Atrial fibrillation (AF) is the most common form of arrhythmia worldwide and a significant health burden. Edoxaban, a recent novel oral anticoagulant (NOAC), is being investigated in the European real-world ETNA-AF study of patients with non-valvular atrial fibrillation (NVAF). The aim of this study was to characterize the Iberian edoxaban-treated cohort of ETNA-AF at baseline and to compare it with previously retrieved Portuguese data.
MethodsPatients with NVAF treated with edoxaban and followed in Portuguese and Spanish centers were consecutively enrolled between June 2017 and January 2018. Only patients with a previous clinical decision to receive edoxaban were included. Patients’ baseline demographic and clinical parameters, medical history, and AF-related characteristics were retrieved.
ResultsA total of 892 NVAF patients, with a mean age of 73.9 years, were included, 75.3% of whom received high-dose (60 mg) and 24.7% low-dose (30 mg) edoxaban. Most patients (55.9%) were male. Of the patients receiving 30 mg and 60 mg edoxaban, 55.9% and 37.9%, respectively, had an estimated CHA2DS2-VASc score ≥4. Previous bleeding event rates were low, with a predominance of clinically relevant non-major bleeding (1.9%). Most patients (47.5%) with NVAF had paroxysmal AF, followed by 26.4% with permanent AF. Median overall CHA2DS2-VASc score was 3.0 and median HAS-BLED score was 2.0. Previous treatments mostly included vitamin K antagonists (35.7%). A considerably higher proportion of patients on low-dose edoxaban required dose adjustments (71.4% vs. 8.6%). Overall adherence to label dosing recommendations was 86.5%.
ConclusionsThis study provides valuable data on disease and patient profiles and will provide valuable insights into disease management and progression, as well as the safety, effectiveness, and patterns of cardiovascular events associated with edoxaban.
A fibrilhação auricular (FA) é a arritmia mais comum globalmente e associa-se a um substancial ónus de saúde. O novo anticoagulante oral (NOAC) edoxabano está a ser investigado no estudo europeu de vida real ETNA-AF em doentes com fibrilhação auricular não valvular (FANV). O objetivo deste estudo é caracterizar a coorte ibérica do ETNA-AF na base de dados e compará-la com dados portugueses prévios.
MétodosDoentes com FANV tratados com edoxabano e seguidos em centros portugueses e espanhóis foram consecutivamente incluídos no estudo entre junho de 2017 e janeiro de 2018. Apenas doentes com indicação prévia para receber edoxabano foram incluídos e os seus dados clínicos e demográficos, história clínica e características relacionadas com a FA foram registados.
ResultadosFora, incluídos 892 doentes com FANV, com média de 73,9 anos, 75,3% dos quais receberam a dose elevada (60 mg) e 24,7% a dose reduzida (30 mg) de edoxabano. A maioria dos doentes (55,9%) era do sexo masculino. Dos doentes que receberam 30 mg e 60 mg de edoxabano, 55,9% e 37,9%, respetivamente, tinham CHA2DS2-VASc estimado ≥ 4. As taxas de eventos hemorrágicos prévios foram baixas, com predominância de hemorragia não major clinicamente relevante (1,9%). A maioria (47,5%) dos doentes com FANV tinha FA paroxística, seguidos de 26,4% com FA permanente. A mediana de CHA2DS2-VASc global foi 3,0 e a mediana de HAS-BLED, 2,0. Os tratamentos prévios incluíram sobretudo VKAs (35,7%). Uma proporção consideravelmente maior de doentes a receber dose reduzida de edoxabano necessitou de ajustes de dose (71,4% versus 8,6%). A adesão global às recomendações de dose foi de 86,5%.
ConclusõesEste estudo fornece dados importantes sobre a doença e o perfil dos doentes e possibilitará considerações futuras acerca da gestão e progressão da doença, bem como de segurança, efetividade e padrões de eventos cardiovasculares associados a edoxabano.
Atrial fibrillation (AF) is the most common form of arrhythmia worldwide and represents a significant health burden.1 Its prevalence increases with age and with conditions such as hypertension, valvular heart disease, obesity, diabetes, and chronic kidney disease (CKD).2
AF is associated with an increased risk of ischemic stroke, heart failure, and all-cause mortality,3–5 as well as hospital admissions, with 10-40% of patients hospitalized annually,6 which has been shown to be an independent risk factor for cardiovascular (CV) death in AF patients.7 With the aging of populations and a higher incidence of CV disease in the elderly, the number of patients with AF is expected to grow exponentially over the coming decades.8 Furthermore, patients with AF have poorer quality of life and may experience vascular dementia and a decline in cognitive function despite anticoagulation for stroke prevention.2
The prevalence of AF is reported to be increasing steadily worldwide, but there is considerable variation between studies and countries.6 In Europe, the USA, and Australia, AF has an estimated prevalence of 1-4%, but it is markedly lower in Asia (0.49-1.9%).6
In Portugal in 2010, the large-scale cross-sectional observational FAMA study reported a 2.5% prevalence of AF in individuals aged 40 years and over.9 No differences were found between genders, but AF prevalence increased with age, and was significantly higher in the ≥70-year-old group.9 Subsequently, two other studies – Primo et al.10 and the SAFIRA study11 – reported a 12.4% overall prevalence of AF/atrial flutter in a younger population of individuals aged ≥40 years10 and a 9% prevalence of AF in an older population, aged ≥65 years,11 respectively.
Differences were also found in antithrombotic treatment use between these studies. SAFIRA reported that 56.3% of patients were non-anticoagulated,11 higher than the 37.8% reported in FAMA.9
The significantly higher incidence of stroke and systemic embolism observed in AF patients can be reduced with appropriately adjusted anticoagulant therapy. Although vitamin K antagonists (VKAs) have been recognized to effectively decrease the risk of thromboembolic events in these patients, their effect is influenced by many factors and new options were needed. The development of novel oral anticoagulants (NOACs) in the last few years has offered new treatment opportunities for clinicians.
Edoxaban is the most recent agent of this new class. Its approval was based on a large phase III trial of NOACs for stroke prevention in patients with non-valvular atrial fibrillation (NVAF), ENGAGE AF-TIMI 48, in which edoxaban was associated with similar efficacy for preventing stroke or systemic embolism and a significant reduction in bleeding events and death from CV causes compared with warfarin.12
ETNA-AF13 is a large multicenter post-authorization observational study of patients with NVAF treated with edoxaban in routine clinical practice in 10 different European countries and care settings (primary and secondary care and different specialties). Its aim is to investigate the risks and benefits of edoxaban use in patients with NVAF in a real-world clinical setting and to gain insight into the drug's safety (bleeding, liver adverse events, all-cause mortality and other drug-related adverse events). Due to its non-interventional nature, this is a non-randomized study.
The present paper aims to describe and characterize the Iberian (Portuguese and Spanish) edoxaban-treated cohort of ETNA-AF at baseline and to compare it with data previously retrieved on Portuguese patients, mainly from the SAFIRA study.
MethodsETNA-AF is an ongoing observational prospective cohort study of patients with NVAF in Europe. The Iberian cohort included patients from Portuguese and Spanish centers (see Appendix).
Patient selectionPatients with NVAF followed in selected Portuguese and Spanish centers, treated with edoxaban according to the Summary of Product Characteristics (SmPC), aged ≥18 years, who provided written informed consent to participate, were consecutively enrolled in the study between August 2017 and March 2018.
To avoid treatment choice bias, patients were only included after the attending physician had made the clinical decision to prescribe edoxaban, so the physician's treatment option was not influenced.
Patients were excluded from the study if they (i) were simultaneously participating in an interventional study; (ii) had missing baseline data; (iii) had baseline data retrieved after the cut-off date; (iv) were missing information on edoxaban treatment at baseline; or (v) did not fulfill the regional eligibility criteria defined in the SmPC for each region.
Study protocolThe protocol and statistical analysis plan have been described previously.13
A one-year patient recruitment period was planned, with the possibility of extension if patient recruitment numbers were not reached. Patient data were documented at baseline, at one annual data documentation point during the four-year follow up, and at final assessment.
Patients who permanently discontinued edoxaban during the observation period will continue to be followed annually for an additional two-year period, or until the end of the observation period (whichever comes first).
The primary outcome measure of the ETNA-AF study is the percentage of patients experiencing real-world safety data events within four years, which include bleeding events (including intracranial hemorrhage), drug-related adverse events such as liver adverse events, and cardiovascular and all-cause mortality. The secondary outcome measures are (i) percentage of patients with patient-relevant outcomes within four years, including ischemic and hemorrhagic stroke, systemic embolic events, transient ischemic attack, major adverse cardiovascular events, venous thromboembolism, acute coronary syndrome, and hospitalizations related to a CV condition; and (ii) percentage of patients compliant with edoxaban within four years (compliance categories: always, almost always, most of the time, less than half the time, unknown).
Patient demographic and clinical parameters, as well as medical history, were collected at baseline. Type of NVAF at first diagnosis and at baseline was also retrieved.
Stroke risk assessment was performed using the CHADS214 and CHA2DS2-VASc15–17 scores, and bleeding risk was estimated with the HAS-BLED score.18 Patients were considered high-risk if they had at least one of the following conditions: prior stroke, prior major bleeding, prior intracranial hemorrhage, or CHA2DS2-VASc ≥4.
The ETNA-AF study is registered under ClinicalTrials.gov number NCT02944019.
Statistical analysisA descriptive analysis of numerical variables was performed, consisting of estimates of the mean and respective standard deviation. Categorical variables were expressed as absolute and relative frequencies. The analysis was performed with IBM SPSS version 25.
ResultsA total of 960 patients from Portugal and Spain were prescribed edoxaban by their attending physicians and were initially enrolled in the study. Of these, 68 patients were excluded for not meeting eligibility criteria: two were missing baseline data, 62 were missing information on edoxaban treatment at baseline, six did not fulfill the regional eligibility criteria, and four were excluded for other reasons. A total of 892 patients were included in the study and are currently undergoing follow-up and being analyzed for study outcome measures.
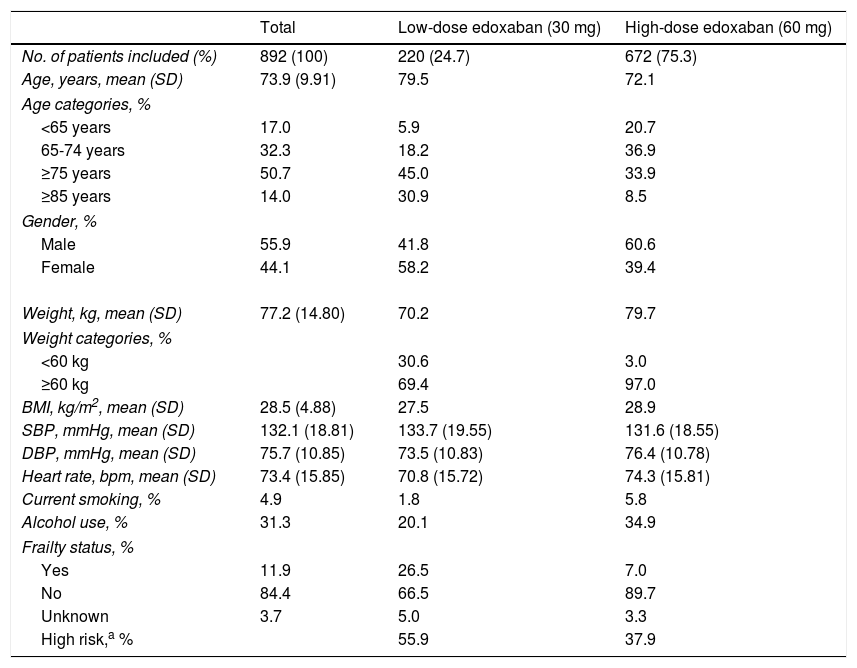
Demographic and clinical characteristicsBaseline demographic and clinical characteristics of patients included in ETNA-AF Iberia are depicted in Table 1. A total of 672 patients (75.3%) received high-dose (60 mg) edoxaban and 220 patients (24.7%) received the lower dose (30 mg).
Baseline demographic and clinical characteristics of the study population.
| Total | Low-dose edoxaban (30 mg) | High-dose edoxaban (60 mg) | |
|---|---|---|---|
| No. of patients included (%) | 892 (100) | 220 (24.7) | 672 (75.3) |
| Age, years, mean (SD) | 73.9 (9.91) | 79.5 | 72.1 |
| Age categories, % | |||
| <65 years | 17.0 | 5.9 | 20.7 |
| 65-74 years | 32.3 | 18.2 | 36.9 |
| ≥75 years | 50.7 | 45.0 | 33.9 |
| ≥85 years | 14.0 | 30.9 | 8.5 |
| Gender, % | |||
| Male | 55.9 | 41.8 | 60.6 |
| Female | 44.1 | 58.2 | 39.4 |
| Weight, kg, mean (SD) | 77.2 (14.80) | 70.2 | 79.7 |
| Weight categories, % | |||
| <60 kg | 30.6 | 3.0 | |
| ≥60 kg | 69.4 | 97.0 | |
| BMI, kg/m2, mean (SD) | 28.5 (4.88) | 27.5 | 28.9 |
| SBP, mmHg, mean (SD) | 132.1 (18.81) | 133.7 (19.55) | 131.6 (18.55) |
| DBP, mmHg, mean (SD) | 75.7 (10.85) | 73.5 (10.83) | 76.4 (10.78) |
| Heart rate, bpm, mean (SD) | 73.4 (15.85) | 70.8 (15.72) | 74.3 (15.81) |
| Current smoking, % | 4.9 | 1.8 | 5.8 |
| Alcohol use, % | 31.3 | 20.1 | 34.9 |
| Frailty status, % | |||
| Yes | 11.9 | 26.5 | 7.0 |
| No | 84.4 | 66.5 | 89.7 |
| Unknown | 3.7 | 5.0 | 3.3 |
| High risk,a % | 55.9 | 37.9 | |
BMI: body mass index; bpm: beats per minute; DBP: diastolic blood pressure; SBP: systolic blood pressure; SD: standard deviation.
At baseline, patients had a mean age of 73.9 years, with those receiving low-dose edoxaban being older (mean 79.5 years) than those receiving the higher dose. Overall, more than half of patients included (50.7%) were aged ≥75 years, this also being the most prevalent age group in patients prescribed low-dose edoxaban (45.0%). Most patients receiving high-dose edoxaban (36.9%) were in the 65-74 age group.
Patients prescribed edoxaban in clinical practice were mostly male (55.9%), but more female patients (58.2%) received low-dose and more male patients (60.6%) received high-dose edoxaban. Patients on high-dose edoxaban had higher mean weight (79.7 kg) and higher body mass index (28.9 kg/m2) than their low-dose counterparts.
This population had a mean systolic blood pressure (BP) of 132.1 mmHg and a mean diastolic BP of 75.7 mmHg, with patients in the low-dose group reporting higher systolic and lower diastolic values than those in the high-dose group. Mean heart rate was 73.4 beats per minute (bpm), which was higher in patients on high-dose treatment (74.3 vs. 70.8 bpm).
A minority of patients were current smokers (4.9%) or alcohol users (31.3%), with more patients in the high-dose group reporting these habits than in the low-dose group.
According to the European Society of Cardiology guidelines for the management of atrial fibrillation, many patients present AF at older ages (e.g. >75 or >80 years) and are considered ‘frail’ patients, due to the presence of comorbidities such as dementia, tendency to falls, CKD, anemia, hypertension, diabetes, and cognitive dysfunction.2 In the ETNA-AF Iberia study, a minority of NVAF patients were considered frail (11.9%), with the proportion of these patients being higher in the low-dose (26.5%) than in the high-dose (7.0%) edoxaban group.
A total of 55.9% and 37.9% patients receiving 30 mg and 60 mg edoxaban, respectively, were considered high-risk according to the estimated CHA2DS2-VASc score (≥4).
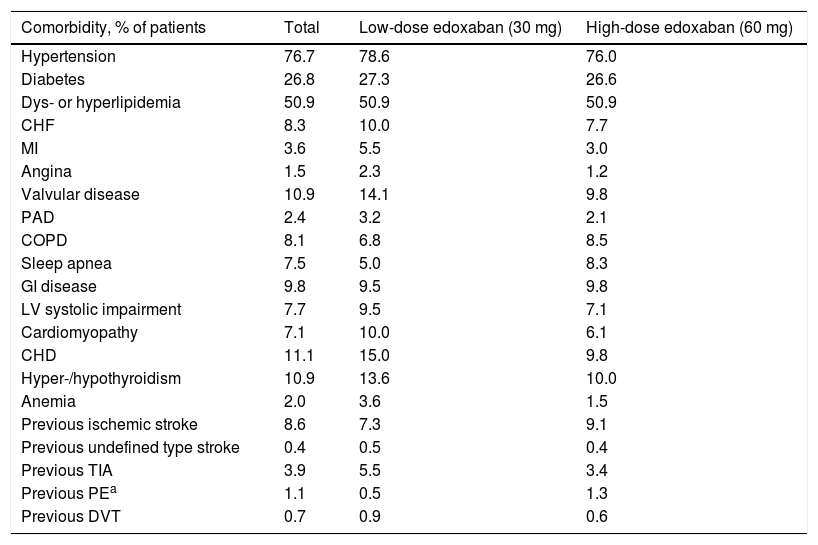
Medical historyThe most prevalent comorbidities at baseline in this cohort of patients with NVAF were hypertension (76.7%), dyslipidemia or hyperlipidemia (50.9%), and diabetes (26.8%), followed by coronary heart disease (11.1%), valvular disease, and hyper- or hypothyroidism (10.9% each) (Table 2). Patients who received low-dose edoxaban were more likely to have more comorbidities, except for chronic obstructive pulmonary disease, sleep apnea, gastrointestinal (GI) disease, ischemic stroke, and pulmonary embolism, which were less prevalent in this group than in the high-dose group.
Comorbidities in the study population.
| Comorbidity, % of patients | Total | Low-dose edoxaban (30 mg) | High-dose edoxaban (60 mg) |
|---|---|---|---|
| Hypertension | 76.7 | 78.6 | 76.0 |
| Diabetes | 26.8 | 27.3 | 26.6 |
| Dys- or hyperlipidemia | 50.9 | 50.9 | 50.9 |
| CHF | 8.3 | 10.0 | 7.7 |
| MI | 3.6 | 5.5 | 3.0 |
| Angina | 1.5 | 2.3 | 1.2 |
| Valvular disease | 10.9 | 14.1 | 9.8 |
| PAD | 2.4 | 3.2 | 2.1 |
| COPD | 8.1 | 6.8 | 8.5 |
| Sleep apnea | 7.5 | 5.0 | 8.3 |
| GI disease | 9.8 | 9.5 | 9.8 |
| LV systolic impairment | 7.7 | 9.5 | 7.1 |
| Cardiomyopathy | 7.1 | 10.0 | 6.1 |
| CHD | 11.1 | 15.0 | 9.8 |
| Hyper-/hypothyroidism | 10.9 | 13.6 | 10.0 |
| Anemia | 2.0 | 3.6 | 1.5 |
| Previous ischemic stroke | 8.6 | 7.3 | 9.1 |
| Previous undefined type stroke | 0.4 | 0.5 | 0.4 |
| Previous TIA | 3.9 | 5.5 | 3.4 |
| Previous PEa | 1.1 | 0.5 | 1.3 |
| Previous DVT | 0.7 | 0.9 | 0.6 |
CHD: coronary heart disease; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; DVT: deep vein thrombosis; GI: gastrointestinal; LV: left ventricular; MI: myocardial infarction; PAD: peripheral arterial disease; PE: pulmonary embolism; TIA: transient ischemic attack.
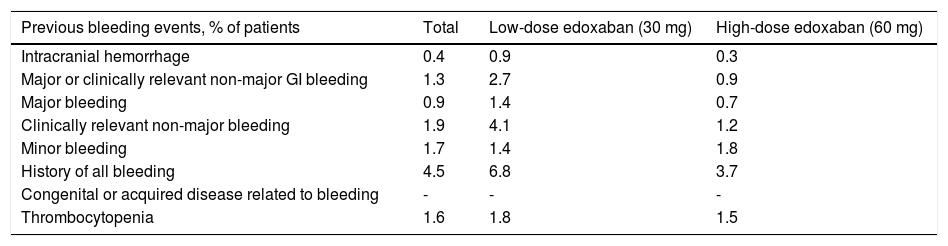
Overall, the number of bleeding events prior to baseline was low, with a predominance of clinically relevant non-major bleeding (1.9% of patients), followed by minor bleeding (1.7%), thrombocytopenia (1.6%), and major or clinically relevant non-major GI bleeding (1.3%) (Table 3). Rates of previous bleeding events that were higher in the low-dose than in the high-dose edoxaban group included intracranial hemorrhage (0.9% vs. 0.3%), major or clinically relevant non-major GI bleeding (2.7% vs. 0.9%), major bleeding (1.4% vs. 0.7%), clinically relevant non-major bleeding (4.1% vs. 1.2%), history of all bleeding (6.8% vs. 3.7%), and thrombocytopenia (1.8% vs. 1.5%), while the opposite was true for minor bleeding (1.4% vs. 1.8%, respectively).
Bleeding history.
| Previous bleeding events, % of patients | Total | Low-dose edoxaban (30 mg) | High-dose edoxaban (60 mg) |
|---|---|---|---|
| Intracranial hemorrhage | 0.4 | 0.9 | 0.3 |
| Major or clinically relevant non-major GI bleeding | 1.3 | 2.7 | 0.9 |
| Major bleeding | 0.9 | 1.4 | 0.7 |
| Clinically relevant non-major bleeding | 1.9 | 4.1 | 1.2 |
| Minor bleeding | 1.7 | 1.4 | 1.8 |
| History of all bleeding | 4.5 | 6.8 | 3.7 |
| Congenital or acquired disease related to bleeding | - | - | - |
| Thrombocytopenia | 1.6 | 1.8 | 1.5 |
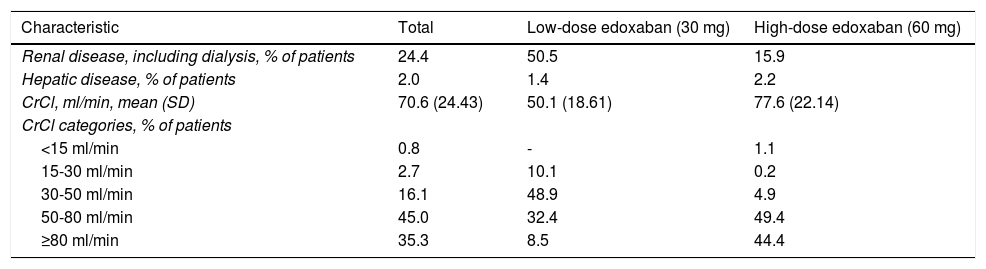
Renal dysfunction was observed in 24.4% of patients included in the study, and hepatic dysfunction in only 2.0%, as reported by patients themselves (Table 4). More patients assigned to receive low-dose edoxaban had renal dysfunction (50.5% vs. 15.9%), while more patients assigned to receive high-dose edoxaban had hepatic dysfunction. Mean creatinine clearance (CrCl) was 70.6 ml/min in the overall study cohort, with most patients (45.0%) falling in the 50-80 ml/min category. Patients set to receive high-dose edoxaban had a higher mean CrCl (77.6 ml/min) than those assigned to the low-dose group (50.1 ml/min).
Renal and hepatic disease, as reported by patients.
| Characteristic | Total | Low-dose edoxaban (30 mg) | High-dose edoxaban (60 mg) |
|---|---|---|---|
| Renal disease, including dialysis, % of patients | 24.4 | 50.5 | 15.9 |
| Hepatic disease, % of patients | 2.0 | 1.4 | 2.2 |
| CrCl, ml/min, mean (SD) | 70.6 (24.43) | 50.1 (18.61) | 77.6 (22.14) |
| CrCl categories, % of patients | |||
| <15 ml/min | 0.8 | - | 1.1 |
| 15-30 ml/min | 2.7 | 10.1 | 0.2 |
| 30-50 ml/min | 16.1 | 48.9 | 4.9 |
| 50-80 ml/min | 45.0 | 32.4 | 49.4 |
| ≥80 ml/min | 35.3 | 8.5 | 44.4 |
CrCl: creatinine clearance; SD: standard deviation.
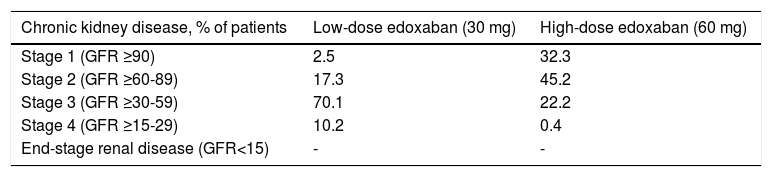
Chronic kidney disease estimated by the Cockcroft-Gault formula indicated a large proportion of patients (70.1%) in the low-dose edoxaban group with stage 3 CKD, followed by a considerably lower proportion (17.3%) with stage 2 CKD (Table 5). In the high-dose group, more patients had stage 2 CKD (45.2%), followed by stage 1 (32.3%) and stage 3 (22.2%).
Chronic kidney disease stage estimated by the Cockcroft-Gault equation.
| Chronic kidney disease, % of patients | Low-dose edoxaban (30 mg) | High-dose edoxaban (60 mg) |
|---|---|---|
| Stage 1 (GFR ≥90) | 2.5 | 32.3 |
| Stage 2 (GFR ≥60-89) | 17.3 | 45.2 |
| Stage 3 (GFR ≥30-59) | 70.1 | 22.2 |
| Stage 4 (GFR ≥15-29) | 10.2 | 0.4 |
| End-stage renal disease (GFR<15) | - | - |
GFR: glomerular filtration rate (ml/min/1.73 m2).
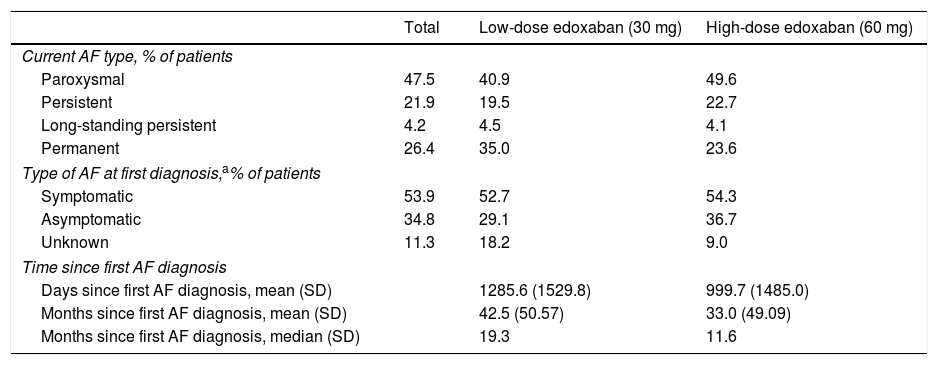
Patients were classified according to the current type of NVAF as having paroxysmal (recurrent episodes that self-terminate within seven days, usually within 48 hours), persistent (of more than seven days duration), long-standing persistent (lasting for >1 year when a decision is made to adopt a rhythm control strategy), or permanent AF (accepted by the patient and physician). Most patients (47.5%) with NVAF had paroxysmal AF at baseline, followed by 26.4% with permanent AF (Table 6). This was also the case for the edoxaban treatment groups individually, with 40.9% and 49.6% of patients in the low- and high-dose groups having paroxysmal AF, and 35.0% and 23.6% of patients having permanent AF, respectively. At first diagnosis, most patients (53.9%) were symptomatic, with a mean time since the first AF diagnosis of 42.5 months for low-dose edoxaban and 33.0 months for high-dose edoxaban patients.
Baseline atrial fibrillation-related characteristics.
| Total | Low-dose edoxaban (30 mg) | High-dose edoxaban (60 mg) | |
|---|---|---|---|
| Current AF type, % of patients | |||
| Paroxysmal | 47.5 | 40.9 | 49.6 |
| Persistent | 21.9 | 19.5 | 22.7 |
| Long-standing persistent | 4.2 | 4.5 | 4.1 |
| Permanent | 26.4 | 35.0 | 23.6 |
| Type of AF at first diagnosis,a% of patients | |||
| Symptomatic | 53.9 | 52.7 | 54.3 |
| Asymptomatic | 34.8 | 29.1 | 36.7 |
| Unknown | 11.3 | 18.2 | 9.0 |
| Time since first AF diagnosis | |||
| Days since first AF diagnosis, mean (SD) | 1285.6 (1529.8) | 999.7 (1485.0) | |
| Months since first AF diagnosis, mean (SD) | 42.5 (50.57) | 33.0 (49.09) | |
| Months since first AF diagnosis, median (SD) | 19.3 | 11.6 | |
AF: atrial fibrillation; SD: standard deviation.
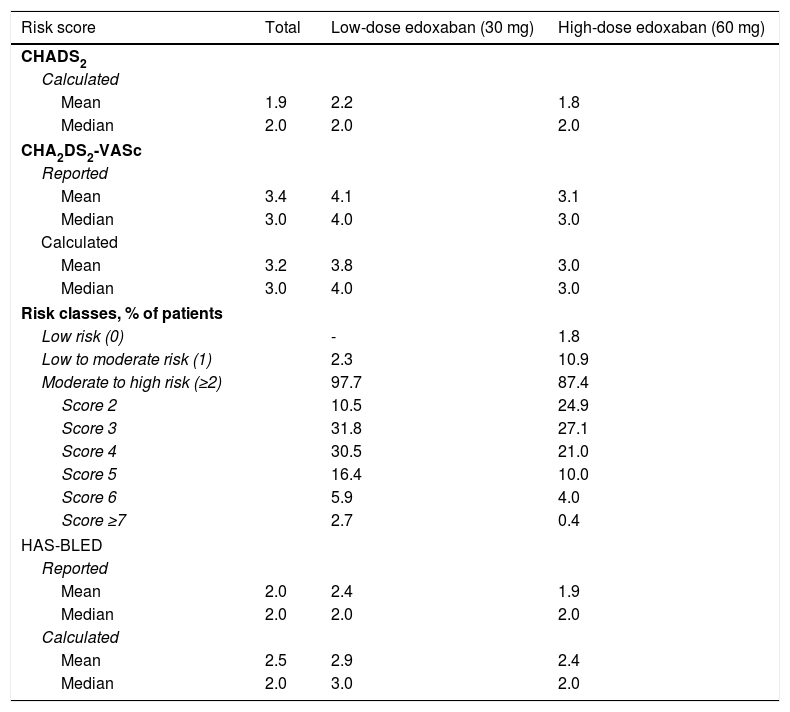
Table 7 shows the risk of stroke and major bleeding for patients with NVAF, as calculated by physicians and reported by patients. A mean CHADS2 score of 1.9 was estimated for the entire cohort, ranging between 1.8 for patients on high-dose treatment and 2.2 for patients on low-dose treatment. The median CHADS2 score was 2.0 for the three study groups, indicating a moderate risk of stroke for this patient population.
Baseline (reported and calculated) risk of stroke and major bleeding for patients with atrial fibrillation.
| Risk score | Total | Low-dose edoxaban (30 mg) | High-dose edoxaban (60 mg) |
|---|---|---|---|
| CHADS2 | |||
| Calculated | |||
| Mean | 1.9 | 2.2 | 1.8 |
| Median | 2.0 | 2.0 | 2.0 |
| CHA2DS2-VASc | |||
| Reported | |||
| Mean | 3.4 | 4.1 | 3.1 |
| Median | 3.0 | 4.0 | 3.0 |
| Calculated | |||
| Mean | 3.2 | 3.8 | 3.0 |
| Median | 3.0 | 4.0 | 3.0 |
| Risk classes, % of patients | |||
| Low risk (0) | - | 1.8 | |
| Low to moderate risk (1) | 2.3 | 10.9 | |
| Moderate to high risk (≥2) | 97.7 | 87.4 | |
| Score 2 | 10.5 | 24.9 | |
| Score 3 | 31.8 | 27.1 | |
| Score 4 | 30.5 | 21.0 | |
| Score 5 | 16.4 | 10.0 | |
| Score 6 | 5.9 | 4.0 | |
| Score ≥7 | 2.7 | 0.4 | |
| HAS-BLED | |||
| Reported | |||
| Mean | 2.0 | 2.4 | 1.9 |
| Median | 2.0 | 2.0 | 2.0 |
| Calculated | |||
| Mean | 2.5 | 2.9 | 2.4 |
| Median | 2.0 | 3.0 | 2.0 |
AF: atrial fibrillation; CHADS2: atrial fibrillation stroke risk; CHA2DS2-VASc: updated atrial fibrillation stroke risk; HAS-BLED: major bleeding risk.
The median overall CHA2DS2-VASc score was 3.0, higher in the low-dose (4.0) than in the high-dose (3.0) group. When patients were stratified according to stroke risk, no patients in the low-dose group were considered to have low stroke risk (score of 0) as opposed to 1.8% of patients in the high-dose group, 2.3% of patients in the low-dose group were considered to have low to moderate stroke risk (score of 1) compared to 10.9% of patients in the high-dose group, and most patients in both groups had moderate to high stroke risk (scores of 2 or higher; 97.7% of patients in the low-dose group vs. 87.4% of those in the high-dose group). Most patients in both treatment groups scored 3 on CHA2DS2-VASc (31.8% and 27.1%, respectively), suggesting a moderate stroke risk.
The median HAS-BLED bleeding score was 2.0 for the overall population and the high-dose edoxaban cohort, indicating moderate bleeding risk, and 3.0 for the low-dose edoxaban cohort, suggesting a high bleeding risk for this group.
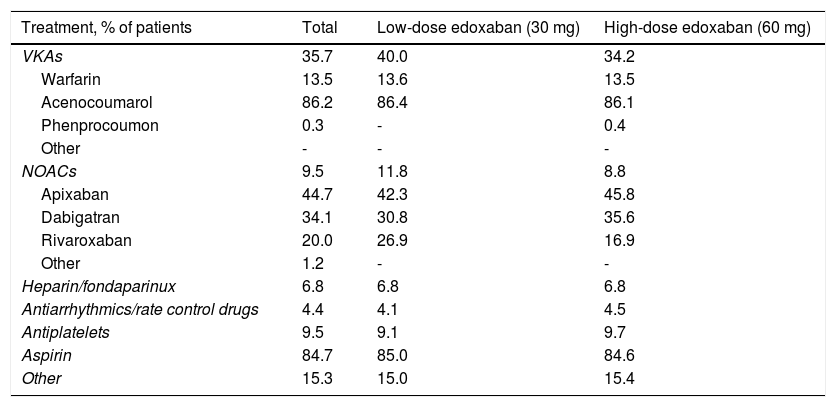
Previous and concomitant atrial fibrillation treatmentPrevious treatments, suspended at or before the baseline enrollment date, mostly consisted of VKAs (35.7%) and, among these, mostly acenocoumarol (Table 8). NOACs and antiplatelets had been previously used by 9.5% of patients each, apixaban (44.7%) and aspirin (84.7%) being the most frequently used drugs within each class, respectively. Heparin or fondaparinux had been previously used by 6.8% of patients and antiarrhythmics and rate control drugs by only 4.4%.
Previous treatments for atrial fibrillation (suspended at or before baseline date).
| Treatment, % of patients | Total | Low-dose edoxaban (30 mg) | High-dose edoxaban (60 mg) |
|---|---|---|---|
| VKAs | 35.7 | 40.0 | 34.2 |
| Warfarin | 13.5 | 13.6 | 13.5 |
| Acenocoumarol | 86.2 | 86.4 | 86.1 |
| Phenprocoumon | 0.3 | - | 0.4 |
| Other | - | - | - |
| NOACs | 9.5 | 11.8 | 8.8 |
| Apixaban | 44.7 | 42.3 | 45.8 |
| Dabigatran | 34.1 | 30.8 | 35.6 |
| Rivaroxaban | 20.0 | 26.9 | 16.9 |
| Other | 1.2 | - | - |
| Heparin/fondaparinux | 6.8 | 6.8 | 6.8 |
| Antiarrhythmics/rate control drugs | 4.4 | 4.1 | 4.5 |
| Antiplatelets | 9.5 | 9.1 | 9.7 |
| Aspirin | 84.7 | 85.0 | 84.6 |
| Other | 15.3 | 15.0 | 15.4 |
NOACs: novel oral anticoagulants; VKAs: vitamin K antagonists.
Before study entry, patients assigned to low-dose treatment were mostly receiving VKAs (40.0%), followed by NOACs (11.8%), and antiplatelets (9.1%), and patients assigned to high-dose treatment were also mostly receiving VKAs (34.2%), but antiplatelets were the second most frequently used agents in the latter group (9.7%), followed by NOACs (8.8%).
Concurrent use of antiarrhythmics and rate control drugs was reported in 39.3% of patients overall, accounting for 46.4% of patients on low-dose and 37.1% of patients on high-dose edoxaban. Antiplatelets, non-steroidal anti-inflammatory drugs, heparin or fondaparinux, and p-glycoprotein inhibitors were also concurrently reported by smaller proportions of patients.
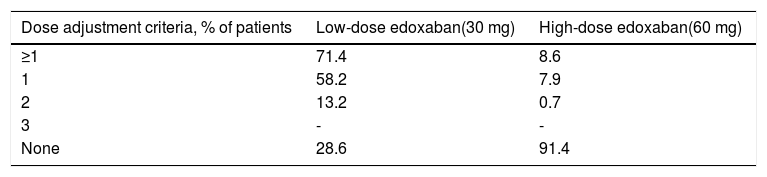
Edoxaban treatmentA considerably higher proportion of patients in the low-dose group required adjustments to the initial edoxaban dose compared to the high-dose group (71.4% vs. 8.6%, respectively) (Table 9).
Only one dose adjustment criterion was required for most patients in both treatment groups (58.2% and 7.9%, respectively), with 13.2% and 0.7% of patients in both groups requiring two dose adjustment criteria, respectively.
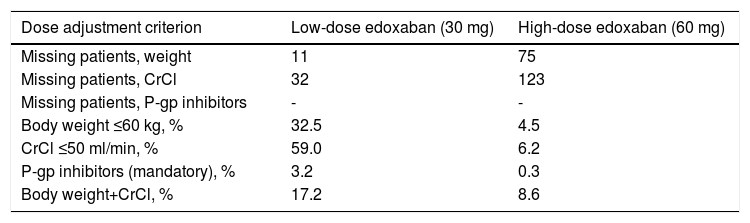
Reasons for dose adjustments in the low-dose treatment group were mainly having CrCl ≤50 ml/min (59.0%) and body weight ≤60 kg (32.5%), while in the high-dose treatment group they were mostly both body weight and CrCl (8.6%) and CrCl ≤50 ml/min (6.2%) (Table 10).
Overview of edoxaban dose adjustment criteria.
| Dose adjustment criterion | Low-dose edoxaban (30 mg) | High-dose edoxaban (60 mg) |
|---|---|---|
| Missing patients, weight | 11 | 75 |
| Missing patients, CrCl | 32 | 123 |
| Missing patients, P-gp inhibitors | - | - |
| Body weight ≤60 kg, % | 32.5 | 4.5 |
| CrCl ≤50 ml/min, % | 59.0 | 6.2 |
| P-gp inhibitors (mandatory), % | 3.2 | 0.3 |
| Body weight+CrCl, % | 17.2 | 8.6 |
CrCl: creatinine clearance; P-gp: P-glycoprotein.
Considering that 28.6% of patients receiving 30 mg (63 out of 220 patients) had no dose reduction criteria (underdosed) and that 8.6% of patients receiving 60 mg (58 out of 672) had at least one reduction criterion (overdosed), 7.1% of all anticoagulated subjects who were underdosed (63 out of 892) and 6.5% were overdosed (58 out of 892). Overall, the data suggest good adherence by the attending physicians to the SmPC recommendations with respect to dose reduction criteria.
DiscussionThe present study aimed to describe and characterize the baseline parameters of a cohort of Iberian (Portuguese and Spanish) patients with NVAF treated with edoxaban and included in the large ongoing ETNA-AF study, and to compare it with previously retrieved Portuguese data.
AF is a significant health burden. It accounts for approximately 15% of all ischemic strokes,8 which represent a major cause of mortality in Portugal. The SAFIRA study recently reported an 11.2% stroke rate in the Portuguese population with AF, which is not negligible.11 The present study therefore helps to gain insights and deepen current knowledge of the situation in Portugal concerning this condition, on the assumption that the availability of reliable data on AF prevalence and incidence is key for optimal management and stroke prevention.
The FAMA study set a reference value of 2.5% for the prevalence of AF in Portugal, which has since been acknowledged as representative of the Portuguese population over 40 years of age.9 No differences were found between genders, but AF prevalence increased with age, and was significantly higher in the ≥70-year-old group.9 However, by not considering the frequency of paroxysmal AF, the study may have underestimated the real prevalence of AF in the country. To address this limitation, the study by Primo et al.10 and the SAFIRA study11 set out to document the prevalence of paroxysmal AF, besides the overall prevalence of AF, using 24-hour electrocardiographic monitoring. However, the use of 24-hour Holter in both studies may have biased population selection and the results to some extent, since this is an expensive and complex test which could therefore not be used in a community setting, but only in specialized health units.
Compared with FAMA, SAFIRA analyzed a more elderly population, of patients aged ≥65 years. Although this population was also included in the FAMA study, it was more thoroughly studied in SAFIRA, and revealed a 9% prevalence of AF, which is higher than reported in FAMA.11 Results according to age class were not so disparate between the studies. Both found that AF prevalence increased with age, with FAMA reporting a prevalence of 6.6% in the 70-79 age group and 10.4% in those over 80 years of age,9 and SAFIRA reporting prevalences of 6.8% in those aged 65-69 years, 11.1% in those aged 70-79 years, and 15.2% in those aged over 80 years.11 These values are lower than those found by Primo et al., who reported an overall 12.4% prevalence of AF/atrial flutter in a younger population of individuals (aged ≥40 years).10 Bonhorst19 provides a more thorough comparison between the FAMA and SAFIRA studies.
In the present study, an even older population of patients with AF was included, with an overall mean age of 73.9 years (79.5 and 72.1 years in the low- and high-dose edoxaban groups, respectively) and more than half (50.7%) aged ≥75 years. This patient population is closer to that of the ENGAGE AF-TIMI clinical trial, in which a median age of 72 years was reported in both the high- and low-dose edoxaban groups.12
In the Iberian cohort of ETNA-AF, most patients (47.5%) had paroxysmal AF, followed by 26.4% with permanent, 21.9% with persistent, and 4.2% with long-standing persistent AF, and most patients (53.9%) were symptomatic at first diagnosis. When the two edoxaban dose groups are analyzed individually, a considerably higher proportion of patients receiving both low- and high-dose edoxaban in ETNA-AF Iberia had paroxysmal AF (40.9% and 49.6%) compared with ENGAGE AF-TIMI (26.1% and 24.9%, respectively).12
With regard to antithrombotic treatment, despite the reported evidence of the safety, efficacy, and cost-effectiveness of oral anticoagulants in ischemic stroke prevention, particularly among the elderly,2 there is a widespread perception that they are underused by Portuguese clinicians. FAMA reported that 62.2% of patients with AF were anticoagulated9 compared to only 43.7% of those in the SAFIRA study.11 In ETNA-AF Iberia, all patients were anticoagulated with edoxaban, as per the inclusion criteria, with previous treatment (suspended at or before baseline enrollment) mostly consisting of VKAs (35.7%), followed by NOACs and antiplatelets (9.5% each), heparin or fondaparinux (6.8%) and antiarrhythmics and rate control drugs (4.4%). In SAFIRA, a considerably higher proportion of anticoagulated patients were receiving VKAs (65.7%) and NOACs (34.3%).11 In the study by Primo et al., only 29.9% of patients with persistent AF and 12.8% of those with paroxysmal AF were anticoagulated, but the type of anticoagulation was not reported.10 A systematic review and meta-analysis of observational studies found that 60% of patients with AF in Portugal were not receiving oral anticoagulation therapy.20 Overall, the low prevalence of oral anticoagulation reported in Portuguese clinical practice is worrying and does not meet international guideline recommendations.2 This may be partially explained by patients’ advanced age and possibly the reluctance of clinicians to prescribe anticoagulants due to the potential for bleeding complications.
The inappropriate dosing rate of the present study was relatively low (7.1% and 6.5% of all anticoagulated subjects were under- and overdosed, respectively) and adherence to the label dosing recommendation was good. In SAFIRA, 24.7% of subjects were incorrectly treated, with only 6.1% of overdosing and no reports of underdosing.11
ETNA-AF Iberia found a median overall CHA2DS2-VASc score of 3.0, which was higher in the low-dose (median 4.0) than in the high-dose (median 3.0) edoxaban group. Despite this difference, most patients in both treatment arms (31.8% and 27.1%, respectively) had a CHA2DS2-VASc of 3, suggesting a moderate stroke risk. This is consistent with the results of SAFIRA, which reported a median CHA2DS2-VASc score of 3.5.11 Regarding the relationship between anticoagulation rate and CHA2DS2-VASc score, except for scores of 4 or 5, for which the rates were similar in both studies (46.9% in the low- and 31.0% in the high-dose arm in ETNA-AF Iberia vs. 50.1% in SAFIRA), the results were somewhat different from ETNA-AF Iberia and SAFIRA. The anticoagulation rate in patients with a CHA2DS2-VASc score between 1 and 3 was considerably higher in ETNA-AF Iberia (44.6% in low- and 62.9% in high-dose arms) than in SAFIRA (25.3%),11 but both were considerably lower than the CHADS2 scores in the ENGAGE AF-TIMI trial (77.8% in low- and 77.1% in high-dose arms).12 The opposite was true for patients with a CHA2DS2-VASc score ≥6, for whom considerably lower anticoagulation rates were described in ETNA-AF Iberia (8.6% in low- and 4.4% in high-dose arms) than in SAFIRA (18.6%). ENGAGE AF-TIMI further reported 22.9% and 22.2% of patients in the high- and low-dose treatment groups, respectively, with CHADS2 scores between 4 and 6 and no patients with CHADS2 scores ≥7.12
Concerning bleeding risk, both the overall and the high-dose edoxaban cohorts in ETNA-AF Iberia were found to have a moderate bleeding risk (median HAS-BLED score of 2.0), while a higher bleeding risk (median HAS-BLED score of 3.0) was reported in the low-dose edoxaban cohort.
The prevalence of CV risk factors was high in this study, as expected in an elderly population. Hypertension (76.7%), dys- or hyperlipidemia (50.9%), and diabetes (26.8%) were the most prevalent comorbidities, in agreement with the findings of SAFIRA (85.3%, 75.4% and 22.7%),11 but this was considerably higher than reported in FAMA (43.5%, 36.8% and 13.1%, respectively).10 The ENGAGE AF-TIMI population had more patients with hypertension (93.7% in the high-dose and 93.5% in the low-dose group)12 than in any of the Portuguese studies, and the same was also true for diabetes (36.4% in the high- and 36.2% in the low-dose group).12 Congestive heart failure was the second most prevalent comorbidity in ENGAGE AF-TIMI (58.2% and 56.6% in the high- and low-dose groups, respectively),12 rather than the dys- or hyperlipidemia observed in the Portuguese population.
Overall, ETNA-AF Iberia describes a real-world patient population which is not very different from that of the ENGAGE AF-TIMI clinical trial. Additionally, it confirms the results of the SAFIRA and FAMA Portuguese reference AF studies, in that a significantly high number of patients still fail to receive anticoagulant therapy, highlighting the need to further optimize management of this condition. Lastly, involving both patients and health professionals is crucial to increase health gains in AF.
This study has some limitations, particularly the sample size, which was partially determined by restrictions regarding the end of enrollment, and the limited experience with edoxaban, which may also have reduced sample size. This may be compensated to some extent by the study's longer follow-up, giving the opportunity to study edoxaban in a broad contemporary AF patient population.
ConclusionThese data, although only concerning clinical and epidemiological profiles at admission, offer a snapshot on a contemporary Iberian AF patient population, thus providing a valuable tool to assess disease profile, patients’ concomitant medications and comorbidities, and how edoxaban is used in clinical practice.
In the future, these real-world patient data will provide valuable insights into disease management and progression and the safety and effectiveness of edoxaban, as well as patterns of cardiovascular events, which are crucial to appropriately position edoxaban in the constantly changing landscape of stroke prevention in Iberian AF patients.
FundingDaiichi Sankyo, Inc. provided funding for this study.
Conflicts of interestPedro Monteiro is an investigator and Steering Committee member of ETNA-AF Europe, investigator of the RE-LY and Engage-AF trials, and has received research and lecturing fees from Bayer, Boehringer Ingelheim, Daiichi Sankyo and Pfizer-BMS.
Cardiology Department of Centro Hospitalar de Setúbal - Hospital de São Bernardo
Cardiology Department of Clínica Cuida Mais - Cuidados de Saúde
Cardiology Department of Vergílio Schneider - Clínica de Cardiologia
Cardiology Department of Centro Hospitalar do Baixo Vouga - Hospital de Aveiro
Cardiology Department of Instituto do Coração
Immunotherapy Department of Centro Hospitalar Cova da Beira
Internal Medicine Department of Centro Hospitalar de Vila Nova de Gaia
Cardiology Department of Hospital do Divino Espírito Santo
Internal Medicine Department of Centro Hospitalar de Lisboa Norte - Hospital de Santa Maria
Cardiology Department of Hospital Lusíadas Lisboa
Internal Medicine Department of ULSM - Hospital Pedro Hispano
Cardiology Department of Centro Hospitalar de Lisboa Norte - Hospital de Santa Maria
Cardiology Department of Hospital de Braga
Cardiology Department of Hospital Virgen Macarena
Cardiology Department of Clínica Cardiologia DR. Anguita
Neurology Department of Hospital Universitario Virgen del Rocío (HUVR)
Cardiology Department of Hospital Universitario Virgen del Rocío (HUVR) - National coordinator
Cardiology Department of Hospital Virgen de la Victoria
Cardiology Department of Hospital Costa del Sol
Internal Medicine Department of Hospital Costa del Sol
Internal Medicine Department of Hospital Virgen del Camino
Cardiology Department of Fundación Hospital de Jove
Internal Medicine Department of Hospital Carmen y Severo Ochoa (Centro de Cangas)
Cardiology Department of Hospital Universitario Central de Asturias
Cardiology Department of Hospital Son Llàtzer
Cardiology Department of Hospital de Gran Canaria Dr. Negrin
Cardiology Department of Clinica Vida
Internal Medicine Department of Hospital Universitario de Canarias
Internal Medicine Department of Hospital Insular Las Palmas
Cardiology Department of Clinica Cardiorreal (H. General La Mancha Centro)
Cardiology Department of Hospital General de Albacete
Internal Medicine Department of Hospital Virgen de la Luz
Geriatrics Department of Hospital Perpetuo Socorro
Internal Medicine Department of Hospital Universitario de Burgos
Cardiology Department of Complejo Asistencial Universitario de Salamanca
Internal Medicine Department of Hospital Clínico de Valladolid
Internal Medicine Department of Complejo Asistencial Universitario de Salamanca
Cardiology Department of Hospital Nuestra Sra. de Sonsóles
Hematology Department of Hospital Río Carrión
Hematology Department of Complejo Asistencial Universitario de Salamanca
Internal Medicine Department of Hospital El Bierzo
Cardiology Department of Hospital El Bierzo
Cardiology Department of Consulta Privada
Cardiology Department of Hospital Comarcal Medina del Campo
Internal Medicine Department of Complejo Asistencial Universitario de León
Cardiology Department of Complejo Asistencial Universitario de Léon
Hematology Department of Hospital Universitario de La Vall D’Hebron
Neurology department of Hospital Universitario de La Vall D’Hebron
Neurology Department of Hospital Del Mar
Cardiology Department of Hospital Del Mar
Hematology Department of Hospital Universitari de Bellvitge
Hematology Department of Hospital Clinic de Barcelona
Cardiology Department of Hospital del Vendrell
Hematology Department of Hospital de la Santa Creu I Sant Pau
Hematology Department of Hospital l’Hospitalet
Cardiology Department of Hospital Moises Broggi
Neurology Department of Hospital Clinico Universitario de Valencia
Cardiology Department of Hospital Clinico Universitario de Valencia
Cardiology Department of Hospital General Universitario de Valencia
Internal Medicine Department of Hospital Vega Baja
Cardiology Department of Hospital San Juan de Alicante
Cardiology Department of Hospital General Universitario de Alicante
Cardiology Department of Hospital Universitario de Torrevieja
Cardiology Department of Hospital de la Vega Baja (Orihuela)
Cardiology Department of Hospital General Universitario de Elche
Cardiology Department of Hospital General de Castellon
Internal Medicine Department of Hospital de Sagunto
Hematology Department of Hospital de Sagunto
Internal Medicine Department of Hospital General Universitario Valencia
Cardiology Department of Hospital General Universitario ELDA
Cardiology Department of Hospital Virgen de los Lirios
Cardiology Department of Hospital San Pedro de Alcántara
Cardiology Department of Hospital Clinico Universitario de Santiago (CHUS)
Internal Medicine Department of Complejo Hospitalario Universitario A Coruña (CHUAC)
Hematology Department of Complexo Hospitalario de Pontevedra
Internal Medicine Department of Hospital Clinico San Carlos
Cardiology Department of Hospital La Paz
Cardiology Department of Hospital Ramón y Cajal
Internal Medicine Department of Hospital Puerta de Hiero
Neurology Department of Hospital Puerta de Hiero
Internal Medicine Department of Hospital Universitario de la Princesa
Cardiology Department of Hospital General Universitario Gregorio Marañón
Cardiology Department of Hospital Universitario Madrid Montepríncipe − Centro Integral Enfermedades Cardiovasculares (CIEC)
Hematology Department of Hospital Universitario de la Princesa
Internal Medicine Department of Hospital San Rafael
Cardiology Department of Hospital Universitario 12 de Octubre
Cardiology Department of Hospital HM Madrid
Cardiology Department of Hospital Sanchinaro
Cardiology Department of Hospital Madrid Montepríncipe
Cardiology Department of HM Policlínico
Cardiology Department of HM Vallés
Cardiology Department of HM Torrelodones
Cardiology Department of HM Puerta del Sur
Cardiology Department of Hospital Clinico Universitario Virgen de la Arriaxaca
Hematology Department of Hospital Clinico Universitario Virgen de la Arriaxaca
Cardiology Department of Hospital General Universitario Morales Meseguer
Internal Medicine Department of Hospital Universitario Santa Lucía
Cardiology Department of Hospital General Universitario Santa Lucia (Cartagena)
Cardiology Department of Complejo Hospitalario de Navarra
Cardiology Department of Clinica Universitaria de Navarra
Cardiology Department of Hospital Universitario de Cruces
Cardiology Department of Hospital Universitario de Álava
Cardiology Department of Hospital de Galdakao-Usansolo
The author declares that the publication of this article in the Portuguese Journal of Cardiology was expressly authorized by all the institutions involved.