CRP: high-sensitivity C-reactive protein; IL-6: interleukin-6; LVEF: left ventricular ejection fraction; NT Pro-BNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; RDW: red blood cell distribution width; SBP: systolic blood pressure.
Hierarchical regression between study variables and severity (n=167).
| Variables | Model 1, β | Model 2, β | Model 3, β |
|---|---|---|---|
| Intercept | -2.19 | -2.16 | 0.10 |
| Adjusted R2 | 0.48 | 0.49 | 0.63 |
| RDW | 0.08a | 0.071b | 0.035 |
| hs-CRP | 0.004a | 0.004a | 0.0003 |
| IL-6 | 0.0002 | 0.0001 | -0.0002 |
| Age | 0.013a | 0.014a | 0.01a |
| Gender | 0.09 | 0.05 | 0.05a |
| Heart failure history | - | 0.03 | -0.02 |
| Diabetes history | - | 0.00001 | -0.06 |
| Hyperlipidemia history | - | 0.10 | 0.04 |
| CKD history | - | 0.08 | -0.03 |
| Hypertension history | - | -0.008b | 0.004 |
| COPD history | - | 0.16 | -0.04 |
| Arrhythmia history | - | -0.04 | -0.01 |
| NYHA classification | - | - | 0.03 |
| SBP | - | - | -0.01a |
| Heart rate | - | - | 0.003b |
| Respiratory rate | - | - | 0.007 |
| Blood glucose | - | - | 0.0009b |
| Urea | - | - | 0.0007 |
| NT Pro-BNP | - | - | -7.59b |
| Hemoglobin | - | - | -0.02 |
| LVEF | - | - | -0.002 |
| Number of previous admissions | - | - | 0.06b |
CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; hs-CRP: high-sensitivity C-reactive protein; IL-6: interleukin-6; LVEF: left ventricular ejection fraction; NT Pro-BNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; RDW: red blood cell distribution width; SBP: systolic blood pressure.
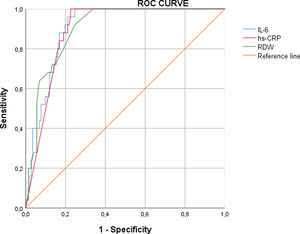
We determined the accuracy profile of biomarkers to predict mortality. The best cut-off for RDW, hs-CRP and IL-6 was 14.8 (sensitivity: 62.37%, specificity: 65.15%, AUC: 0.90), 68.7 (sensitivity: 46.53%, specificity: 80.3%, AUC: 0.90), 52.9 (sensitivity: 100%, specificity: 75.35%, AUC: 0.91), respectively (Supplementary Table 1, Appendix). The ROC curve of biomarkers is presented in Supplementary Figure 1 (Appendix).
The best cut-off of GWTG-HF for mortality was 52 (sensitivity: 92%, specificity: 90.85%, AUC: 0.97). We determined the accuracy profile of biomarkers to predict severity. The best cut-off of RDW, hs-CRP and IL-6 was 15.6 (sensitivity: 93.6%, specificity: 73.33%, AUC: 0.87), 65.4 (sensitivity: 87.2%, specificity: 82.50%, AUC: 0.86), 36.6 (sensitivity: 89%, specificity: 71.6%, AUC: 0.83), respectively (Supplementary Table 2).
DiscussionMain findingsWe explored the role of three biomarkers in a cohort of HF patients. After adjusting for age, gender and comorbidity confounders, IL-6 resulted the only independent factor of in-hospital mortality, however its effect was not independent from other clinical confounders. The IL-6 and RDW presented the best accuracy for mortality and severity, respectively (Figure 1).
Comparison with previous studiesIn our study, clinical confounders controlled the association between IL-6 and mortality, however its best cut-off for mortality prediction showed the best accuracy profile. IL-6 is a pleiotropic cytokine that increases in response to injury and activates immune cells and a signal protective response.14 Haugen et al.16 performed a case-control study, with the cases being the HF patients, and found that IL-6 was the only cytokine that predicted one-year mortality. Some characteristics may explain the differences with our results. First, the case-control study enables a better analysis; then, the group was mainly composed of octogenarian people, and the authors only included severe HF, additionally, authors did not state the confounders, but stated that they did not collect BNP data, which is a well-recognized marker.16 Another study suggested a potential association between IL-6 and mortality, however there were some details that reduce comparability: the population was composed of patients with HF and/or acute coronary syndrome, and the authors did not report the confounders of the multivariate model.17 IL-6 increases with aging,18 COPD,19 hypertension,20 among other diseases, so IL-6 rise may be mediated by the burden of comorbidity. Markousis-Mavrogenis et al.21 analyzed the data of 2516 patients with new-onset or worsening HF and reported an association between IL-6 and CV mortality through a model controlled by sociodemographic data, clinical data, and several comorbidities. In contrast, we added hemoglobin and NT pro-BNP, which might have better controlled the association since they are potential confounders.22,23 No previous studies have reported the accuracy of IL-6 in HF. Considering this context, IL-6 should not be used alone to predict mortality in HF.
In this study, hs-CRP was not an independent factor of mortality but of severity after controlling for age, gender and comorbidities. Nevertheless, other clinical confounders controlled its association. Previous studies have evidenced an association between CRP and mortality in HF with both reduced and preserved ejection fraction.24,25 This could be explained by the related physiopathological events, such as ventricular dysfunction.26 Although high CRP is linked to several chronic diseases,26 we controlled the potential comorbidity confounders, especially COPD, and hypertension. Nevertheless, the sensitivity of the best cut-off for hs-CRP was considerably lower, thus it could be limited in clinical practice. We did not find previous studies that reported the accuracy profile of hs-CRP to predict mortality among HF patients, but Aseri et al.27 reported high specificity and sensitivity in HF prediction among patients with myocardial infarction.
In our study, confounders controlled the association between RDW and mortality, however, it was shown to be highly correlated with disease severity. Konstantinos-Sotiropoulos et al.28 showed that the highest RDW quartile was associated with mortality in HF patients, however the model was only adjusted for age and gender. Not adjusting for potential confounders, such as hemoglobin and chronic diseases,29,30 may be a limitation. On the other hand, their association with ventricular dysfunction could explain the significant correlation with severity.31 In addition, a previous analysis in patients with HF with reduced ejection fraction showed that RDW was associated with lower global longitudinal strain, which estimates the myocardium contractibility.32 Kawasoe et al.33 demonstrated that the combined use of a similar RDW cut-off and BNP≥686 pg/ml was associated with mortality among HF patients. Other studies support that RDW adds a better prognosis capacity to NT pro-BNP.34,35 Nevertheless, there are no proposed potential direct effects of RDW on the myocardium.
We further studied the role of three biomarkers in predicting mortality and severity in HF patients. We do not recommend using these biomarkers alone but using them with other complex risk scores and clinical status. These tools are not expensive, indeed the RDW is a blood count component that can be routinely assessed, however, before applying it in clinical practice, we recommend carrying out cost-effectiveness studies in Peru.
Clinical practiceThe effect of biomarkers on mortality was controlled by clinical confounders, such as vital signs, NT Pro-BNP, and LVEF. However, IL-6 and RDW's effect on severity is independent from comorbidities, age, and gender. If we translated this evidence to a context of lack of laboratory and echocardiographic findings, RDW, an accessible tool, may be useful to classify the patient as severe. Nevertheless, in most situations there is not a lack of NT Pro-BNP or LVEF, consequently we prove that RDW and IL-6 use may not be the most pragmatic. Indeed, with the current evidence, it should not be used as criteria to modify HF therapy as LVEF is used. Although there is plausibility in their use, multi-center studies with a greater number of patients must be carried out to confirm our suggestion.
Strengths and limitationsOur study presents several limitations. First, we only assessed three biomarkers, however currently there are other markers that could be investigated. While in previous studies mortality was assessed up to once a year, we only assessed in-hospital mortality. This could alter the interpretation of results since the effects of some biomarkers may dramatically change over time. For example, it has been suggested that initial IL-6 response protects heart tissue, but when it is chronically activated, it can lead to fibrotic disorders.14 Then, although our sample size ensured optimal statistical power, it was small in comparison to other papers. Finally, our results cannot be extrapolated to the whole Peruvian population since it is a single experience, moreover the external validity only applies to non-ischemic HF patients due to our inclusion criteria.
ConclusionsInterleukin-6 was an independent mortality factor when only considering age, gender, and comorbidity confounders, however, its effect was not dependent on the major clinical variables. Despite its usefulness in clinical practice, better studies must be performed to assess cost-effectiveness.
Conflicts of interestThe authors have no conflicts of interest to declare.