This retrospective study aimed to develop a nomogram to predict the risk of postoperative acute respiratory distress syndrome (ARDS) in patients with Stanford type A acute aortic dissection.
MethodsThe study included patients who underwent surgical repair for Stanford type A acute aortic dissection between January 2020 and December 2023. Demographic data, surgical details, intraoperative information, and postoperative outcomes were collected. Univariate logistic regression was used for preliminary predictor screening, and a multivariate logistic regression model was constructed and presented as a nomogram. The nomogram's performance was evaluated using the area under the receiver operating characteristics (ROC) curve calibration plots, and decision curve analysis (DCA). Internal validation was performed using bootstrap resampling.
ResultsThe study included 142 patients, 41 (28.873%) of whom developed ARDS postoperatively. Multivariate logistic regression identified body mass index (BMI), postoperative procalcitonin (PCT), cardiopulmonary bypass (CPB) time, and low albumin as independent risk factors for postoperative ARDS in type A acute aortic dissection patients. These factors were used to develop the nomogram, which demonstrated good predictive performance with an area under the ROC curve of 0.809 (95% confidence interval: 0.721–0.881). The nomogram was successfully validated by calibration plots and DCA.
ConclusionsBMI, PCT, CPB time, and low albumin are independent risk factors for postoperative ARDS in type A acute aortic dissection patients. The constructed nomogram provides an effective tool for predicting the risk of ARDS, aiding in the prevention and management of this complication in patients undergoing aortic surgery.
Este estudo retrospetivo teve como objetivo desenvolver um nomograma para prever o risco de síndrome do desconforto respiratório agudo (SDRA) no pós-operatório de pacientes com disseção aguda da aorta tipo A de Stanford (TAAD).
MétodosForam incluídos pacientes submetidos à correção cirúrgica para disseção aguda da aorta tipo A de Stanford entre janeiro de 2020 e dezembro de 2023. Foram coletados dados demográficos, detalhes cirúrgicos, informações intraoperatórias e desfechos pós-operatórios. A regressão logística univariada foi utilizada para triagem preliminar dos preditores, seguida pela construção de um modelo multivariado de regressão logística, apresentado como nomograma. O desempenho do nomograma foi avaliado por meio da área sob a curva ROC (Receiver Operating Characteristic), gráficos de calibração e análise da curva de decisão (DCA). A validação interna foi realizada por meio de reamostragem bootstrap.
ResultadosForam incluídos 142 pacientes, dos quais 41 (28,9%) desenvolveram SDRA no pós-operatório. A regressão logística multivariada identificou o índice de massa corporal (IMC), a procalcitonina pós-operatória (PCT), o tempo de circulação extracorpórea (CEC) e a baixa albumina como fatores de risco independentes para SDRA pós-operatória em pacientes com TAAD. Esses fatores foram utilizados para desenvolver o nomograma, que demonstrou bom desempenho preditivo com uma área sob a curva ROC de 0,809 (intervalo de confiança de 95%: 0,721-0,881). O nomograma foi validado com sucesso por meio de curvas de calibração e DCA.
ConclusõesIMC, PCT, tempo de CEC e baixa albumina são fatores de risco independentes para SDRA pós-operatória em pacientes com TAAD. O nomograma desenvolvido fornece uma ferramenta eficaz para prever o risco de SDRA, auxiliando na prevenção e no manejo dessa complicação em pacientes submetidos à cirurgia aórtica.
Acute respiratory distress syndrome (ARDS) is a clinical syndrome characterized by refractory hypoxemia, radiographic severity, and respiratory system compliance. This syndrome is usually associated with sepsis, shock, aspiration, and trauma. Cardiac surgery involving cardiopulmonary bypass (CPB) has been reported to result in ARDS in 0.4–20% of patients, with mortality rates reaching as high as 70–80%.1–3 Beyond mortality, compromised long-term health status and increased medical expenditures could not be ignored contributing to this respiratory failure. Thus, every attempt to decrease the incidence of ARDS should be embraced.
Type A aortic dissection, which involves the ascending aorta irrespective of the site of the intimal tear according to the Stanford system, remains the most common aortic catastrophe despite recent advances in diagnosis and treatment. Prompt surgical repair is required because of an extremely high mortality 22.7% within the first six hours, 33.3% within 12 hours, 50% within 24 hours, and 68.2% within the first two days if left untreated.4 Acute lung injury (ALI) and its more severe form, ARDS, are lethal complications during perioperative management; aortic surgery accounts for 16% of ARDS cases.2 ALI and hypoxemia were observed in more than half of type A acute aortic dissection patients, with ARDS occurring in 10–20% of the population.5,6 Additionally, emergent surgery predisposed type A acute aortic dissection patients to higher risk of ARDS.7 Thus, a better understanding of the independent risk factors for ARDS in type A acute aortic dissection is essential to improve clinical strategies and outcomes.
However, drawing conclusions is challenging because studies on postoperative ARDS have primarily included patients undergoing heterogeneous cardiac surgeries, such as coronary artery bypass grafting (CABG) and multiple valve surgery. Only one recent study reported an occurrence of 15.9% in ARDS following aortic dissection surgery.6 More research is needed to identify pre- and perioperative risk factors for ARDS. Therefore, we firstly established and validated a predictive nomogram to calculate the probability of ARDS for individuals with type A acute aortic dissection, which might enhance the clinical decision-making.
ObjectivesThe study aimed to identify independent risk factors and establish a numerical model for postoperative acute respiratory distress syndrome in type A aortic dissection patients.
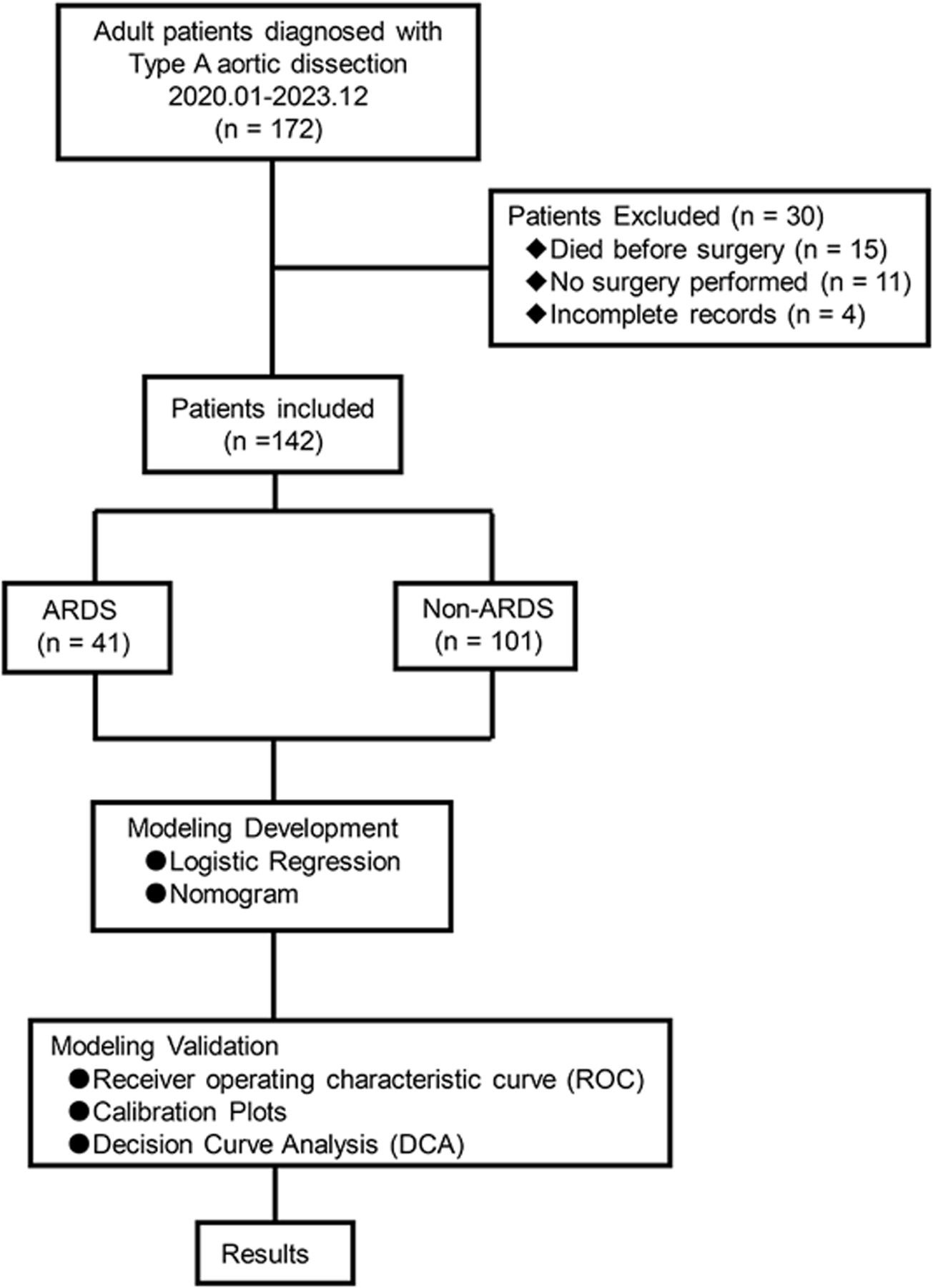
MethodsStudy participants and designThis was a single-center, retrospective, observational study. Clinical data were collected from the hospital database between January 2020 and December 2023. Eligible participants were over 18 years old. The exclusion criteria for this study were as follows: (1) death before surgery; (2) no surgery performed; (3) incomplete records exceeding 20%. One hundred forty-two clinical records were enrolled into this study.
This study was approved by the Institutional Review Board of the First Affiliated Hospital of Jinan University (No.: KY-2023-001). Informed consent was obtained from all patients (or their guardians). All personal information was de-identified prior to analysis. The study was conducted in accordance with the Declaration of Helsinki.
Clinical data and definitionsAcute respiratory distress syndrome was defined in accordance with the 2012 Berlin definition as follows8: (1) hypoxemia, acute onset within first week of exposure to a known clinical insult or new or worsening respiratory symptoms; (2) bilateral opacities on chest image that cannot be fully explained by the presence of pleural effusions, lobar or lung collapse, or nodules; and (3) clinical outcomes not fully explained by fluid overload or heart failure. Imaging findings were reviewed independently by two qualified physicians, and a third one was consulted in cases of disagreement over the diagnosis.
Data on demographic and clinical characteristics, including age, gender, body mass index (BMI), mean arterial pressure, vital signs, and cardiac function as well as comorbidities such as smoking, cerebrovascular disease, hypertension, diabetes, end-stage renal disease, and coronary heart disease were extracted. Surgery details, including the procedure, blood product transfusion, duration of the operation, CPB, mechanical ventilation, and aortic clamping, were recorded. Preoperative laboratory test results, including leukocyte count, hemoglobin, albumin, cardiac troponin I, N-terminal B-type natriuretic peptide, fibrinogen, D-dimer, prothrombin time, and serum creatinine were also collected. Postoperatively, all patients were transferred to the intensive care unit (ICU), where chest X-rays were conducted daily for the first three days. Serum procalcitonin (PCT) levels were measured on the first postoperative day. Postoperative complications, including stroke, deep sternal wound infection, tracheostomy, disseminated intravascular coagulation, liver failure, acute renal failure, and parenternal nultrition, were documented during follow-up until discharge from hospital.
Statistical analysisData were collected and analyzed using R software package (Version 4.2.1) and DCPM (V2.33 Jingding Medical Technology Co., Ltd). Variables with a p-value ≤0.05 in the univariate logistic regression were considered potential predictors for postoperative ARDS and entered into a multivariate logical regression analysis with backward elimination, using the “glm” package in R. Results were presented as odds ratios (OR) with 95% confidence intervals (CIs). The prediction model established through multivariate logistic regression was visualized with a nomogram using the “rms” package in R. Model performance was evaluated using receiver operating characteristic (ROC) curve analysis and calibration plots, with a bootstrap resampling method (bootstrap=500). The area under the working characteristic curve (AUC) was used to assess model discrimination, while calibration was estimated using Hosmer–Lemeshow test. Clinical utility was assessed by decision curve analysis (DCA) combined with a cutoff value. All tests were two-tailed tests and p-values ≤0.05 were considered statistically significant. Variables with missing data exceeding 20% were excluded to ensure data reliability. A flow diagram depicting the study process is provided (Figure 1).
ResultsCharacteristics of the study populationA total of 172 patients diagnosed with Stanford type A aortic dissection preliminarily fulfilled the entry criteria for this study. Of these, 30 were excluded due to the following reasons: 15 cases died before surgery, 11 cases did not undergo surgery, and four cases had incomplete records. One hundred forty-two adult patients were enrolled, with a mean age of 52.387±11.587 years; 87.324% were male (18 females and 124 males). Among the enrolled patients, 41 (28.873%) developed postoperative ARDS. The study population exhibited various comorbidities: a history of smoking was observed in 40 patients (28.169%), cerebrovascular disease in 13 patients (9.155%), hypertension in 95 patients (66.901%), diabetes in six patients (4.225%), end-stage renal disease in seven patients (4.930%) and coronary heart disease in four patients (2.817%). According to the New York Heart Association (NYHA) classification, nine patients (6.338%) were considered class II, 43 patients (30.282%) were considered class III, and 90 patients (63.380%) were considered class IV.
All patients underwent aortic repair surgery through median sternotomy, with CPB established through femoral artery and right axillary artery cannulation. Thirty-four patients (23.944%) received Bentall surgery, which involved replacing the aortic valve with a mechanical valve; nine patients (6.338%) underwent CABG, and two cases (1.401%) had mitral and/or tricuspid valve repair. The mean durations of the operations, CPB, aortic clamping, and mechanical ventilation were 473.754±99.719 minutes, 237.831±65.652 minutes, 129.690±34.941 minutes, and 45.500 (20.500, 107.750) hours, respectively. The average length of ICU stay was 9.620±9.238 days. The all-cause mortality rate in hospital was 7.745% (11 cases).
Risk factors and the prediction modelPatients in the ARDS group had significantly higher BMI (26.935±4.650 vs. 25.034±3.782 kg/m2, p=0.012), leukocyte counts (13.165±4.448 vs. 11.417±4.228×109, p=0.030), PCT [21.960 (4.557, 43.000) vs. 4.643 (2.408, 12.855) ng/mL, p<0.001], as well as longer durations of operation (505.122±105.575 vs. 461.020±94.843 min, p=0.016) and CPB time (268.244±69.305 vs. 225.485±60.191 min, p<0.001) compared to patients in the non-ARDS group. A higher proportion of patients who underwent CABG [6 (14.634%) vs. 3 (2.970%), p=0.027] was observed in the ARDS group. Additionally, albumin levels were notably decreased in ARDS patients (34.341±5.376 vs. 36.629±4.456 g/L, p=0.010).
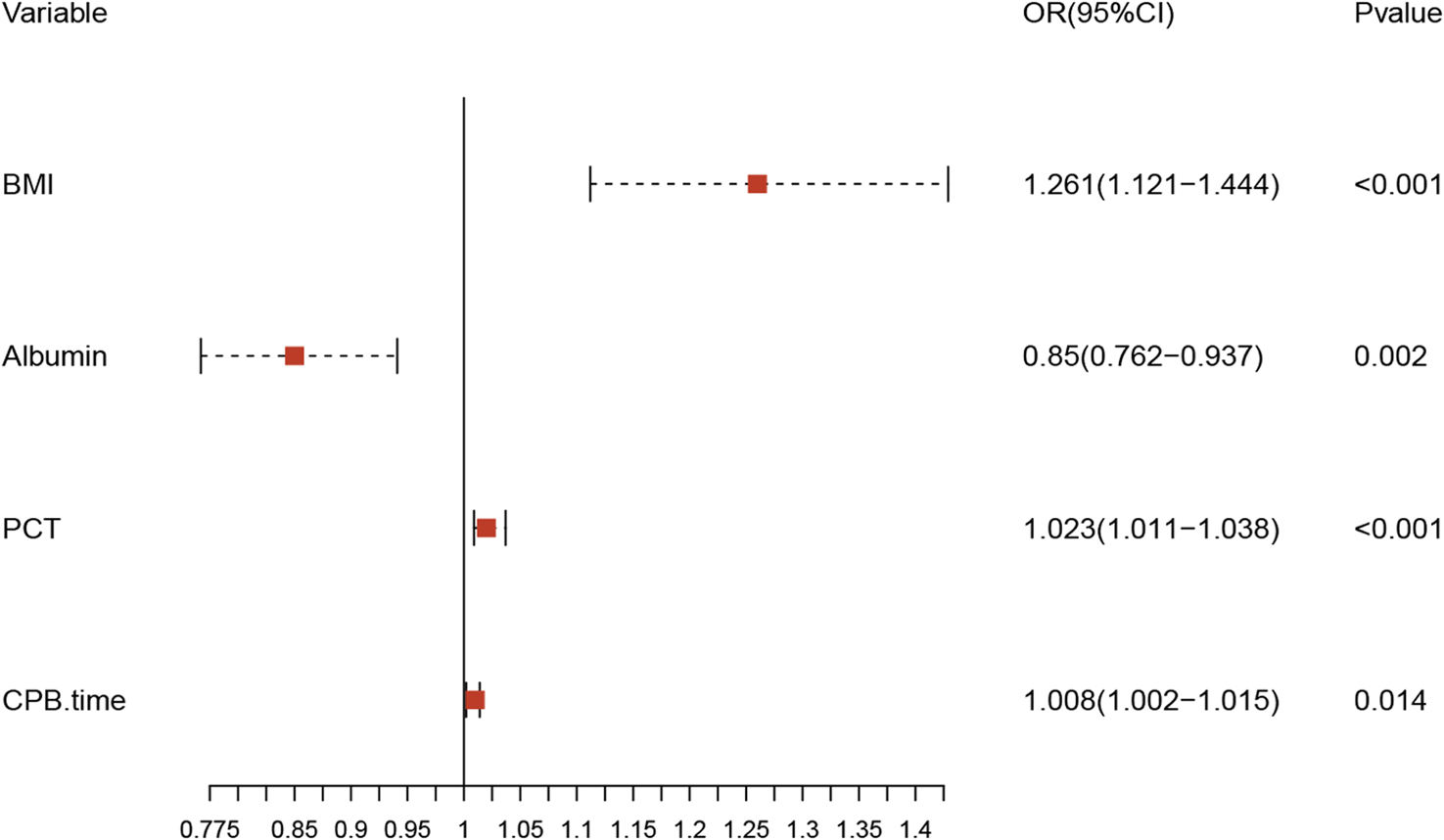
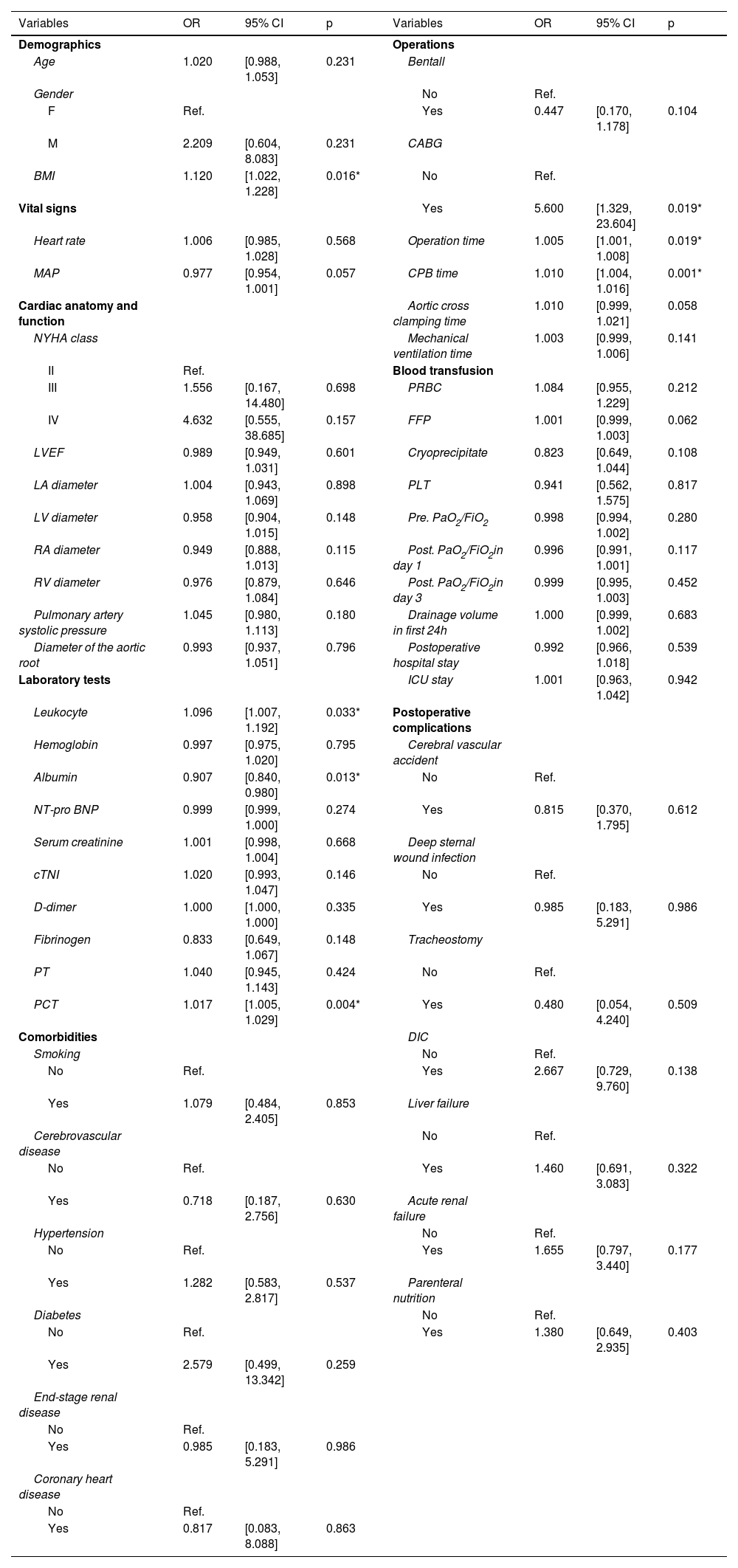
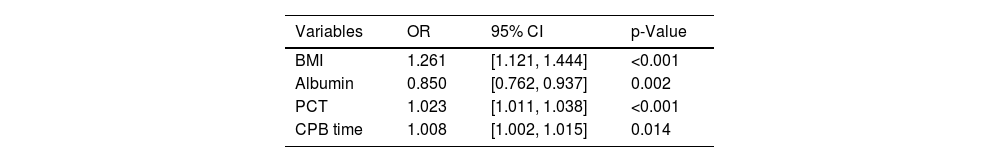
Univariate analysis identified several potential risk factors for postoperative ARDS (Table 1): BMI (OR: 1.120; 95% CI: 1.022–1.228), leukocyte (1.096; 1.007–1.192), albumin (0.907; 0.840–0.980), PCT (1.017; 1.005–1.029), CABG (5.600; 1.329–23.604), operation time (1.005; 1.001–1.008), and CPB time (1.010; 1.004–1.016). Variables with a p-value ≤0.05 were entered into a multivariate logical regression analysis. The results of the multivariate analysis, illustrated in Table 2 and visualized in a forest plot (Figure 2), identified four predictors of postoperative ARDS: BMI (OR: 1.261; 95% CI: 1.121–1.444), albumin (0.850; 0.762–0.937), PCT (1.023; 1.011–1.038), and CPB time (1.008; 1.002–1.015).
Univariate analysis of variables related to postoperative ARDS in TAAD patients.
| Variables | OR | 95% CI | p | Variables | OR | 95% CI | p |
|---|---|---|---|---|---|---|---|
| Demographics | Operations | ||||||
| Age | 1.020 | [0.988, 1.053] | 0.231 | Bentall | |||
| Gender | No | Ref. | |||||
| F | Ref. | Yes | 0.447 | [0.170, 1.178] | 0.104 | ||
| M | 2.209 | [0.604, 8.083] | 0.231 | CABG | |||
| BMI | 1.120 | [1.022, 1.228] | 0.016* | No | Ref. | ||
| Vital signs | Yes | 5.600 | [1.329, 23.604] | 0.019* | |||
| Heart rate | 1.006 | [0.985, 1.028] | 0.568 | Operation time | 1.005 | [1.001, 1.008] | 0.019* |
| MAP | 0.977 | [0.954, 1.001] | 0.057 | CPB time | 1.010 | [1.004, 1.016] | 0.001* |
| Cardiac anatomy and function | Aortic cross clamping time | 1.010 | [0.999, 1.021] | 0.058 | |||
| NYHA class | Mechanical ventilation time | 1.003 | [0.999, 1.006] | 0.141 | |||
| II | Ref. | Blood transfusion | |||||
| III | 1.556 | [0.167, 14.480] | 0.698 | PRBC | 1.084 | [0.955, 1.229] | 0.212 |
| IV | 4.632 | [0.555, 38.685] | 0.157 | FFP | 1.001 | [0.999, 1.003] | 0.062 |
| LVEF | 0.989 | [0.949, 1.031] | 0.601 | Cryoprecipitate | 0.823 | [0.649, 1.044] | 0.108 |
| LA diameter | 1.004 | [0.943, 1.069] | 0.898 | PLT | 0.941 | [0.562, 1.575] | 0.817 |
| LV diameter | 0.958 | [0.904, 1.015] | 0.148 | Pre. PaO2/FiO2 | 0.998 | [0.994, 1.002] | 0.280 |
| RA diameter | 0.949 | [0.888, 1.013] | 0.115 | Post. PaO2/FiO2in day 1 | 0.996 | [0.991, 1.001] | 0.117 |
| RV diameter | 0.976 | [0.879, 1.084] | 0.646 | Post. PaO2/FiO2in day 3 | 0.999 | [0.995, 1.003] | 0.452 |
| Pulmonary artery systolic pressure | 1.045 | [0.980, 1.113] | 0.180 | Drainage volume in first 24h | 1.000 | [0.999, 1.002] | 0.683 |
| Diameter of the aortic root | 0.993 | [0.937, 1.051] | 0.796 | Postoperative hospital stay | 0.992 | [0.966, 1.018] | 0.539 |
| Laboratory tests | ICU stay | 1.001 | [0.963, 1.042] | 0.942 | |||
| Leukocyte | 1.096 | [1.007, 1.192] | 0.033* | Postoperative complications | |||
| Hemoglobin | 0.997 | [0.975, 1.020] | 0.795 | Cerebral vascular accident | |||
| Albumin | 0.907 | [0.840, 0.980] | 0.013* | No | Ref. | ||
| NT-pro BNP | 0.999 | [0.999, 1.000] | 0.274 | Yes | 0.815 | [0.370, 1.795] | 0.612 |
| Serum creatinine | 1.001 | [0.998, 1.004] | 0.668 | Deep sternal wound infection | |||
| cTNI | 1.020 | [0.993, 1.047] | 0.146 | No | Ref. | ||
| D-dimer | 1.000 | [1.000, 1.000] | 0.335 | Yes | 0.985 | [0.183, 5.291] | 0.986 |
| Fibrinogen | 0.833 | [0.649, 1.067] | 0.148 | Tracheostomy | |||
| PT | 1.040 | [0.945, 1.143] | 0.424 | No | Ref. | ||
| PCT | 1.017 | [1.005, 1.029] | 0.004* | Yes | 0.480 | [0.054, 4.240] | 0.509 |
| Comorbidities | DIC | ||||||
| Smoking | No | Ref. | |||||
| No | Ref. | Yes | 2.667 | [0.729, 9.760] | 0.138 | ||
| Yes | 1.079 | [0.484, 2.405] | 0.853 | Liver failure | |||
| Cerebrovascular disease | No | Ref. | |||||
| No | Ref. | Yes | 1.460 | [0.691, 3.083] | 0.322 | ||
| Yes | 0.718 | [0.187, 2.756] | 0.630 | Acute renal failure | |||
| Hypertension | No | Ref. | |||||
| No | Ref. | Yes | 1.655 | [0.797, 3.440] | 0.177 | ||
| Yes | 1.282 | [0.583, 2.817] | 0.537 | Parenteral nutrition | |||
| Diabetes | No | Ref. | |||||
| No | Ref. | Yes | 1.380 | [0.649, 2.935] | 0.403 | ||
| Yes | 2.579 | [0.499, 13.342] | 0.259 | ||||
| End-stage renal disease | |||||||
| No | Ref. | ||||||
| Yes | 0.985 | [0.183, 5.291] | 0.986 | ||||
| Coronary heart disease | |||||||
| No | Ref. | ||||||
| Yes | 0.817 | [0.083, 8.088] | 0.863 |
ARDS: acute respiratory distress syndrome; BMI: body mass index; CABG: coronary artery bypass grafting; CI: confidential interval; CPB: cardiopulmonary bypass; cTNI: cardiac troponin I; DIC: disseminated intravascular coagulation; FFP: fresh frozen plasma; FiO2: fraction of inspired oxygen; ICU: intensive care unit; LA: left atrium; LV: left ventricle; LVEF: left ventricular ejection fraction; MAP: mean arterial pressure; NT-pro BNP: N-terminal B-type natriuretic peptide; NYHA: New York Heart Association function classification; OR: odds ratio; PaO2: partial pressure of oxygen in the arterial blood; PCT: procalcitonin; PLT: platelet; PRBC: packed red blood cell; PT: prothrombin time; RA: right atrium; RV: right ventricle; TAAD: type A aortic dissection.
Multivariate logistic regression analysis of independent risk factors for postoperative ARDS in TAAD patients.
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| BMI | 1.261 | [1.121, 1.444] | <0.001 |
| Albumin | 0.850 | [0.762, 0.937] | 0.002 |
| PCT | 1.023 | [1.011, 1.038] | <0.001 |
| CPB time | 1.008 | [1.002, 1.015] | 0.014 |
BMI: body mass index; CPB: cardiopulmonary bypass; PCT: procalcitonin.
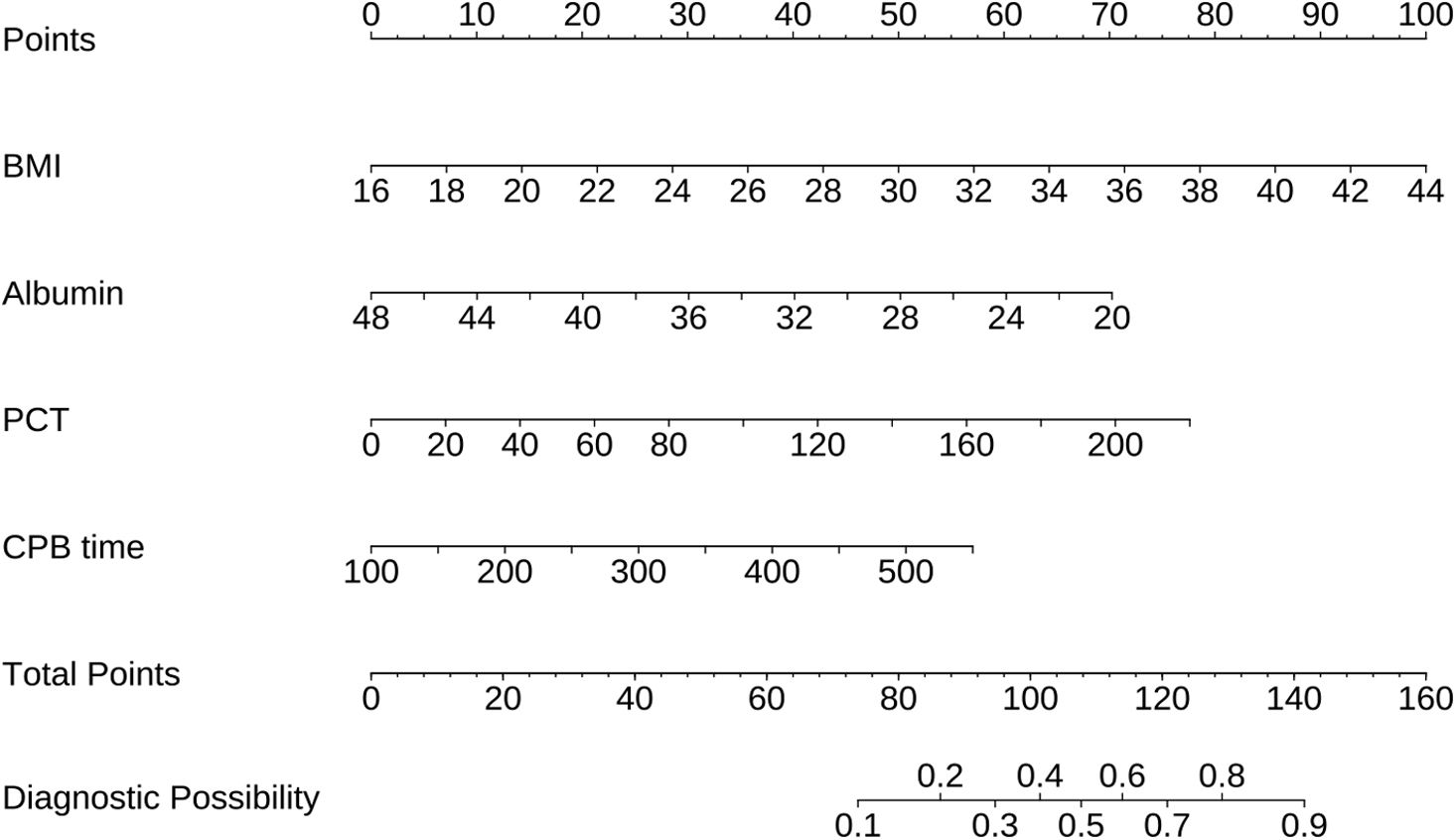
A nomogram was developed incorporating BMI, albumin, PCT, and CPB time to predict the risk of postoperative ARDS in type A acute aortic dissection patients (Figure 3). Each predictor was assigned a score range based on its value, and the total score, derived from summing the points for each factor, can be used to estimate the probability of ARDS.
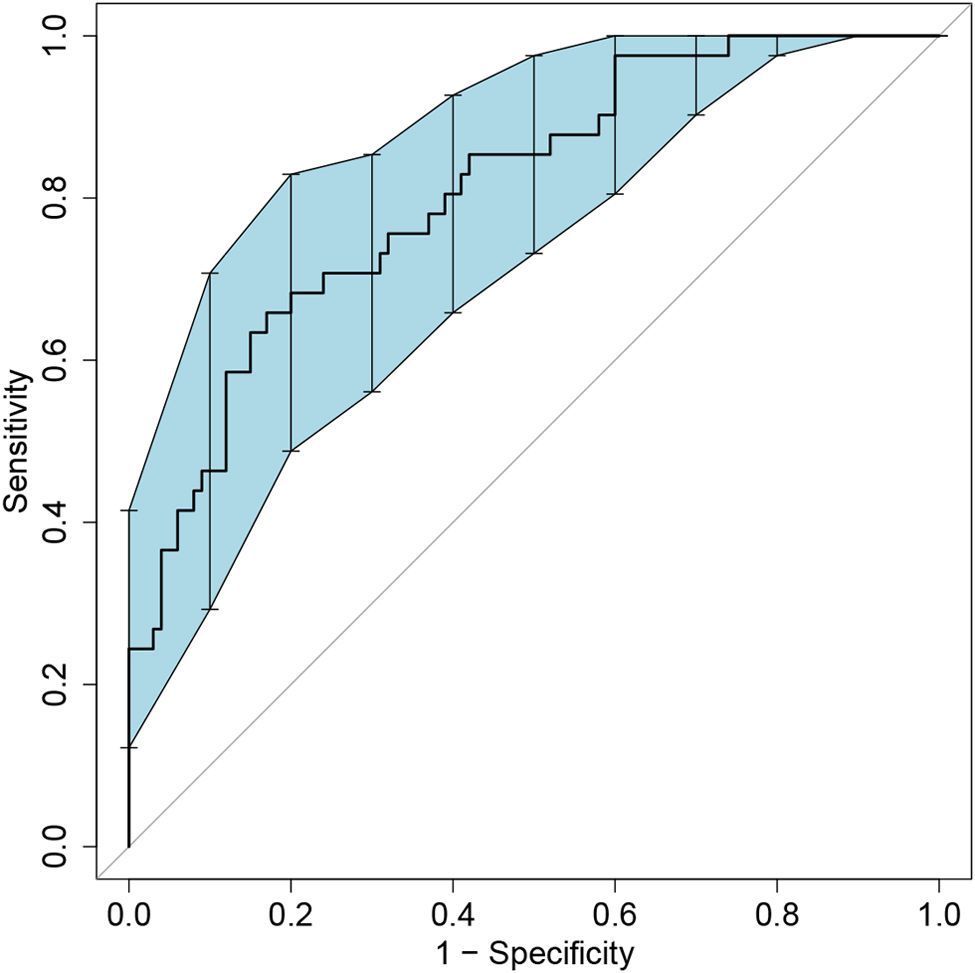
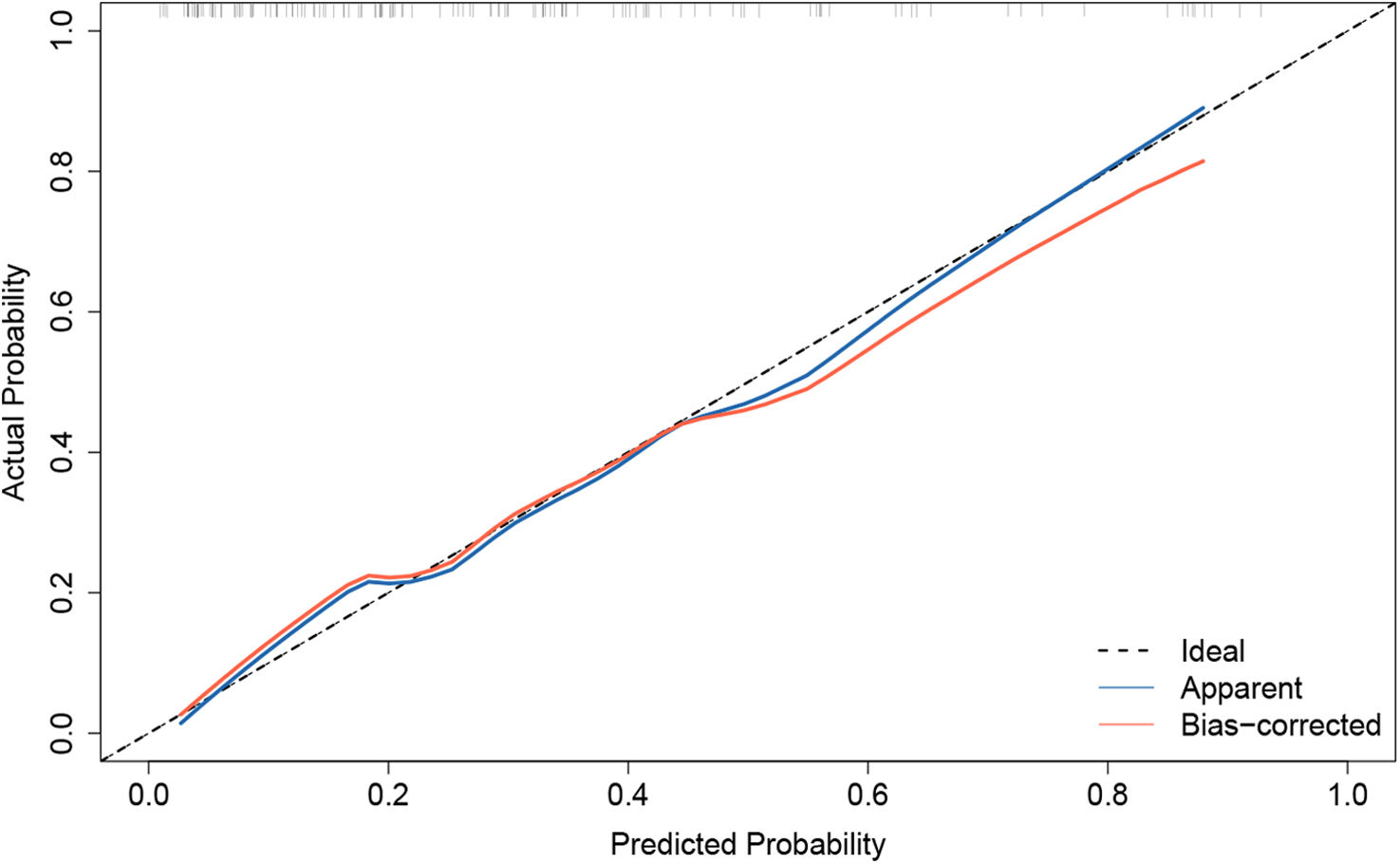
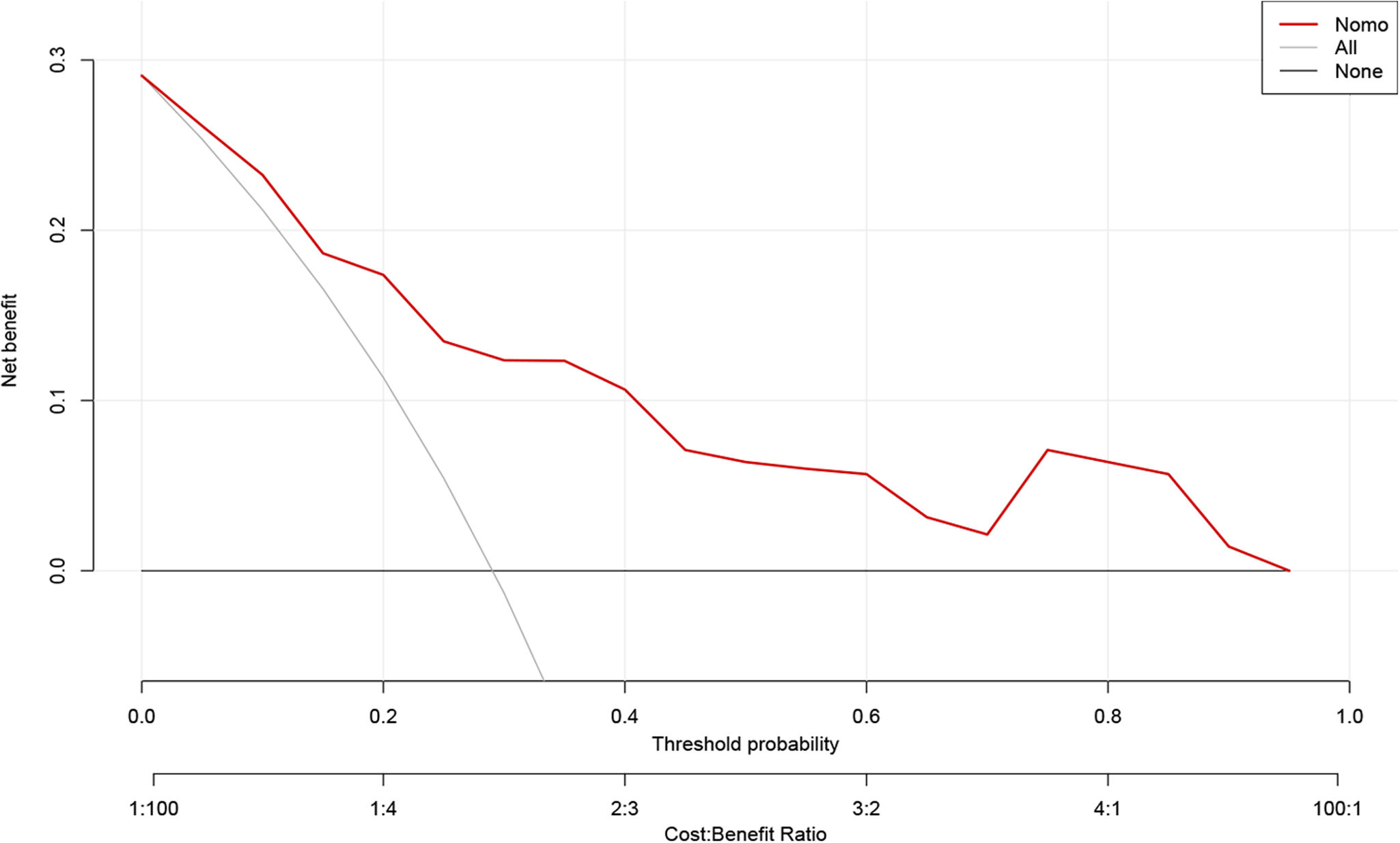
Model validation and net benefit predictionThe predictive model for estimating the occurrence of ARDS after aortic surgery demonstrated stable performance, as validated by bootstrapping with 500 replications (Figure 4). The pooled area under the ROC curve was 0.809 (95% CI: 0.721–0.881). The optimal cutoff value for predicting postoperative ARDS was 0.343, providing a high specificity of 0.830 and a sensitivity of 0.659. The Hosmer–Lemeshow test (χ2=3.664, p=0.932) indicated a good concordance between the predicted probabilities and the observed postoperative ARDS in type A acute aortic dissection patients (Figure 5). Boosted DCA curve displayed clinical benefit in Figure 6, with a threshold probability range of 0.01–0.95. The horizontal line represents the scenario in which no patients develop ARDS and thus no benefit from intervention. The diagonal line represents the scenario in which all patients develop ARDS, showing potential benefit from treatment. Therefore, the DCA confirms the clinical practicality of the prediction model within this range.
This study is the first to develop a nomogram of postoperative ARDS in type A acute aortic dissection patients, which illustrates the regression model in an accessible way and thus simplifies the risk assessment. We analyzed clinical characteristics, laboratory values and postoperative outcomes to identify the most relevant variables for multivariate regression analysis. Four independent predictors for postoperative ARDS were identified: BMI, albumin, PCT, and CPB time.
Postoperative ARDS carries a high mortality rate, ranging from 10% to 70%, resulting from the differences in ARDS definitions, types of surgeries, and study populations.1,6,9–11 Previous definitions of ARDS, such as the Murray's Lung Injury Score from 1988, the American–European Consensus Conference definition (AECC) from 1994, and the Berlin definition from 2012, offer varying criteria for diagnosis and intervention.8,12,13 The Berlin definition, which is the most recent one, provided practical guidelines for early recognition and intervention of ARDS. However, the application of the Berlin definition to type A acute aortic dissection patients specifically has not been thoroughly investigated. Studies on ARDS following cardiac surgery often included a mix of procedures involving CPB, such as valvular surgery and CABG. These varied combinations can lead to different incidence and mortality rates. For instance, a study reported that 8.1% of 457 patients undergoing isolated valvular heart surgery developed postoperative ARDS, with a mortality rate of 29.7%.10 In contrast, a broader study including CABG, valve, and combined procedures found mortality of 68.4%.11 Our study observed an ARDS incidence of 28.873% in type A acute aortic dissection patients, which was slightly higher than reported in some studies, possibly due to the high proportion of patients classified as NYHA class IV.6
The pathophysiology of ARDS following CPB has not been totally understood. The prevailing hypothesis involves activation of the inflammatory cascade, including polymorphonuclear leukocyte and cytokine activation, which increases pulmonary permeability and vascular resistance, leading to impaired gas exchange.14 Our findings indicate that CPB time is a significant risk factor in ARDS. Besides, protamine-heparine complexes may trigger immune reactions by releasing anaphylatoxins such as C4a and C3a, potentially increasing ARDS risk.1 Patients with ARDS were reported to have lower preoperative albumin, which was similar in our study, and higher plasminogen activator inhibitor-1, interleukin-8 and surfactant protein-D.15 Up-regulation of tumor necrosis factor-alpha and elevation of serum endocan are also believed to get involved in the early phase of ARDS.16,17 Albumin in the extracorporeal prime can preserve platelet count and reduce chest tube drainage by passivating the synthetic surfaces of the extracorporeal circuit.18 Elevated PCT levels were also associated with ARDS in our study, suggesting it could serve as a potential diagnostic biomarker.19 Timely infection control and interruption of inflammatory processes are crucial for ARDS prevention. Corticosteroids have been shown to improve cardiopulmonary function within three to seven days and alter the ARDS course, with early treatment improving ICU and ventilator outcomes with minimal safety concerns.20
Transfusion-related complications, including transfusion-related acute lung injury and transfusion-associated circulatory overload, are significant concerns. Multiple transfusions have been linked to ARDS risk, with packed red blood cell transfusions exhibiting a dose-dependent effect on pulmonary capillary permeability.21 In a large observational cohort of CABG patients (11963), red blood cell transfusion was associated with higher mortality, renal failure, prolonged ventilatory support.22 A retrospective nested case–control study demonstrated extended durations of CPB, deep hypothermic circulatory arrest, ICU stay, and larger perioperative transfusions volumes (red blood cell, platelet and fresh frozen plasma) in ARDS group of type A acute aortic dissection population.23 Although our study did not identify blood product transfusion as an independent predictor of ARDS, possibly due to sample size limitations and the urgent nature of aortic surgery, meticulous hemostasis and restrictive transfusion strategies could mitigate ARDS risk. A restrictive transfusion strategy targeting a hematocrit of 24% has been shown to be as safe as a liberal strategy in elective cardiac surgery, though caution is needed for elderly patients due to cardiovascular risks.24,25
Obesity's role in ARDS remains debated and is known as the “obesity paradox”. While excess body weight has not consistently been linked to increased mortality in acute lung injury cases,26,27 it is associated with longer hospital stays.28,29 A study of 5023 patients undergoing heart surgery with CPB found that obese patients were at higher risk for postoperative hypoxia, but were somewhat protected against adverse effects from hemodilution and transfusions.30 Our study confirmed that BMI is an independent risk factor of ARDS after aortic surgery. A case–control study identified a BMI cutoff of 24.78 kg/m2, with ARDS likelihood increasing above this threshold.31 While BMI is challenging to modify in emergency settings, weight control strategies could benefit postoperative outcomes.
This study has limitations, including the inability to split the data into training and validation sets due to a limited sample size. Consequently, model validation was performed using bootstrap methods. Additionally, among data from a monocenter there may be some degree of bias. Further research with multicenter data and larger sample sizes is needed to enhance the validity and generalizability of these findings.
ConclusionWe established a straightforward predictive nomogram to estimate the probability of ARDS in patients with type A acute aortic dissection by incorporating four perioperative variables, including BMI, albumin, procalcitonin and CPB time. The accuracy and stability of the model were validated internally using the AUC, calibration plot, and DCA. This nomogram has the potential to serve as a valuable prognostic tool for assessing the risk of ARDS in type A acute aortic dissection patients.
FundingNone declared.
Conflicts of interestNone declared.