To investigate the incidence and clinical relevance of the presence of mobile echogenic images (MEI) during transesophageal echocardiography (TEE) for monitoring of transcatheter aortic valve implantation (TAVI).
MethodsConsecutive patients referred to our center for transfemoral or transapical TAVI were included. The procedure was monitored by three-dimensional (3D) TEE and images were analyzed by two independent experts. In-hospital follow-up was carried out and correlated with imaging findings.
ResultsA total of 104 patients were included. MEI were visualized in 11 patients during the procedure (11%) and in over 50% of cases were identified as thrombi, however no differences in periprocedural stroke were found in follow-up.
ConclusionsVisualization of MEI during 3D TEE monitoring of TAVI is relatively common (11%) and in over 50% of cases they are identified as thrombi. The clinical implications of this finding are uncertain, as despite their frequency, the incidence of clinical stroke in this patient population was no higher. 3D TEE is a useful tool for diagnosis of MEI and can alert the operator to their presence.
Investigar a incidência e relevância clínica da presença de imagens ecogénicas móveis (IEM), através de ecocardiografia transesofágica (ETE) durante a implantação valvular aórtica percutânea (TAVI).
MétodosForam incluídos doentes consecutivos referenciados para o nosso centro para realização de TAVI por via transapical ou transfemoral. Foi realizada a monitorização da ETE 3 D e as imagens foram analisadas por dois peritos individualmente. O seguimento foi realizado no hospital e foi correlacionado com achados de imagem.
ResultadosForam definitivamente incluídos 104 doentes. Durante o procedimento foram visualizadas IEM em 11 pacientes (11%) e,em mais de 50% dos casos, foram identificadas como trombos, não tendo, no entanto, sido encontradas diferenças no seguimento dos doentes.
ConclusõesA visualização das IEM através da monitorização por ETE 3 D durante a TAVI é relativamente comum (11%) e, em mais de 50% dos casos, são identificadas como trombos. As implicações clínicas deste achado são incertas, porque apesar da sua frequência não há maior incidência de acidente vascular cerebral clínico nesta população de doentes. A ETE é uma ferramenta útil para o diagnóstico e pode alertar o operador da sua presença.
Aortic stenosis (AS) is the most prevalent valvular disease in the Western world, and surgical valve replacement remains the standard treatment. However, approximately one third of patients are not referred or are rejected for surgical treatment due to high risk. In recent years, transcatheter aortic valve implantation (TAVI) has emerged as an alternative treatment for these patients.1–3
Different cardiac imaging techniques are essential in the proper selection of patients for TAVI, in intraprocedural monitoring and in subsequent follow-up. Of these techniques, transesophageal echocardiography (TEE) is very helpful for selecting prosthesis size, guiding valve positioning and release, checking the final result and detecting complications.4–6 Moreover, three-dimensional (3D) TEE, due to its ability to characterize soft structures, has a growing role during percutaneous interventions of structural heart disease.
During all the steps of TAVI, mobile echogenic images (MEI) can be observed in some patients. These are defined as small mobile masses, mainly attached to catheters, after valvuloplasty or prosthetic vale implantation, or as remains of the former valve structure.
The aim of our study was to clarify the incidence and clinical significance of MEI detected by 3D TEE, as there are no specifically designed studies for this purpose.
MethodsStudy population and procedureOne hundred and four consecutive patients referred to our center for TAVI were included. The device used in all cases was the Edwards SAPIEN prosthesis, and all procedures were performed under general anesthesia. A transfemoral approach was the first choice, although in cases with minimum peripheral vascular diameter <6 mm or excessive tortuosity implantation was performed by a transapical route.
In transfemoral procedures, surgical exposure of the femoral artery was performed in all except six cases and 100 IU/kg intravenous heparin was administered. Prior to valve implantation, balloon valvuloplasty was performed under rapid ventricular pacing using a temporary pacemaker inserted in the right ventricle.
Echocardiographic monitoring3D TEE monitoring was performed in all cases, using an iE 33 ultrasound system (Philips).
The presence of MEI during the procedure was assessed, as well as their main echocardiographic characteristics and time of occurrence.
MEI were defined as small mobile masses, mainly attached to catheters, after valvuloplasty or prosthetic valve implantation, or as remains of the former valve structure.
The echocardiographic images were assessed independently by two experts in imaging and monitoring of structural interventional procedures.
Statistical analysisThe data collected were analyzed using SPSS version 20.0 for Mac (SPSS Inc., Chicago, IL, USA). Qualitative variables were expressed as percentages, and quantitative variables as mean ± standard deviation. The chi-square test with Fisher's correction was used for comparison between qualitative variables. A multivariate analysis was performed using logistic regression. Comparison between quantitative variables was performed using the Student's t test. A value of p<0.05 was considered statistically significant.
ResultsOut of the 104 patients, 81 (78%) underwent TAVI by a transfemoral approach. The clinical characteristics of the study population are summarized in Table 1.
Baseline characteristics of the study population.
| Transfemoral approach | 81 (78%) |
| Transapical approach | 23 (22%) |
| Hypertension | 85 (82%) |
| Diabetes | 42 (41%) |
| Dyslipidemia | 54 (52%) |
| Previous stroke | 19 (18%) |
| Coronary disease | 49 (47%) |
| Previous MI | 13 (12%) |
| Renal failure | 25 (24%) |
| Peripheral vascular disease | 14 (13%) |
| Atrial fibrillation | 44 (42%) |
| Intracardiac thrombus | 1 (1%) |
| MEI | 11 (11%) |
| In-hospital stroke | 3 (3%) |
MEI: mobile echogenic masses; MI: myocardial infarction.
MEI were visualized during the procedure in 11 patients (11%). Echocardiographic characteristics, including dimensions, time of appearance and disappearance and operator's interpretation, are summarized in Table 2.
Characteristics of mobile echogenic masses.
| Yes | No | |
|---|---|---|
| Appearance | ||
| Valvuloplasty | 1 (10%) | 10 (90%) |
| Post valvuloplasty | 3 (27%) | 8 (73%) |
| Prosthesis implantation | 7 (64%) | 4 (36%) |
| Disappearance | 5 (45%) | 6 (55%) |
| Location | ||
| Ventricle | 2 (18%) | 9 (82%) |
| Catheter | 3 (27%) | 8 (73%) |
| Prosthesis | 1 (9%) | 10 (90%) |
| Aortic root | 4 (36%) | 7 (64%) |
| Ascending aorta | 1 (9%) | 10 (90%) |
| Mobility | 11 (100%) | 0 |
| Calcium | 5 (45%) | 6 (55%) |
| Morphology | ||
| Valve structures | 5 (45%) | 6 (55%) |
| Thrombus | 6 (55%) | 5 (45%) |
In four cases, MEI were observed before valve implantation (one before and three after valvuloplasty), whereas in while cases (64%) they appeared during valve implantation.
Of note, 45% of MEI (n=5) disappeared at the end of the procedure.
MEI were located at the aortic root in four cases (36%), in the left ventricular cavity in two (18%), attached to the catheter in three (27%), and in other locations (the prosthetic valve and the aortic arch) in two patients (18%).
Echocardiographic features of mobile echogenic massesThere was consensus in the identification of MEIs between the two observers in 100% of cases, including their description and main echocardiographic characteristics. They were interpreted as thrombus in 55% of cases, and as remains of native valve structures in 45%.
The size of MEI ranged from 3 mm to 30 mm, and 45% of them had the echocardiographic density of calcium.
Clinical implications of mobile echogenic massesNo statistically significant differences were identified between patients with and without MEI regarding baseline characteristics, type of access, incidence of in-hospital stroke, or in-hospital mortality (Table 3). Three out of the 104 patients had an in-hospital stroke, but in only one of these cases were MEI observed during valve implantation (Table 4). Previous myocardial infarction was the only predictor significantly associated with the occurrence of stroke during hospitalization.
Characteristics of patients with and without mobile echogenic masses.
| MEI (n=12) | No MEI (n=92) | p | |
|---|---|---|---|
| Transfemoral approach | 10 (83%) | 79 (91%) | 1 |
| Transapical approach | 2 (17%) | 21 (23%) | 1 |
| Hypertension | 10 (83%) | 75 (81%) | 1 |
| Diabetes | 3 (25%) | 39 (42%) | 0.352 |
| Dyslipidemia | 6 (50%) | 48 (52%) | 1 |
| Previous stroke | 3 (25%) | 16 (17%) | 0.456 |
| Coronary disease | 6 (50%) | 43 (47%) | 1 |
| Previous MI | 1 (8%) | 12 (13%) | 1 |
| Renal failure | 2 (17%) | 23 (25%) | 0.726 |
| Peripheral vascular disease | 3 (25%) | 11 (12%) | 0.203 |
| Previous AF | 5 (42%) | 39 (42%) | 1 |
| Intracardiac thrombus | 0 | 1 (1%) | 1 |
| In-hospital stroke | 1 (8%) | 11 (12%) | 0.310 |
| In-hospital mortality | 0 | 9 (10%) | 0.506 |
AF: atrial fibrillation; MEI: mobile echogenic masses; MI: myocardial infarction.
Characteristics of patients with in-hospital stroke.
| Stroke (n=3) | No stroke (n=101) | p | |
|---|---|---|---|
| Transfemoral approach | 2 (67%) | 79 (78%) | 0.635 |
| Transapical approach | 1 (33%) | 22 (22%) | 0.635 |
| Hypertension | 2 (67%) | 83 (82%) | 0.493 |
| Diabetes | 1 (33%) | 41 (41%) | 0.640 |
| Dyslipidemia | 1 (33%) | 53 (52%) | 0.513 |
| Previous stroke | 0 | 19 (19%) | 0.406 |
| Coronary disease | 3 (100%) | 46 (45%) | 0.063 |
| Previous MI | 2 (67%) | 11 (10%) | 0.004 |
| Renal failure | 0 | 25 (24%) | 0.323 |
| Peripheral vascular disease | 1 (33%) | 13 (12%) | 0.306 |
| Previous AF | 2 (67%) | 42 (41%) | 0.386 |
| Intracardiac thrombus | 0 | 1 (0.9%) | 0.863 |
| MEI | 1 (33%) | 10 (9%) | 0.230 |
| In-hospital mortality | 0 | 9 (8%) | 0.774 |
| Prosthesis embolization | 0 | 4 (3%) | 0.908 |
AF: atrial fibrillation; MEI: mobile echogenic masses; MI: myocardial infarction.
This is the first study specifically focused on MEI during TAVI with TEE monitoring. Our findings show that in up to 11% of cases MEI can be observed during 3D TEE monitoring of TAVI procedures. These images are in most cases a thrombus attached to catheters or remains of former valve structures, which represent a potential source of emboli.7 However, we did not observe an increase in the incidence of periprocedural stroke in patients with MEI (Figure 1).
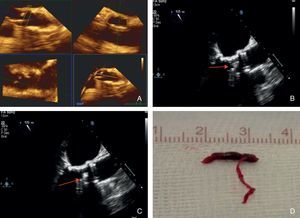
An 85-year-old woman with a history of severe aortic stenosis who was accepted for transfemoral valve replacement. After the valve was crossed with the hydrophilic wire, a mobile echogenic image (MEI) was observed by the TEE operator and identified as thrombus. 2000 U of heparin were administered, but unfortunately the thrombus embolized. Cerebral angiography was then performed and showed occlusion of the right mid cerebral artery, and thrombectomy was successfully performed. The patient was discharged without neurological deficit. (A) Three-dimensional determination of aortic valvular area (0.6 cm2); (B and C) mobile echogenic image observed during the procedure and identified as thrombus; (D) macroscopic image of the thrombus that was extracted from the right mid cerebral artery.
TEE monitoring of non-coronary interventional procedures is an essential safety tool and is considered one of the great challenges of cardiac imaging. Traditional fluoroscopy provides an inaccurate display of soft cardiac structures and is dependent on high doses of iodinated contrast and long exposure to ionizing radiation. Sector scanning and safety of the procedure are better with TEE than with intracardiac echocardiography. In our series, the presence of MEI was highly reproducible by experienced TEE operators, and there was a 100% correlation between two qualified physicians.8 In our experience, 3D TEE is particularly useful for characterization, since real-time imaging provides information about location of MEI, time of onset and relation to cardiac structures, and devices used during the procedure. The characterization of MEI can in some cases help to correct their precipitating factors and thereby minimize the risk of embolism. With the use of 3D TEE, up to 55% of MEI in our series were identified as thrombus attached to the catheters, and probably allowed the operator to modify the precipitating factors and increase the dose of heparin used, thus preventing clinical consequences (stroke or systemic embolism).9,10
Although TAVI is considered an effective and safe therapeutic option for patients with aortic stenosis and high surgical risk, 30-day mortality of patients undergoing this procedure is still around 8%–10%.2 This mortality is largely associated with the main complications inherent to the procedure: vascular complications, device embolization and stroke.11 The prognosis of patients with periprocedural stroke after TAVI is poor and remains one of the limitations of the technique. Previous studies estimated 30-day mortality in these patients at 42.3%, compared with patients without stroke of around 5.1%.12–14
The PARTNER B study showed a higher incidence of stroke at 30 days in patients undergoing TAVI compared with those randomized to medical therapy (5.0% vs. 1.1%, p=0.06).3 The main contributing factors are post-valvuloplasty valve remains, formation of thrombus attached to catheters, erosion of aortic plaques or even thrombosis of the implanted valve. Other factors, such as the presence of spontaneous echo contrast in the left atrium or supraventricular arrhythmias such as atrial fibrillation or flutter, are common and may increase the risk of embolism.15–17 These phenomena were not observed in the PARTNER A study, in which patients undergoing TAVI and surgical aortic valve replacement showed no significant differences in periprocedural stroke rates (3.8% vs. 2.1%, p=0.20).2
In our study, we observed MEI mainly during valve implantation. The Edwards SAPIEN device used in all patients is designed similarly to a stent, being mounted on a balloon, which is inflated to high pressure once it is positioned at the level of the annular plane. Analogously to the compression involved in coronary balloon angioplasty, valve implantation excludes the calcified aortic leaflets, which are compressed between the ring and the valve. Total or partial disintegration of the leaflets, aggravated in most cases by high calcification, makes them into pedunculated mobile structures, with embolic potential.18,19
It is noteworthy that in 45% of cases, the MEI disappeared during the procedure and no correlation with a higher incidence of periprocedural stroke was observed. Previous studies have shown high incidences of brain microbleeds associated with TAVI, with a variable incidence that can approach 70%. There is considerable interest in the clinical, cognitive and functional impairment that these injuries may cause and that in most cases remains unnoticed. The role played in this context by MEI visualized during the procedure remains unclear.20–24
ConclusionsVisualization of MEI during 3D TEE monitoring of TAVI is relatively common (11% of cases in our study). Echocardiographic characterization identifies them in over 50% of cases as thrombi. The clinical implications of this finding are uncertain, as despite their frequency, there was no increased incidence of clinical stroke in this patient population. 3D TEE is a useful tool for diagnosis and can alert the operator to their presence.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.