Recent randomized controlled trials have evaluated the benefit of extended antithrombotic therapy in secondary prevention of acute coronary syndrome (ACS). However, the numerous and strict enrollment criteria may limit the validity and reproducibility of the published results in clinical practice. Our goal was to estimate the eligibility for participation in two randomized clinical trials in a group of patients followed for ACS.
MethodsWe applied the enrollment criteria of two randomized clinical trials (PEGASUS and COMPASS) to consecutive patients who underwent percutaneous coronary intervention in an ACS registry between January 2016 and June 2017.
ResultsA total of 780 patients were included in the final analysis. The proportion of patients fulfilling the trial enrollment criteria was 35.9% for PEGASUS and 32.1% for COMPASS. The proportion of patients eligible for both trials was 17.7% and 49.7% of patients were eligible for at least one trial. The need for anticoagulant therapy was the most common reason for exclusion on the PEGASUS criteria (46.2%) and the presence of high bleeding risk was the most common reason for exclusion on the COMPASS criteria (61.5%).
ConclusionsApproximately 50% of real-world patients are not eligible for the antithrombotic strategies applied in these trials. Since this non-eligible population is at greater risk of events, further studies are needed to confirm the applicability of these strategies.
Foram publicados recentemente os resultados dos ensaios clínicos aleatorizados que avaliaram o benefício da extensão da terapêutica antitrombótica na prevenção secundária da síndrome coronária aguda. Contudo, os variados e restritos critérios de elegibilidade usados poderão limitar a validade e a reprodutibilidade dos resultados publicados na prática clínica. O objetivo deste estudo consiste em estimar a elegibilidade para inclusão nos ensaios clínicos aleatorizados de terapêutica antitrombótica numa coorte de doentes admitidos por síndrome coronária aguda.
MétodosForam aplicados os critérios de inclusão e exclusão de dois ensaios clínicos (PEGASUS e COMPASS) a um coorte de doentes consecutivos incluídos num registo de síndromes coronárias agudas e submetidos a intervenção coronária percutânea entre janeiro de 2016 e junho de 2017.
ResultadosForam incluídos 780 doentes para análise final. A proporção de doentes que cumpriram os critérios de elegibilidade foi de 35,9% para o ensaio PEGASUS e 32,1% para o ensaio COMPASS. A proporção de doentes elegíveis para ambos os ensaios clínicos foi de 17,7%; 49,7% dos doentes cumpriram critérios de elegibilidade para pelo menos um ensaio clínico. Os motivos mais frequentes para exclusão dos estudos foram a necessidade de terapêutica anticoagulante (46,2%) no estudo PEGASUS e a presença de elevado risco hemorrágico (61,5%) no estudo COMPASS.
ConclusõesAproximadamente 50% dos doentes do «mundo real» não são elegíveis para as estratégias antitrombóticas estudadas nestes ensaios. Sendo a população não elegível a que apresenta maior risco de eventos, é necessária maior evidência para confirmar a aplicabilidade das estratégicas estudas.
Currently, the standard antithrombotic therapy for secondary cardiovascular prevention after an acute coronary syndrome (ACS) consists of dual antiplatelet therapy (DAPT) for up to 12 months.1–3 However, there is still much uncertainty regarding the ideal duration of antithrombotic therapy, particularly when considering patients’ different ischemic and bleeding risk profiles.4–6 Beyond 12 months, there is still a considerable risk of ischemic events, and a small number of studies have examined the outcomes of extended DAPT.7–11 This led to the publication of a focused update on DAPT by the European Society of Cardiology and the European Association of Cardio-Thoracic Surgery.12
Until recently, the role of the new oral anticoagulants and ticagrelor in long-term regimens in patients with ACS was uncertain. However, these drugs were assessed in two recent randomized clinical trials (RCTs) that set out to improve on the existing standard of care for secondary cardiovascular prevention after an ACS. Both trials indicated significant improvements regarding cardiovascular outcomes and death. However, the large number of eligibility criteria used in these RCTs may limit the applicability of their results in clinical practice.
The purpose of our study was to assess the eligibility for long-term use of low-dose (LD) ticagrelor (60mg twice daily) or LD rivaroxaban (2.5mg twice daily) according to the enrollment criteria of two trials of extended antithrombotic treatment after ACS, Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction (PEGASUS-TIMI 54)13 and Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease (COMPASS),14 respectively, in a cohort of consecutive ACS patients.
MethodsPatient selectionThis was a retrospective cohort study based on consecutive patients admitted for ACS to a single center in an 18-month period (January 2016 to June 2017). Patients were included if: (1) they were diagnosed with ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI), or unstable angina (UA), and (2) were followed for at least 12 months after discharge. The study protocol was reviewed and approved by the ethics committee.
Work-upPatients’ electronic medical records regarding in-hospital treatment and subsequent appointments after discharge were reviewed to obtain data on demographics, medical history, physical examination, medical exams, therapies, in-hospital complications and readmissions. Follow-up information was obtained from the national heath registry. Variables that could potentially influence mortality and ischemic events were recorded for each patient, including age, gender, serum creatinine, cardiac arrest, ST-segment deviation on the electrocardiogram, abnormal cardiac enzymes, Killip class, congestive heart failure, hypertension, diabetes, prior stroke, transient ischemic attack or thromboembolism, and evidence of previous vascular disease. In this study, we used the ATRIA and HAS-BLED scores for bleeding risk prediction instead of the CRUSADE score, to avoid possible data collection bias, since this score includes blood pressure and heart rate on admission. Since the bleeding risk assumption in the COMPASS trial was left to the principal investigator's discretion, we deemed it significant if ≥5.0% (ATRIA score ≥5 or HAS-BLED score ≥3).
Eligibility for participation in the trialsWe applied the enrollment criteria of PEGASUS-TIMI 5413 and COMPASS.14 The inclusion and exclusion criteria of the two RCTs (Table 1) were investigated for each patient and eligibility for participation in the stated trials was determined.
Inclusion and exclusion criteria of the two trials.
| PEGASUS-TIMI 54 | COMPASS | |
|---|---|---|
| Inclusion criteria | ||
| 1) Age ≥50 years2) MI occurring 1-3 years prior to randomization and at least 1 of the following risk factors:a. Age >65 yearsb. Diabetesc. Significant multivessel CADd. CrCl <60 ml/min/1.73 m23) Patient prescribed and tolerating aspirin 75-150 mg/day4) WOCP with a negative pregnancy test and willing to use a medically accepted method of contraception | 1) Age >65 years2) PAD3) CAD + age >65 years4) CAD + age <65 years and at least one of the following risk factors:a. Involvement of 2 vascular bedsb. Current smokerc. Diabetesd. CrCl <60 ml/min/1.73 m2e. Heart failuref. Non-lacunar ischemic stroke >1 month | |
| Exclusion criteria | ||
| Bleeding risk | ||
| History of intracranial bleeding | Exclude | Exclude |
| CNS tumor or vascular abnormality | Exclude | a |
| Intracranial or spinal cord injury within 5 years | Exclude | a |
| GI bleed in the past 6 months | Exclude | a |
| Major surgery within 30 days | Exclude | a |
| Ischemic stroke <1 month | Exclude | Exclude |
| Comorbidities | ||
| CrCl <15 ml/min/1.73 m2 | Exclude if requiring or anticipating need for dialysis | Exclude |
| Severe liver disease | Exclude | Exclude |
| Need for chronic OAC or LMWH | Exclude | Exclude |
| Coagulopathy | Exclude | Exclude |
| Risk of bradycardic events unless already treated with a permanent pacemaker | Exclude | --- |
| History of hypersensitivity to or known contraindication for rivaroxaban, aspirin, pantoprazole, or excipients, if applicable | --- | Exclude |
| Severe HF with known LVEF <30% or NYHA class III or IV symptoms | --- | Exclude |
| CABG in the past 5 years, unless the patient has experienced a spontaneous MI | Exclude | --- |
| Study participation | ||
| Need for continuous treatment with a proton pump inhibitor | --- | Exclude |
| Systemic treatment with strong inhibitors of both CYP 3A4 and p-glycoprotein or strong CYP 3A4 inducers | Exclude | Exclude |
| Planned coronary, cerebrovascular, or peripheral arterial revascularization | Exclude | --- |
| Planned use of ADP receptor blockers | Exclude | Exclude |
| Known non-cardiovascular disease associated with poor prognosis | Exclude | Exclude |
| Life expectancy <1 year | Exclude | Exclude |
| Pregnancy or lactation | Exclude | Exclude |
| WOCP not practicing an effective method of birth control | --- | Exclude |
| Concern for inability of the patient to comply with study procedures and/or follow-up | Exclude | --- |
| Concomitant participation in another study with investigational drug | Exclude if within 30 days | Exclude |
| Participation in previous study with ticagrelor if treated with ticagrelor | Exclude | --- |
| Involvement in the planning and/or conduct of the study | Exclude | --- |
| Previous assignment to treatment | Exclude | Exclude |
Bleeding risk assessment left to the principal investigator's discretion.
ADP: adenoside diphosphate; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CNS: central nervous system; COMPASS: Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease; CrCl: creatinine clearance; GI: gastrointestinal; HF: heart failure; LMWH: low molecular weight heparin; LVEF: ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association class; OAC: oral anticoagulation; PAD: peripheral arterial disease; PEGASUS-TIMI 54: Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction; WOCP: women of childbearing potential.
Continuous variables with normal distribution were expressed as means and standard deviation. Continuous variables with non-normal distribution were expressed as median and interquartile range (IQR). Normality was tested with the Kolmogorov-Smirnov test and Q-Q plot visual assessment. Categorical variables were expressed as percentages. Differences in clinical and laboratory parameters between eligible and ineligible patients were assessed by the Student's t test or one-way analysis of variance for continuous variables with normal distribution, and the Mann-Whitney U test for continuous variables with non-normal distribution. Categorical variables were compared using the chi-square test, with Yates’ correction when appropriate. All statistical analyses were performed using commercially available software (IBM SPSS for Windows, version 23.0). Statistical significance was defined as p<0.05.
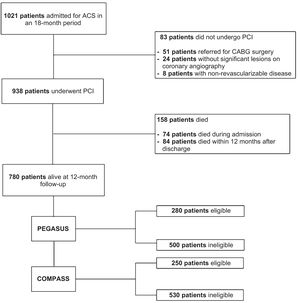
ResultsA total of 1021 patients were admitted for ACS during the 18-month inclusion period. Of those, 938 underwent PCI. Median length of stay was 6.0 days (IQR 3.0-11.0). Regarding the type of ACS, 30.3% were classified as STEMI, 54.4% as NSTEMI and 15.3% as UA. In-hospital mortality was 7.9%. Mortality within 12 months of discharge was 9.7%.
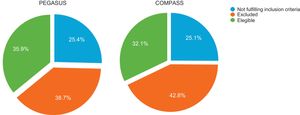
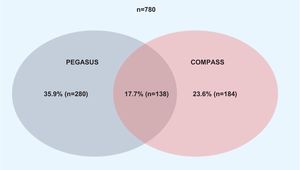
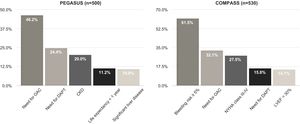
A total of 780 patients were screened for enrollment in the RCTs after 12 months of follow-up (Figure 1). Baseline characteristics are summarized in Table 2. The proportion of patients fulfilling the trial enrollment criteria was not significantly different between the trials (35.9%, n=250 for PEGASUS vs. 32.1%, n=280 for COMPASS, p=0.113) (Figure 2). The proportion of patients eligible for both RCTs was 17.7% (n=138) and 49.7% (n=388) of patients were eligible for at least one of them (Figure 3). The main reasons for exclusion from each RCT were the need for oral anticoagulation (OAC) in PEGASUS (46.2%, n=231) and the presence of high bleeding risk in COMPASS (61.5%, n=326). The need for OAC was also an important reason for exclusion in COMPASS (32.1%, n=170) and occurred in 22.4% (n=210) of the total study population. The main reasons for OAC are summarized in Table 3. Other significant reasons for exclusion are illustrated in Figure 4. Patients not fulfilling enrollment criteria for either RCT (58.4%) were older (71.4±9.7 vs. 65.2±10.2 years, p<0.0001) and had worse renal function (creatinine clearance 47.8±28.8 vs. 73.9±21.2ml/min/1.73 m2, p<0.0001), lower left ventricular ejection fraction (LVEF) (44.4±11.4 vs. 51.9±7.5%, p<0.0001) and longer hospital stay (7 [IQR 4-13] vs. 5 days [IQR 3-8], p<0.0001). The main differences between patients eligible and ineligible for both RCTs are summarized in Table 4. Compared with non-eligible patients (n=388), patients who were eligible for both RCTs (n=138) were typically younger (64.4±8.7 vs. 70.3±10.1 years, p<0.001) and had higher LVEF (53.8±5.1 vs. 44.6±11.5%, p<0.001), higher creatinine clearance (73.6±18.2 vs. 47.7±29.1ml/min/1.73 m2), and lower prevalence of cardiovascular risk factors (Table 5).
Patient characteristics after 12 months of follow-up (n=780).
| Age, years, mean ± SD | 67.7±10.5 |
| Male, n (%) | 512 (65.6) |
| Hypertension, n (%) | 678 (86.9) |
| BMI, kg/m2, mean ± SD | 27.9±4.3 |
| Hypercholesterolemia, n (%) | 550 (70.5) |
| Current smoker, n (%) | 160 (20.5) |
| Diabetes, n (%) | 254 (32.6) |
| Previous PCI, n (%) | 201 (25.7) |
| Previous CABG, n (%) | 71 (9.1) |
| Previous stroke or TIA, n (%) | 88 (11.2) |
| PAD, n (%) | 56 (7.1) |
| HF, n (%) | 126 (16.1) |
| CrCl <60 ml/min/1.73 m2, n (%) | 148 (18.9) |
| Type of ACS | |
| STEMI, n (%) | 230 (29.5) |
| NSTEMI, n (%) | 398 (51.0) |
| UA, n (%) | 152 (19.4) |
ACS: acute coronary syndrome; BMI: body mass index; CABG: coronary artery bypass grafting; CrCl: creatinine clearance; HF: heart failure; NSTEMI: non-ST segment elevation myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction; TIA: transient ischemic attack; UA: unstable angina.
Differences in patient characteristics according to eligibility for the trials.
| PEGASUS | COMPASS | |||||
|---|---|---|---|---|---|---|
| Eligible (n=280) | Ineligible (n=500) | p | Eligible (n=250) | Ineligible (n=530) | p | |
| Age, years, mean ± SD | 66.9±9.3 | 68.2±11.0 | 0.124 | 62.8±9.9 | 70.1±9.8 | <0.0001 |
| Male, n (%) | 192 (68.6%) | 328 (65.6%) | 0.222 | 196 (78.4%) | 324 (61.1%) | <0.0001 |
| Symptomatic HF, n (%) | 10 (7.1%) | 53 (21.2%) | <0.0001 | 9 (7.2%) | 54 (23.4%) | 0.001 |
| LVEF, % | 52.1±7.7 | 46.2±10.9 | <0.0001 | 52.8±5.9 | 46.2±11.2 | <0.0001 |
| CKD, n (%) | 5 (3.6%) | 69 (27.6%) | <0.0001 | 6 (4.8%) | 68 (25.7%) | <0.0001 |
| CrCl, ml/min/1.73 m2, mean ± SD | 71.2±21.6 | 55.1±30.4 | <0.0001 | 76.8±18.6 | 53.4±29.4 | <0.0001 |
| Hypertension, n (%) | 222 (79.3%) | 444 (88.8%) | <0.0001 | 214 (85.6%) | 452 (85.3%) | 0.669 |
| Diabetes, n (%) | 76 (27.1%) | 186 (37.2%) | 0.004 | 38 (15.2%) | 224 (42.3%) | <0.0001 |
| Hypercholestrolemia, n (%) | 190 (67.9%) | 358 (71.6%) | 0.018 | 176 (70.4%) | 372 (70.2%) | 0.107 |
| Current smoker, n (%) | 58 (20.7%) | 102 (20.4%) | 0.493 | 88 (35.2%) | 72 (13.6%) | <0.0001 |
| STEMI, n (%) | 58 (20.7%) | 172 (34.4%) | <0.0001 | 48 (19.2%) | 182 (34.3%) | <0.0001 |
| NSTEMI, n (%) | 164 (58.6%) | 242 (48.4%) | 0.007 | 132 (52.8%) | 274 (51.7%) | 0.417 |
| UA, n (%) | 58 (20.7%) | 94 (18.8%) | 0.511 | 70 (28.0%) | 82 (15.5%) | <0.0001 |
| Previous PCI, n (%) | 120 (42.9%) | 210 (42.0%) | 0.542 | 138 (55.2%) | 192 (36.2%) | <0.0001 |
| Previous CABG, n (%) | 28 (10.0%) | 108 (21.6%) | <0.0001 | 12 (4.8%) | 124 (23.4%) | <0.0001 |
| Anemia, n (%) | 39 (27.8%) | 135 (54.0%) | <0.0001 | 11 (8.8%) | 163 (61.5%) | <0.0001 |
| Hemoglobin (g/dl) | 11.8±2.5 | 10.4±2.9 | 0.001 | 13.1±1.2 | 10.2±3.4 | <0.0001 |
| ATRIA score, mean ± SD | 2.2±2.1 | 3.9±2.8 | <0.0001 | 1.4±1.1 | 4.2±2.8 | <0.0001 |
| HAS-BLED score, mean ± SD | 2.3±0.9 | 3.1±1.1 | <0.0001 | 1.9±0.6 | 3.3±1.0 | <0.0001 |
| HEMORR2HAGES score, mean ± SD | 1.6±0.8 | 2.1±1.1 | <0.0001 | 1.4±0.7 | 2.2±1.1 | <0.0001 |
| GRACE score | 115±25 | 132±31 | <0.0001 | 105±21 | 136±29 | <0.0001 |
CABG: coronary artery bypass grafting; CKD: chronic kidney disease; CrCl: creatinine clearance; HF: heart failure; NSTEMI: non-ST segment elevation myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction; UA: unstable angina.
Characteristics of patients eligible for both trials and those non-eligible for either trial.
| Eligible for both trials (n=138) | Ineligible for either trial (n=500) | p | |
|---|---|---|---|
| Age, years, mean ± SD | 64.4±8.7 | 70.3±10.1 | <0.001 |
| Male, n (%) | 102 (73.9%) | 234 (60.3%) | 0.003 |
| LVEF (%) | 58.1±5.1 | 44.6±11.5 | <0.001 |
| CrCl, ml/min/1.73 m2, mean ± SD | 73.6±18.2 | 47.7±29.1 | <0.001 |
| Hypertension, n (%) | 110 (79.7%) | 352 (90.7%) | <0.001 |
| Diabetes, n (%) | 28 (20.1%) | 176 (45.3%) | <0.001 |
| Hypercholestrolemia, n (%) | 90 (65.2%) | 274 (70.1%) | 0.363 |
| STEMI, n (%) | 26 (18.8%) | 150 (38.6%) | <0.001 |
| NSTEMI, n (%) | 86 (62.3%) | 196 (48.4%) | 0.017 |
| UA, n (%) | 26 (18.8%) | 50 (12.9%) | 0.061 |
| ATRIA score, mean ± SD | 1.4±1.0 | 4.6±2.8 | <0.0001 |
| HAS-BLED score, mean ± SD | 1.6±0.6 | 3.5±0.9 | <0.001 |
| GRACE score, mean ± SD | 105.5±20.0 | 139.9±29.6 | <0.001 |
CrCl: creatinine clearance; HF: heart failure; NSTEMI: non-ST segment elevation myocardial infarction; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction; UA: unstable angina.
Our results show that, when the eligibility criteria of the PEGASUS-TIMI 54 and COMPASS trials are applied to a real-world population of consecutive patients with previous ACS, only around one third are eligible for the novel long-term antithrombotic therapies. The authors suggest that as soon as these drugs are available in Portugal, prospective registries should be established to assess their overall benefit in patients not eligible for the two RCTs under study, as has been done with the new oral anticoagulants.
It can be difficult to perform external validation and to determine the applicability of RCTs, since the strict enrollment criteria they use often result in a highly selected population that is usually at lower risk than patients in everyday practice, and who may not be representative of real-world populations regarding characteristics, risk factors and outcomes.15–18
It is known that more intense antithrombotic therapy reduces ischemic complications, but this comes at the cost of more bleeding events.13,19,20 Trial designers therefore often aim to exclude patients with high bleeding risk. However, our results show that real-world patients with previous ACS have higher risk scores for both cardiovascular and bleeding events than those enrolled in the PEGASUS and COMPASS trials.
As previously reported, patients who are excluded from RCTs usually represent high-risk subgroups with poor outcomes.21,22 In our study, patients who met the exclusion criteria for both the RCTs under study were those with the highest ischemic risk, as indicated by the significant differences in GRACE scores, and hence those who would most likely benefit from extended antithrombotic therapy. However, compared with eligible patients, they were older and had higher bleeding risk scores and more severely impaired renal function. This paradoxical relation between bleeding and thrombotic risk is reported in the literature.23,24
The need for chronic anticoagulation was an important reason for exclusion, found in 22.4% of the total study population (46.2% of excluded patients on the PEGASUS criteria and 32.1% of excluded patients on the COMPASS criteria). The exclusion of patients due to high bleeding risk is in line with other registries, however the proportion of patients ineligible for the COMPASS trial due to bleeding risk in our population was higher (28.9% in the REACH registry and 17% in the FAST-MI registry).25,26 This could be explained by the addition of bleeding score risks in our population, since the bleeding risk assumption in the COMPASS trial was left to the investigators’ discretion.14 Nevertheless, in both trials, patients had to undergo at least 12 months of DAPT without bleeding events to be eligible. This may have selected a specific population with lower bleeding risk.
In our study, we applied the enrollment criteria to patients 12 months after an ACS. This population also does not correspond to that reported in the COMPASS trial, inasmuch as although 70% of the patients enrolled had a previous myocardial infarction, around half of these had occurred more than five years prior to randomization, which may have altered their long-term ischemic outcomes compared with a real-world population. On the other hand, more than half of ACS patients in PEGASUS had STEMI and were at high risk for ischemic events. Hence, patients at low risk for ischemic events may not benefit as much as seen in the trial.
This real-world cohort registry included patients with similar characteristics to those reported in other published studies on the maintenance of prolonged DAPT27,28 that reported no significant differences in mortality, despite reductions in ischemic events, due to significant increases in bleeding events.
The patients eligible for trial enrollment were similar across the RCTs, suggesting that the deciding factor in choosing between LD ticagrelor or LD rivaroxaban is not specific patient characteristics, but mainly the time elapsed since the last ACS.
To our knowledge, this is the first study to apply the enrollment criteria of the extended antithrombotic therapy trials to a Portuguese population, and the first anywhere to apply the criteria from both trials in the same population. The strength of the study lies in the inclusion of a reasonable number of unselected and consecutively enrolled patients. However, the limitations resulting from a single-center observation should also be considered. Further studies enrolling high-risk patients are needed to clarify the benefit of these interventions in more representative populations. As these therapies are still not available in Portugal, the authors suggest the development of prospective registries after these drugs are approved and enter the market, to assess their overall benefit in patients not eligible for the RCTs under study, as has been done with the new oral anticoagulants.29
ConclusionsPatients eligible for trial enrollment in PEGASUS and COMPASS are only partly representative of the real population with a recent history of ACS. Hence, their results are limited if applied to the real-world patients that are at greater risk of future events, who represent approximately half of the total population. Further studies with less restrictive enrollment criteria are needed to determine if this approach is still beneficial in these patients.
Conflicts of interestDaniel Faria has received speaker fees from AstraZeneca, Miguel Santos has received speaker fees from AstraZeneca and Bayer, and Sérgio Baptista has received research grants from AstraZeneca and Bayer.