The 2023 European Acute Coronary Syndrome (ACS) guidelines state that prasugrel should be considered in preference to ticagrelor in patients undergoing percutaneous coronary intervention (PCI).1 In Portugal, prasugrel is less available due to initial evidence suggesting higher risk of bleeding. Nevertheless, prasugrel 10 mg is currently available as a generic drug, while ticagrelor has no generic formulation at the time of writing. Given the results of the ISAR-REACT 5 trial,2 we aimed to evaluate the proportion of patients that could be switched from ticagrelor to prasugrel, and the economic impact of widespread national adoption.
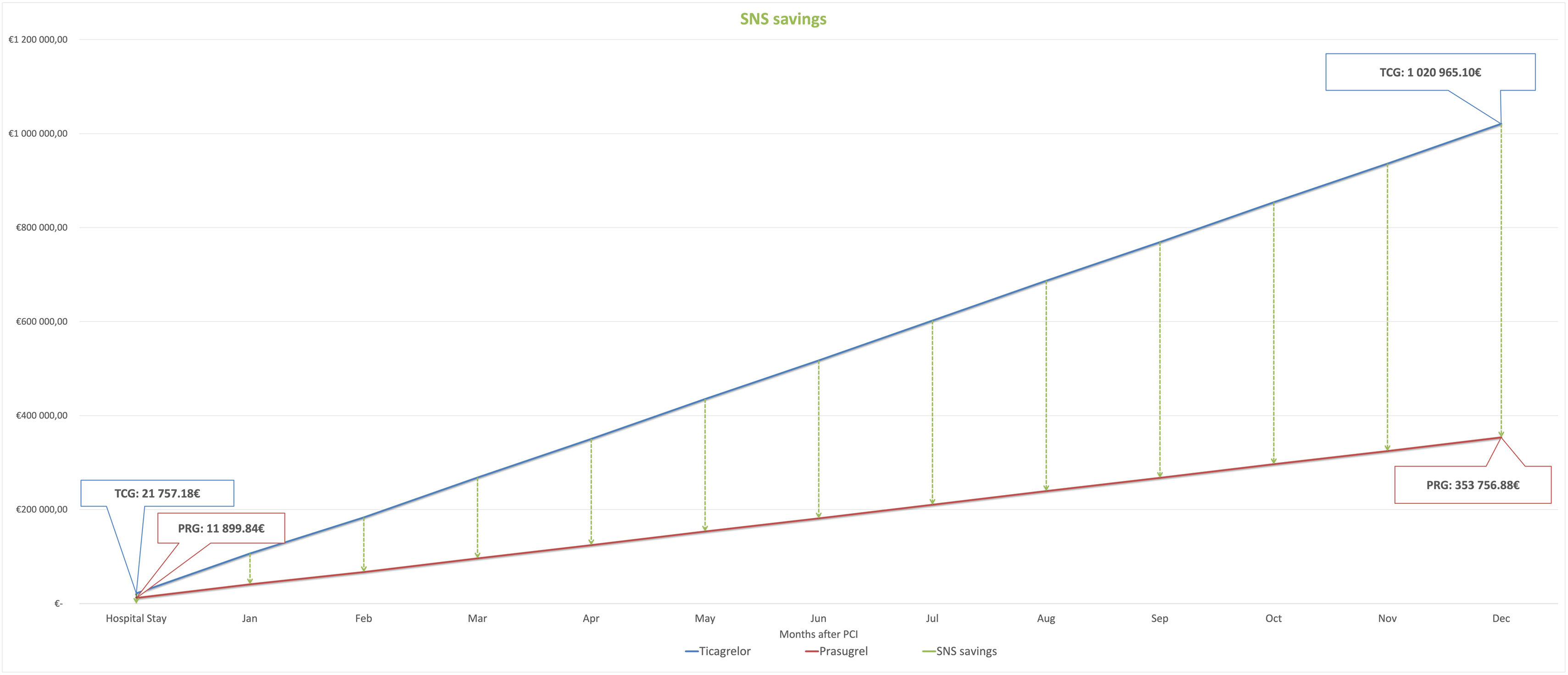
To determine eligibility for generic prasugrel treatment, we examined a single center retrospective database of consecutive ST segment elevation myocardial infarction (STEMI) patients undergoing primary PCI from January 2016 to December 2016. Eligibility for ticagrelor and prasugrel was based on (1) drug-specific contraindications – previous stroke for prasugrel, chronic liver disease for ticagrelor, and the need for anticoagulation (contraindication for both prasugrel and ticagrelor) and (2) indication for prasugrel dose reduction (<60 kg and >75 years).1 To estimate hospital costs, we used the price per pill indicated by the pharmacy of our hospital (0.98€ ticagrelor, 0.67€ prasugrel). To obtain post-discharge and Portuguese National Health Service (SNS) savings, we used the national institute for drug pricing (INFARMED)3 public information on the patient and SNS copayments per pill, respectively ticagrelor 1.34€/0.41€ and prasugrel 0.92€/0.28€. A total of 284 patients were included, median age 70±11.4 years, 170 (60%) were male, 82 (29%) had type 2 diabetes, 200 (70%) had dyslipidemia and 255 (90%) had arterial hypertension. Median hospitalization time was 7 (IQR 5-13) days. Considering prasugrel and ticagrelor contraindications, 62 (22%) had a previous history of stroke and 54 (19%) had atrial fibrillation and need for anticoagulation. There were no identified patients with chronic liver disease. Regarding prasugrel dose reduction criteria, 94 (33%) patients were older than 75 years and 28 (16%) weighed <60 kg. Of the complete cohort, 120 (42%) patients were eligible for prasugrel 10 mg and ticagrelor 90 mg. If we take the most recently published data from 2013 from the Portugal National Acute Coronary Syndrome register,4 where 3500 STEMI patients underwent PCI at a national level, we forecast potential cost reductions considering 42% of patients eligible for both prasugrel and ticagrelor. Cost per admission for a seven-day median admission time would be 21757.18€ for ticagrelor and 11899.84€ for prasugrel. At a national level, in-hospital drug costs could be reduced only by 9857.33€. Considering a one-year outpatient setting and widespread adoption of generic prasugrel 10 mg whenever possible, SNS savings are estimated at 657350.89€, and patient savings at 293614.75€, annually. The one-year individual patient savings would be 198.38€.
Acute coronary syndrome patients in real-world scenarios exhibit only moderate eligibility for transition of ticagrelor to prasugrel 10 mg. Considering the lower cost, the SNS could potentially save 667208.22€ (Figure 1) annually through the widespread adoption of generic prasugrel 10 mg instead of ticagrelor. We acknowledge there are price fluctuations and note that the introduction of a generic version of ticagrelor will likely impact the cost analysis presented in this paper. Nevertheless, these findings advocate for broader adoption of generic prasugrel, in keeping with economic considerations and contemporary guidelines.
Conflicts of interestThe authors have no conflicts of interest to declare.