Sudden cardiac arrest (SCA) affects individuals across all age groups and is defined as the sudden cessation of normal cardiac activity, leading to hemodynamic collapse. Determining the etiology of SCA is challenging due to its wide range of cardiac and noncardiac causes. Structural heart disease, mainly coronary artery disease, is predominant in older adults, while cardiomyopathies and primary electrical diseases are more common in younger individuals. Noncardiac causes, such as intracranial hemorrhage and pulmonary embolism, account for 15–25% of cases. This review examines the epidemiology, etiology, and investigation of SCA and proposes a diagnostic approach for SCA patients admitted to the emergency department and intensive care unit. The study protocol is divided into four main stages: (1) initial evaluation, identification of reversible causes, and exclusion of ischemic heart disease and extracardiac disease; (2) assessment of nonischemic cardiac causes; (3) neuroprognostication; and (4) clinical autopsy and/or genetic testing, if appropriate. We emphasize the importance of a multidisciplinary approach, involving an intensivist, cardiologist, neurologist, geneticist, and pathologist, as well as early genetic testing to identify potential heritable diseases and facilitate early referral of patient relatives. By providing this structured diagnostic algorithm, we aim to improve the management and outcomes of SCA patients.
A morte súbita cardíaca (MSC) afeta indivíduos de todas as faixas etárias e é definida como a cessação súbita da atividade cardíaca normal, levando ao colapso hemodinâmico. A determinação da etiologia da MSC é desafiante, dada a existência de múltiplas causas cardíacas e não cardíacas. A doença cardíaca estrutural (e sobretudo a doença arterial coronária) predomina em adultos com idade mais avançada, enquanto as miocardiopatias e doenças elétricas primárias são mais comuns em indivíduos mais jovens. Causas não cardíacas como a hemorragia intracraniana e o tromboembolismo pulmonar representam cerca de 15 a 25% dos casos de MSC. Nesta revisão, é apresentada a epidemiologia, etiologia e investigação da MSC e é proposto um algoritmo diagnóstico em doentes com MSC, admitidos no serviço de urgência e na Unidade de Cuidados Intensivos. O protocolo de estudo é dividido em quatro etapas principais: (1) avaliação inicial, identificação de causas reversíveis e exclusão de doença cardíaca isquémica e doença extracardíaca; (2) avaliação de causa cardíaca não isquémica; (3) neuroprognosticação; e (4) autópsia clínica e/ou testes genéticos, se apropriado. Enfatizamos a importância de uma abordagem multidisciplinar, envolvendo as especialidades de Medicina Intensiva, Cardiologia, Neurología, Genética Médica e Anatomia Patológica, bem como a realização de estudo genético precoce, por forma a identificar doenças potencialmente hereditárias e facilitar a referenciação precoce dos familiares. Com este algoritmo diagnóstico, pretendemos melhorar a gestão e o desfecho clínico dos doentes com MSC.
Sudden cardiac arrest (SCA) can occur in any individual, regardless of age, often without any prior signs or warnings.1 According to the 2022 European Society of Cardiology (ESC) Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death, SCA is defined as the abrupt cessation of normal cardiac activity, leading to hemodynamic collapse, and sudden cardiac death (SCD) refers to a sudden natural death presumed to be of cardiac cause.2
Determining the etiology of SCA is often challenging, as the most frequent causes vary by age and population (Table 1). These causes can be broadly categorized into cardiac and noncardiac origins. SCA most commonly occurs in the context of structural heart disease, particularly coronary artery disease. This is often due to the development of malignant arrhythmias such as ventricular fibrillation, polymorphic ventricular tachycardia, or monomorphic ventricular tachycardia, leading to hemodynamic collapse.3,4
Major causes of sudden cardiac arrest.
| Structural heart disease |
| Coronary artery disease – acute and chronic coronary syndrome |
| Coronary dissection |
| Coronary vasospasm |
| Congenital coronary anomalies |
| Valvular heart disease |
| Hypertrophic cardiomyopathy |
| Dilated cardiomyopathy |
| Arrhythmogenic right ventricular dysplasia |
| Aortic dissection |
| Myocarditis |
| Non-structural heart disease |
| QT syndrome |
| Brugada syndrome |
| Preexcitation syndrome |
| Complete heart block |
| Polymorphic catecholaminergic tachycardia |
| Idiopathic ventricular fibrillation |
| Noncardiac causes |
| Pulmonary embolism |
| Ischemic stroke |
| Intracerebral hemorrhage |
| Airway obstruction |
| Drug overdose |
| Pneumothorax |
| Hypoxia |
| Anaphylaxis |
| Hypovolemia |
Currently, it is estimated that 10–20% of all deaths in Europe are due to SCD.5,6 Additionally, approximately 300000 people with out-of-hospital cardiac arrest are treated by emergency medical teams every year, with eight out of ten cases attributed to a cardiac cause.7,8 This represents a major public health problem, characterized by high mortality rates and poor neurological outcomes.5–9 A comprehensive etiological investigation of SCA is essential to promptly identify and treat the underlying cause, thereby improving patient outcomes. Moreover, investigating sudden unexpected death in the young presents an opportunity to prevent further deaths. Many SCA victims have silent structural heart disease (SHD), primary electrical diseases (PED), cardiomyopathies, familial hypercholesterolemia, and premature ischemic heart disease; SCA is the first manifestation of cardiovascular disease in 50% of cases. Therefore, screening relatives is crucial for primary prevention, as it may facilitate preventive treatments (medication, lifestyle changes, defibrillator implantation) and ongoing medical follow-up.10–12
Therefore, we recognize the need for a structured diagnostic approach for SCA patients. Our study protocol aims to establish a standardized workflow for the etiological investigation of SCA patients admitted to the emergency department or intensive care unit (ICU), considering local practices/resources and international guidelines. The protocol promotes cooperation among clinicians from the emergency department, ICU, cardiology, genetics, and pathology, for the short- and long-term management of SCA survivors and their relatives.
EpidemiologyThe incidence of SCA significantly increases with age, underlying cardiovascular disease, and specific risk factors such as hypertension, hyperlipidemia, diabetes, cigarette smoking, and a family history of coronary disease.2 The incidence of SCA is low during childhood and young adulthood, reaching values of 50 per 100000 person-years between the ages of 45 and 75 years.2 The highest incidence is reported during the eighth decade of life, at 200 per 100000 person-years.2 Additionally, men have a two to three times higher rate of SCA compared to women.13 In up to 60% of patients with established coronary artery disease, SCD is the mechanism of death. In Europe, up to 50% of all cardiovascular deaths are attributed to SCD.2,14–18
EtiologyIt is well established that most SCD cases occur in patients with underlying SHD and primarily coronary artery disease accounts for up to 70% of cases.19 According to the 2022 ESC Guidelines, different cardiac etiologies should be considered based on the patient's age distribution.2 In patients <40 years, predominant etiologies include hypertrophic and arrhythmogenic cardiomyopathies, myocarditis, congenital coronary anomalies, and congenital heart diseases.2,19–24 However, SCA can occur in patients without any documented SHD, with reported incidences of up to 12% in patients younger than 40–45 years, mainly due to PED.25–27 In older patients, coronary artery disease is the predominant cause of SCA, occurring in the setting of an acute coronary syndrome or due to chronic coronary artery disease, with formation of myocardial scar tissue and subsequent development of malignant arrhythmias. Other common nonischemic SHDs include valvular heart disease and heart failure.19–28
Between 15 and 25% of SCA cases are due to noncardiac causes such as bleeding, drug overdose, trauma, intracranial hemorrhage, pulmonary embolism, or airway obstruction (Table 1) and should be investigated according to clinical presentation.29,30
If the cause of SCD remains unknown after negative pathological and toxicological assessment, it is classified as sudden arrhythmic death syndrome.2,25,26
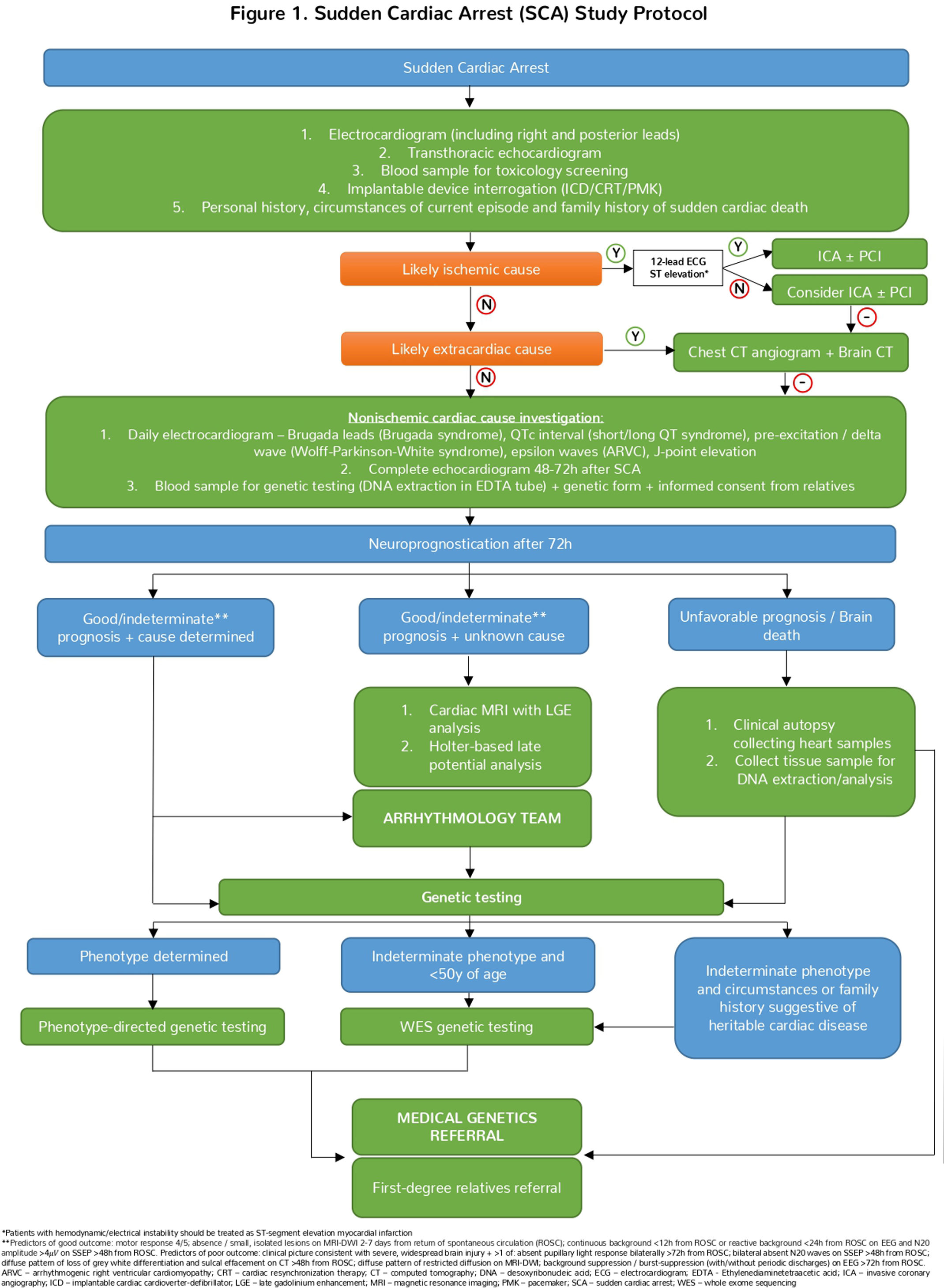
Protocol overviewThe rationale for creating and implementing our study protocol is the need to promptly identify and treat the cause of SCA to improve post-resuscitation care, a key determinant of survival. Additionally, having an etiologic diagnosis facilitates the screening and follow-up of relatives. Therefore, we aimed to create a standardized tool to provide a comprehensive and systematic approach to SCA patients (Figure 1).
The evaluation of an SCA patient begins immediately after resuscitation and initial stabilization. Our protocol encompasses the different evaluation stages of SCA/SCD patients and highlights the most appropriate diagnostic tests/procedures at each stage. The evaluation of SCA patients is divided into four stages. Although presented sequentially, some stages can occur simultaneously if appropriate:
- •
Initial evaluation and identification of reversible causes
- •
Assessment of nonischemic cardiac causes, including advanced cardiac diagnostics and multidisciplinary review
- •
Neurologic assessment at least 72 hours post-resuscitation
- •
Clinical autopsy and/or genetic testing with referral to medical genetics for patients and relatives where a cardiac heritable disease is present or suspected.
In the first stage of the protocol, which focuses on assessing reversible causes (both cardiac and noncardiac), a 12-lead electrocardiogram (ECG) with right and posterior precordial leads should be obtained immediately after resuscitation. This should be repeated if necessary to exclude acute coronary syndrome and identify malignant arrythmias or abnormalities suggestive of PED. In this context, retrieving previous ECGs and ECGs from pre-hospital emergency services may provide important information regarding the circumstances of SCA.
Since the majority of SCA are unwitnessed, in the absence of cardiac monitoring, the exact cardiac rhythm is often difficult to establish.31 The presence of implantable cardiac devices (pacemakers, defibrillators, or resynchronizers) can aid in this diagnosis and should be interrogated. Most episodes are attributed to ventricular arrhythmias, and in minority of cases, bradyarrhythmia is responsible, often associated with nonischemic cardiomyopathy or noncardiac causes.32–38
As a readily available and noninvasive diagnostic tool, a bedside transthoracic echocardiogram should be performed immediately to exclude reversible causes of cardiac arrest, such as cardiac tamponade, and significant SHD (e.g., major valve disease, coronary artery disease, and cardiomyopathies), which can be present in up to 90% of patients with SCD.39,40 Left ventricle dysfunction may initially be present solely due to myocardial stunning induced by SCA and resuscitation maneuvers. Therefore, a complete echocardiogram should be repeated 48–72 hours after admission.41
Careful history taking (with patient and/or relatives) is warranted and should focus on identifying “red flags” of cardiac origin (e.g., absence of prodromes, chest pain, past history of heart disease, family history of SCD) or noncardiac origin (e.g., headache and seizures, new neurological deficit, shortness of breath, risk factors for pulmonary embolism, known respiratory disease). The clinical history should also consider the ingestion of toxins/illicit drugs and precursor events/triggers in PED, such as fever or pro-arrhythmic medication.2,42
Blood samples should be collected for toxicology screening, as it may reveal polypharmacy or drug overdose in up to 56% of cases, especially in young patients.2
According to the 2023 ESC Guidelines for the management of acute coronary syndromes, urgent invasive coronary angiography is recommended in patients with resuscitated SCA who are estimated to have high probability of acute coronary occlusion (e.g., 12-lead ECG with persistent ST-segment elevation (or equivalent) or hemodynamic/electrical instability in the absence of ST-segment elevation).43–47 In the absence of such findings, the decision to perform ICA should be individualized. Based on data from several recent randomized controlled trials, routine urgent ICA is not superior to a delayed invasive strategy, in the absence of ST-segment elevation (or equivalents) or hemodynamic and/or electrical instability and is therefore not recommended. In these cases, patient management in an urgent setting should be directed to exclude the most common noncardiac causes followed by ICU admission and clinical stabilization.48–53 ICA should be considered in the latter stages of management in SCA survivors, using, if needed, advanced coronary imaging techniques (optical coherence tomography/intravascular ultrasound) or intracoronary provocative tests, to exclude coronary artery disease, coronary dissection, coronary anomalies, and coronary vasospasm with higher sensitivity.39,40,54–57
If there is clinical suspicion of noncardiac origin, brain and chest imaging with computed tomography scan should be performed in the acute setting to exclude intracranial hemorrhage and pulmonary embolism, respectively. These entities are the two most common noncardiac causes of SCA and should be managed urgently.43,58
Assessment of nonischemic cardiac causesThe second stage of the protocol focuses on investigation of patients with a negative preliminary etiologic study and no evidence of ischemic disease. In such cases, a more comprehensive echocardiogram should be performed, and other advanced imaging modalities (mentioned below) should be considered.
Approximately 5–10% of SCD survivors have no evidence of a noncardiac etiology or SHD.25 In these patients, the diagnosis of PED is more likely.25 Continuous cardiac monitoring and daily 12-lead ECG (including Brugada leads)59 during stable rhythm are recommended to look for characteristic ECG findings, such as long or short corrected QT intervals, preexcitation syndromes, early repolarization, epsilon waves and Brugada patterns, which may aid in establishing a definitive diagnosis.39,60
At this stage of the protocol, we recommend blood collection for genetic testing.54,61,62 We included this step early in the protocol to systematically guarantee that a sample is preserved, allowing for the establishment of a possible genetic/hereditary diagnosis, with implications for both patients and their relatives.
Neuroprognostication at 72 hours post-resuscitationNeuroprognostication is crucial for making informed decisions about patient care, including the potential for recovery and the appropriateness of continuing life-support measures. In patients who remain comatose after the return of spontaneous circulation (about 50%), assessment of brain injury and neuroprognostication are of utmost importance, since they may impact the medical team's decision to pursue additional investigation.63 These patients should undergo daily neurological examination to ascertain the extent of neurological impairment that resulted from the SCA.63
Current evidence suggests that during the first 72 hours post-SCA, no combination of diagnostic tests results and/or clinical signs can reliably predict neurological outcomes.63,64 Therefore, 72 hours post-SCA is the temporal hallmark used to start multimodal neuroprognostication,10 and to determine which diagnostic tests should be pursued, especially when the decision to withdraw life-sustaining measures is made.63,64
Current guidelines recommend assessment of neuroprognostication with daily neurological examination, continuous / routine electroencephalography (EEG), non-contrast computed tomography (CT) of the brain and, if available, somatosensory evoked potentials (SSEP) and magnetic resonance imaging of the brain with diffusion weighted imaging (MRI-DWI)63. Predictors of good outcome include motor response withdrawal / localization; absence or small, isolated lesions on MRI-DWI 2-7 days from return of spontaneous circulation (ROSC); continuous background <12 hours from ROSC or reactive background <24 hours from ROSC on EEG and N20 amplitude > 4μV on SSEP >48 hours from ROSC63. Regarding predictors of poor outcome, the clinical picture must be consistent with severe, widespread brain injury and one of the following should be present: absent pupillary light response bilaterally >72 hours from ROSC; bilateral absent N20 waves on SSEP >48 hours from ROSC; diffuse pattern of loss of grey white differentiation and sulcal effacement on CT >48 hours from ROSC; diffuse pattern of restricted diffusion on MRI-DWI and background suppression or burst-suppression (with or without periodic discharges) on EEG >72 hours from ROSC63.
In the presence of predictors of good neurological outcome or indeterminate neurological prognosis, a longer period of support and continued observation is recommended.63 In the setting of unfavorable neurological prognosis, the decision to withdraw life-sustaining measures, involving patients’ surrogates and family members, is recommended.10
Advanced cardiac diagnostics and multidisciplinary reviewIf the patient presents predictors of good or indeterminate neurological outcome and the etiology of the SCA remains unknown, further investigation is warranted. At this stage of the protocol, we recommend involving a specialized arrhythmology team in the investigation. Additionally, cardiac magnetic resonance imaging with late gadolinium enhancement allows further characterization of ventricular morphology, function, and myocardial tissue, as well as the presence of scar/fibrosis, adding diagnostic value, particularly in cases of concealed cardiomyopathy.39,40
Holter recording over a period of 24–48 hours and signal-averaged ECG with late potentials detection can be useful and might contribute to the diagnosis of arrhythmogenic cardiomyopathy and PED.65,66
If the cause remains unknown, the decision to pursue additional investigations (e.g., electrophysiological study or drug provocative tests) should be taken after discussion with a specialized arrhythmology team, and local expertise and resources.
Clinical autopsyIn cases of unexplained SCD, a comprehensive clinical autopsy with extended cardiac evaluation is recommended in all patients.2 Autopsy should include full macroscopic examination and histopathology of all organs.2 This procedure should preferably be carried out by a cardiac pathologist, since subtle cardiomyopathy cases may be missed in standard autopsies.2 Tissue samples should be collected for pathological examination and DNA extraction for post-mortem genetic analysis, if applicable.67,68 When an autopsy suggests possible heritable cardiac disease, it is recommended to refer first-degree relatives for cardiac assessment at a specialized clinic.2
Heritable cardiac disease: genetic testing and family referralGenetic testing can identify cardiac heritable diseases, with diagnostic and management implications for patients and their relatives. In 25–49% of cases of SCD, a genetic cardiac disease can be identified, especially in younger patients (<50 years).2 Additionally, a diagnostic yield of an underlying genetic cardiac etiology can range from 18 to 53% upon integration of clinical and genetic evaluation of unexplained SCD patients’ relatives.69,70
The widespread use of genetic testing has allowed expansion of the worldwide gene databases, enabling better management of SCA survivors and their families. However, it should always be used with caution and in recommended circumstances, because its interpretation may prove challenging due to the existence of a wide range of gene mutations and variable gene expression and penetrance.
To improve diagnostic yield, genetic testing should be used if the cause is known or suspected to be genetic/heritable.2 If a phenotype is suspected after integration of all clinical findings, a phenotype-directed panel should be used, preferably in specialized centers, with some series reporting pathogenic mutations in approximately one-third of cases.69,70
If there is no phenotype suspicion, genetic testing with whole exome sequencing is recommended if the decedent is younger than 50 years and/or the circumstances of the SCA and family history support the presence of a heritable syndrome.69,70 Testing relatives should then be offered if a pathogenic or likely pathogenic variant is identified. Although hypothesis-free post-mortem genetic testing using whole exome or genome sequencing is not recommended according to recent guidelines,2 it is used in our center due to ease of access and the possibility of reanalysis. The interpretation of the results must involve the medical genetics team.
Finally, referral of patients with pathogenic or likely pathogenic mutations and their first-degree relatives to medical genetic evaluation is indicated.2 It is of upmost importance that clinicians discuss genetic evaluation in a multidisciplinary setting (e.g., in a cardiogenetics team). After explaining benefits, limitations and potential implications to relatives, genetic testing should be done in a shared decision-making fashion and considering local ethical guidelines.
ConclusionsResearch into SCA remains a difficult challenge in daily clinical practice due to the heterogeneity of cardiac and noncardiac causes, often with complex clinical presentations. Our protocol aims to address this problem by providing a structured and comprehensive framework for the systematic investigation of SCA etiology in patients admitted to the emergency department or ICU. We support a multidisciplinary approach, incorporating various medical specialties (Intensive Care, Cardiology, Medical Genetic and Pathology) involved in the care of post-SCA patients.
CRediT authorship contribution statementThe authors João Cravo and Daniel Inácio Cazeiro wrote the manuscript and contributed equally to this work. The remaining authors provided their expert input and contributed to the revision of the manuscript.
Ethical considerationsNo ethical approval was required.
FundingNo authors received financial support for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.