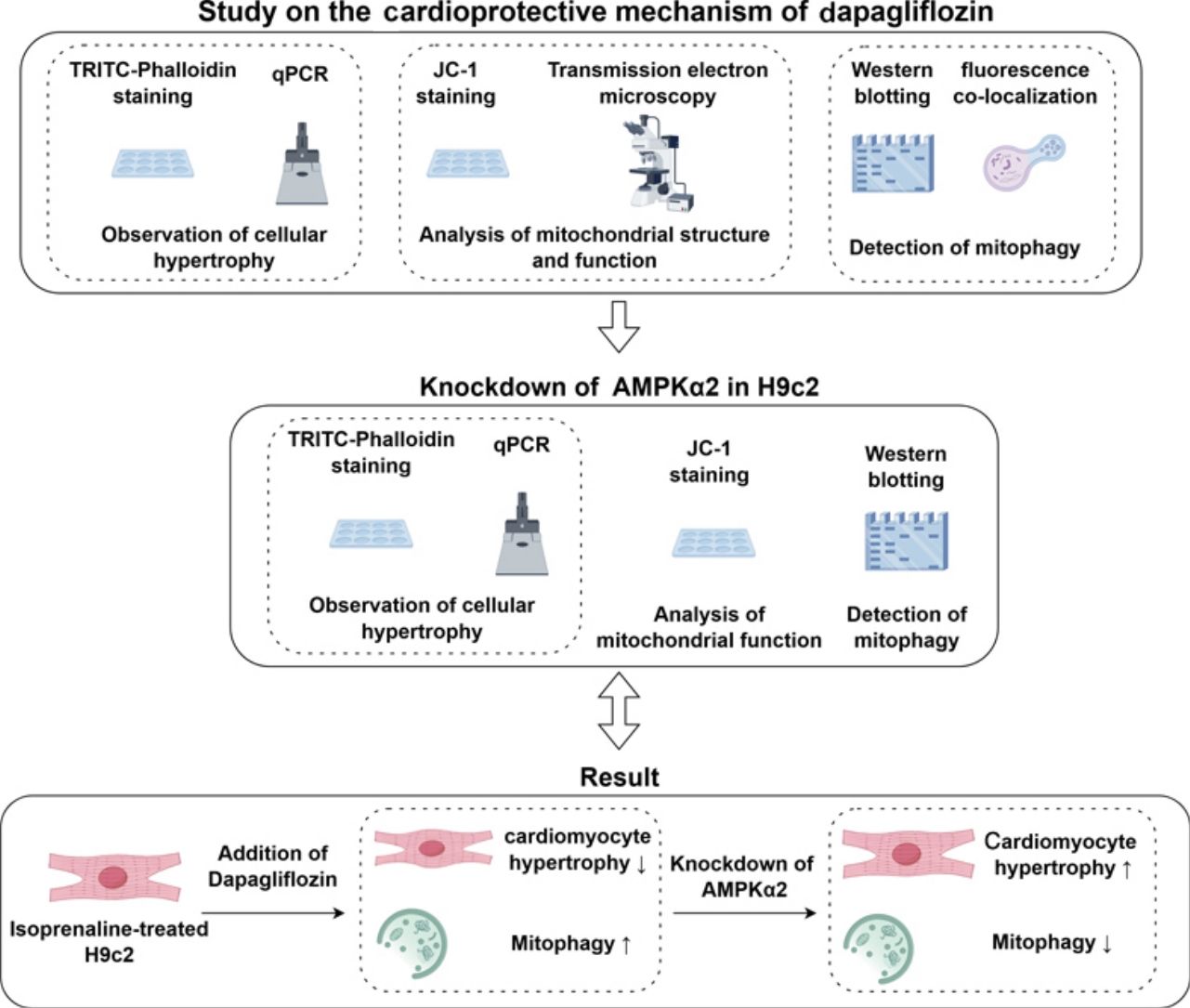
The global prevalence of heart failure (HF) has been increasing in recent years, posing a significant threat to human health. Several studies have shown that impaired mitophagy accelerates HF progression. Dapagliflozin (DAPA) has demonstrated cardioprotective effects in HF patients. This study aims to investigate the therapeutic effects of DAPA on cardiomyocyte hypertrophy and its underlying mechanism.
MethodsThe working concentration of isoprenaline (ISO) was determined through combined quantitative real-time polymerase chain reaction (qPCR) and CCK-8 assays. H9c2 cells were stimulated with ISO to induce a hypertrophy model. Cellular hypertrophy was quantified by qPCR and TRITC-phalloidin staining. Mitochondrial ultrastructure and functional integrity was assessed by transmission electron microscopy and JC-1 staining. Mitophagy levels were measured using Western blotting and co-localization assays. AMPKα2 expression levels were determined via Western blotting. Following AMPKα2 siRNA transfection, cellular hypertrophy and mitophagy levels were reassessed.
ResultsISO markedly induced cardiomyocyte hypertrophy, mitochondrial damage and mitophagy inhibition, whereas DAPA effectively attenuated these pathologica changes, with AMPK agonists demonstrating comparable cardioprotective effects. In ISO-treated H9c2 cells, AMPKα2 expression was reduced, while DAPA significantly upregulated its expression. Notably, AMPKα2 inhibition significantly weakened DAPA's protective effect on ISO-induced hypertrophy and mitochondrial injury.
ConclusionDAPA exerts cardioprotective effects by mitigating ISO-induced cardiac hypertrophy and preserving mitochondrial integrity, mediated through AMPKα2-dependent activation of mitophagy.
Nos últimos anos, a incidência de insuficiência cardíaca (IC) tem vindo a aumentar a nível mundial, representando uma grave ameaça para a saúde humana. Vários estudos demonstram que a disfunção da mitofagia acelera a progressão da IC. A dapagliflozina (DAPA) tem evidenciado efeitos cardioprotetores em doentes com insuficiência cardíaca. Este estudo visa investigar os efeitos terapêuticos da DAPA na hipertrofia dos cardiomiócitos e os seus mecanismos subjacentes.
MétodosA concentração eficaz de isoproterenol (ISO) foi determinada através de PCR quantitativo em tempo real (qPCR) e de ensaio CCK-8. As células H9c2 foram estimuladas com ISO para induzir um modelo de hipertrofia. A hipertrofia celular foi quantificada através de qPCR e coloração com TRITC-Faloidina. A estrutura ultramicroscópica e a integridade funcional das mitocôndrias foram avaliadas por microscopia eletrónica de transmissão e coloração JC-1. Os níveis de mitofagia foram medidos por Western blot e ensaio de co-localização. A expressão de AMPKα2 foi avaliada por Western blot. Após a transfecção com siRNA dirigido a AMPKα2, a hipertrofia celular e os níveis de mitofagia foram reavaliados.
ResultadosO ISO induziu de forma significativa a hipertrofia dos cardiomiócitos, lesão mitocondrial e inibição da mitofagia, enquanto a DAPA atenuou eficazmente estas alterações patológicas. O agonista de AMPK apresentou efeitos cardioprotetores semelhantes. Nas células H9c2 tratadas com ISO, a expressão de AMPKα2 foi reduzida, mas a DAPA aumentou significativamente essa expressão. Importa referir que a inibição de AMPKα2 reduziu de forma marcante os efeitos protetores da DAPA contra a hipertrofia e lesão mitocondrial induzidas por ISO.
ConclusãoA DAPA exerce efeitos cardioprotetores ao ativar a mitofagia mediada por AMPKα2, aliviando a hipertrofia cardíaca induzida por ISO e preservando a integridade mitocondrial.
Heart failure (HF) is the end-stage of various cardiovascular diseases caused by multiple factors. Between 1990 and 2019, the global prevalence of HF surged by 106.3%, reaching 56.2 million cases in 2019, according to the Global Burden of Diseases Study.1 Patients with HF experience numerous symptoms, including dyspnea, fatigue, poor activity tolerance and fluid retention, which seriously impact their quality of life and life expectancy and impose a significant burden on family and society. Currently, the treatments for HF are diverse and include pharmacotherapy, device therapy and lifestyle modifications. Traditional pharmacotherapy, including angiotensin-converting enzyme inhibitors, beta-blockers and diuretics, has been widely used but with limited efficacy.2 Therefore, further exploration of the different pathophysiological mechanisms of HF is needed to identify novel therapeutic targets and drug candidates for more precise treatment.
The heart, a high-energy-demanding organ, relies heavily on mitochondrial function, as mitochondria supply 95% of the heart's ATP.3 Study shows that mitochondrial dysfunction is closely linked to the occurrence and progression of HF, leading to impaired myocardial energy metabolism, increased oxidative stress, cardiomyocyte damage and death and ultimately worsening HF.4 Mitophagy removes damaged mitochondria, maintaining mitochondrial quality and function. When mitochondrial content or function declines, an increase in the cellular AMP:ATP ratio activates AMP-activated protein kinase (AMPK).5 Activated AMPK directly or indirectly phosphorylates Unc-51-like autophagy activating kinase 1, initiating autophagosome formation and promoting the translocation of the autophagy machinery to damaged mitochondria.6 In the heart, AMPK exists in two major isoforms, AMPKα1 and AMPKα2, AMPKα2 stimulates the PTEN-induced putative kinase 1 (PINK1)-Parkin-SQSTM1 pathway involved in cardiac mitophagy via PINK1 phosphorylation.7 Decreased mitophagy is observed in HF, and enhancing AMPKα2-related mitophagy is expected to improve mitochondrial function and alleviate HF. These results denote that AMPKα2 is a potential therapeutic target and that modulating the mitophagy pathway may be a way to treat HF.
Dapagliflozin (DAPA), a sodium-glucose cotransporter-2 inhibitor, reduces the risk of cardiovascular death or worsening HF in patients with chronic HF and reduced ejection fraction.8 Several mechanisms, including reversal of cardiac remodeling, improvement of cardiac energy metabolism, reduction of cardiac fibrosis and inflammation and amelioration of endothelial dysfunction, have been proposed to explain its cardioprotective effects, though many remain incompletely defined.9 DAPA activates AMPK phosphorylation in vitro and in vivo to exert cardiac benefits, and AMPKα2-dependent mitophagy has been shown to protect the myocardium against pressure overload-induced HF.7,10 However, it remains uncertain whether AMPKα2-associated mitophagy contributes to DAPA's protective effects. Shen et al. found that DAPA could dephosphorylate FUNDC1 in a PKM2-dependent manner, thereby restoring mitophagy and improving mitochondrial function.11 Given this evidence, we propose that DAPA might protect against HF by promoting mitophagy via the AMPKα2 signaling pathway.
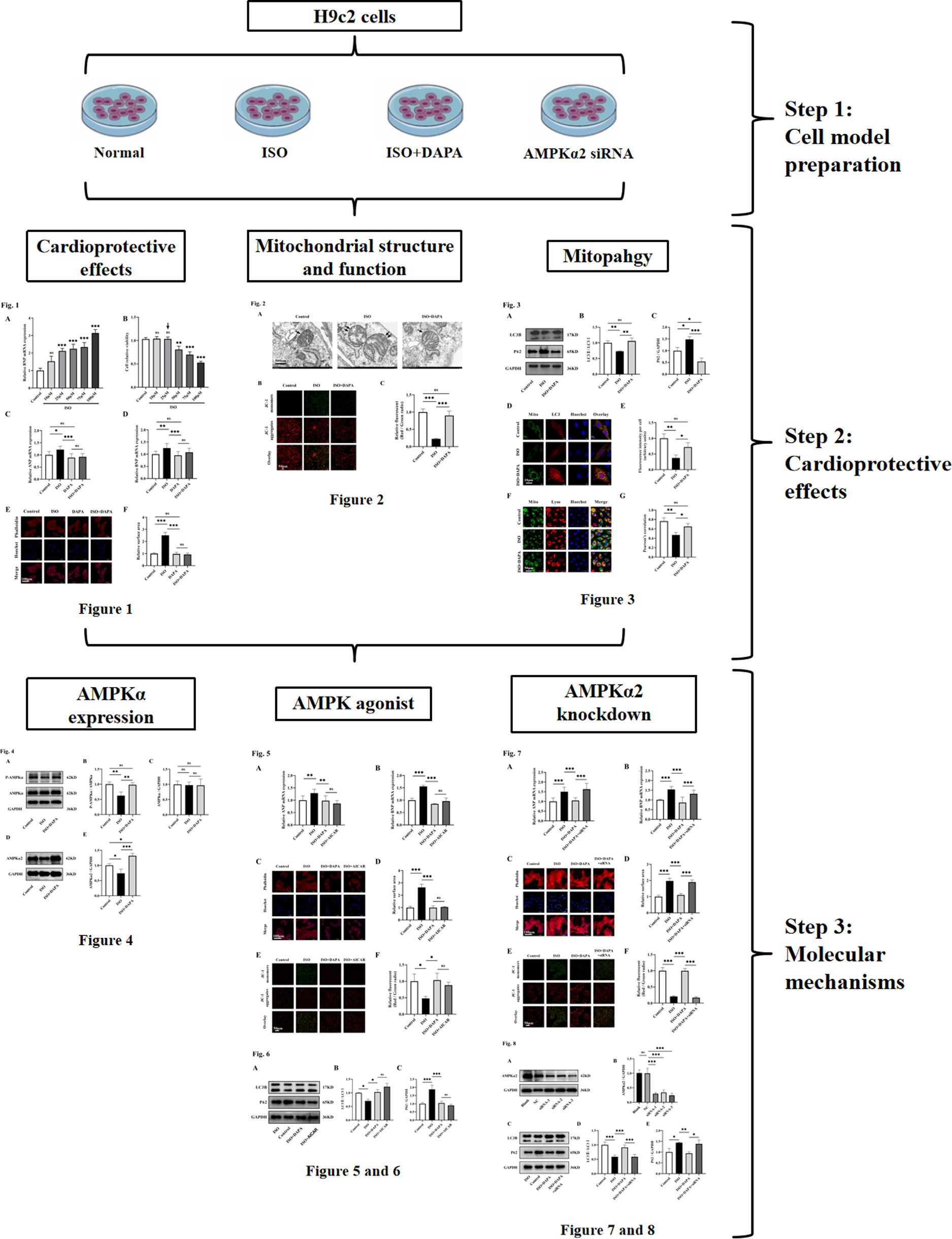
ObjectivesThis study employed an isoproterenol-induced H9c2 cardiomyocyte hypertrophy model to investigate dapagliflozin's cardioprotective mechanisms against heart failure pathogenesis, providing mechanistic insights for dapagliflozin's therapeutic potential in heart failure management.
MethodsCell culture and treatmentRat embryonic cardiomyoblast-derived H9c2 cells (TCR-C607; Cas9X, Suzhou, China) were maintained in high-glucose Dulbecco's modified Eagle's medium (DMEM; Boster, Wuhan, China) containing 10% fetal bovine serum (FBS, CF01S02; CellBox, Hunan, China) and 1% penicillin–streptomycin antibiotic cocktail (PYG0016; Boster, Wuhan, China). Cells were incubated at a constant temperature of 37°C under a humidified atmosphere of 5% CO2 and 95% air.
Isoproterenol (ISO, I5627; Sigma-Aldrich, St. Louis, MO, USA) was first dissolved in water and diluted to 25 μM in culture medium. Dapagliflozin (DAPA, HY-10450; MedChemExpress, Monmouth Junction, NJ, USA), provided as a 10 mM stock solution in DMSO, was thawed and diluted to 10 μM. The AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR, HY-13417; MedChemExpress, Monmouth Junction, NJ, USA) was initially dissolved in water, followed by dilution to 500 μM. All pharmacological treatments were administered to H9c2 cells for 24 hours under standard culture conditions (37°C, 5% CO2).
Cell transfectionUpon reaching 80–100% confluence, cells were seeded into six-well plates and allowed to adhere until 30–50% confluence was achieved. Transfected with 100 nM AMPKα2-specific siRNA (Tsingke, Shanghai, China) was performed using GenMute™ siRNA Transfection Reagent (SL100568; SignaGen, Shanghai, China) following the manufacturer's protocol. Forty-eight hours post-transfection, total protein was extracted and subjected to Western blot analysis to assess AMPKα2 knockdown efficiency.
Cell viabilityH9c2 cells were seeded in 96-well plates at an optimal density of 3×103 cells/well in 100 μL culture medium. Following 24-hour ISO exposure, CCK-8 reagent (BS350B; Biosharp, AnHui, China) was added to each well at 10% of the total culture volume (10 μL/well), followed by incubation for 90 min at 37°C. Absorbance measurements were then performed at 450 nm using a multiskan skyhigh microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
TRITC-phalloidin stainingThe cultured H9c2 cells were fixed with 4% paraformaldehyde for 15 min, washed twice in PBS, and permeabilized with 0.05% Triton X-100 (9002-93-1; Solarbio, Beijing, China) for five minutes. They were subsequently incubated with 100 nM TRITC-phalloidin (CA1610; Solarbio, Beijing, China) in 1% BSA for 30 min, followed by three 5-min PBS washes. Nuclei were counterstained with 1 μg/mL Hoechst 33342 (C1028; Beyotime, Shanghai, China) for five minutes, then washed twice with PBS. Stained cells were captured with an inverted fluorescent microscope (ECLIPSE Ts2R, Shanghai, China). Cell surface area was quantified using ImageJ software (version 1.5.3; National Institutes of Health, Bethesda, USA) by analyzing ≥30 cells per condition.
Transmission electron microscopeFor ultrastructural analysis, treated H9c2 cells were fixed in 2.5% glutaraldehyde (G1102; Servicebio, Wuhan, China) at 4°C overnight, then post-fixed with 1% osmium tetroxide (18456; Ted Pella, Redding, CA, USA) for 2 hours at room temperature (RT). After graded dehydration by ethanol series, the cells were infiltrated with EMBed 812 resin (90529-77-4; SPI, West Chester, PA, USA). Subsequently, the resin blocks were cut to 60–80 nm thin sections, and then double-stained with 2% uranium acetate (02624-AB; SPI, West Chester, PA, USA) and 2.6% lead citrate. Ultimately, the mitochondrial ultrastructure was examined and images were captured by TEM (HITACHI, H-7500, Japan).
JC-1 stainingH9c2 cells were seeded into six well plates at a density of 1×105 cells/well (30–50% confluence). Configure the JC-1 solution (C2006; Beyotime, Shanghai, China) according to the manufacturer's instructions, 1 mL of JC-1 working solution was added to H9c2 cells, which were then incubated in a 37°C incubator for 30 min. Following incubation, cells were gently rinsed twice with ice-cold JC-1 staining buffer. Finally, fluorescence images were acquired using a fluorescence microscope (ECLIPSE Ts2R, Shanghai, China). The change in the ratio of red fluorescence (J-aggregate) to green fluorescence (monomer) represented an alteration in the level of mitochondrial membrane potential.
Analysis of mitophagyH9c2 cells were seeded in confocal dishes and 6-well plates and cultured overnight. For autophagosome visualization, confocal dish-cultured cells in confocal dishes were transduced with mCherry-LC3 lentivirus particles (G120A; Hanbio, Shanghai, China), strictly adhering to manufacturer's 1/2 smal-volume infection instructions. After 48 hours, mCherry-LC3-expressing cells were loaded with 100 nM MitoTracker™ Green FM (C1048; Beyotime, Shanghai, China) to label mitochondria, whereas cells in 6-well plates received combinatorial staining with 100 nM MitoTracker™ Green FM and 75 nM LysoTracker Red (C1046; Beyotime, Shanghai, China) for mitochondrial and lysosome labeling for 15 min at 37°C. Nuclear counterstaining was performed with 10 μg/mL Hoechst 33342 (C1028, Beyotime, Shanghai, China) for five minutes at 37°C. Lastly, co-localization images of mitochondria with autophagosomes (mCherry-LC3 puncta) and lysosomes (LysoTracker Red) were acquired using confocal microscopy (Zeiss LSM, Baden-Württemberg, Germany) and fluorescence microscopy (ECLIPSE Ts2R, Shanghai, China), respectively.
Quantitative real-time polymerase chain reactionTotal RNA was isolated from H9c2 cells using the Trizol reagent (G3013; Servicebio, Wuhan, China). Subsequently, the extracted RNA was reverse-transcribed into complementary DNA (cDNA) using a qPCR reverse transcription kit (MF166; Mei5 Biotechnology, Beijing, China) equipped with a genomic DNA (gDNA) removal functionality. Finally, cDNA amplification was performed using the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) with HiPer SYBR Premix EsTaq (MF787; Mei5 Biotechnology, Beijing, China) under optimized cycling conditions. All qPCR assays were conducted in triplicate and normalized to the reference gene GAPDH. Relative gene expression levels were calculated using the 2^-ΔΔCT method. The gene-specific primer sequences used in this study were as follows:
ANP Forward: 5′-CCT GGA CTG GGG AAG TCA AC-3′; Reverse: 5′-GTC AAT CCT ACC CCC GAA GC-3′;
BNP Forward: 5′-ATT CCA AGA TGG CAC ATA GT-3′; Reverse: 5′-AGG AGG TCT TCC TAA AAC AAC-3′;
GAPDH Forward: 5′-GAG ACA GCC GCA TCT TCT TG-3′; Reverse: 5′-TGA CTG TGC CGT TGA ACT TG-3′.
Western blottingTotal cellular proteins were lysed from H9c2 cells using RIPA lysis buffer (AR0102; Boster, Wuhan, China) supplemented with complete protease and phosphatase inhibitors. Protein concentrations were quantified using a bicinchoninic acid (BCA) assay kit (AR0146; Boster, Wuhan, China). Following quantification, protein lysates were mixed with 5X protein loading buffer (AR1112; Boster, Wuhan, China) at a 4:1 ratio. After denaturation at 95°C for five minutes, 10 μg of proteins lysates were resolved on 8–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently electrotransferred onto a polyvinylidene fluoride (PVDF) membrane (ISEQ00010; Merck Millipore, Billerica, MA, USA). Membranes were blocked with rapid blocking buffer (G2052; Servicebio, Wuhan, China) for 15 min, then incubated overnight at 4°C with the following primary antibodies: anti-LC3B (CY5992; Abways, Shanghai, China), anti-P62/SQSTM1 (CY5546; Abways, Shanghai, China), anti-AMPKα1+AMPKα2 (ab207442; Abcam, Cambridge, MA, USA), anti-AMPKα1 (phospho T183)+AMPKα2 (phospho T172) (ab133448; Abcam, Cambridge, MA, USA), anti-AMPKα2 (ab97275; Abcam, Cambridge, MA, USA) and GAPDH (AB0037; Abways, Shanghai, China). After incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (BA1054; Boster, Wuhan, China) for 90 min at RT, protein bands were detected using enhanced chemiluminescence (ECL) substrate (MA0186; MeilunBio, Dalian, China) with one minute exposure. Eventually, protein band intensities were quantified by densitometric analysis using Image Lab™ software (version 6.1.0; Bio-Rad, Hercules, CA, USA).
Statistical analysisAll experiments were conducted independently at least three times. Data are presented as mean±standard deviation (SD) and were statistically analyzed using GraphPad Prism Software (version 9.0; GraphPad Software, San Diego, CA, USA). For comparisons among three or more groups, statistical significance was determined by one-way analysis of variance ith Tukey's honestly significant difference post hoc test. A probability value of p<0.05 was considered statistically significant for all analyses.
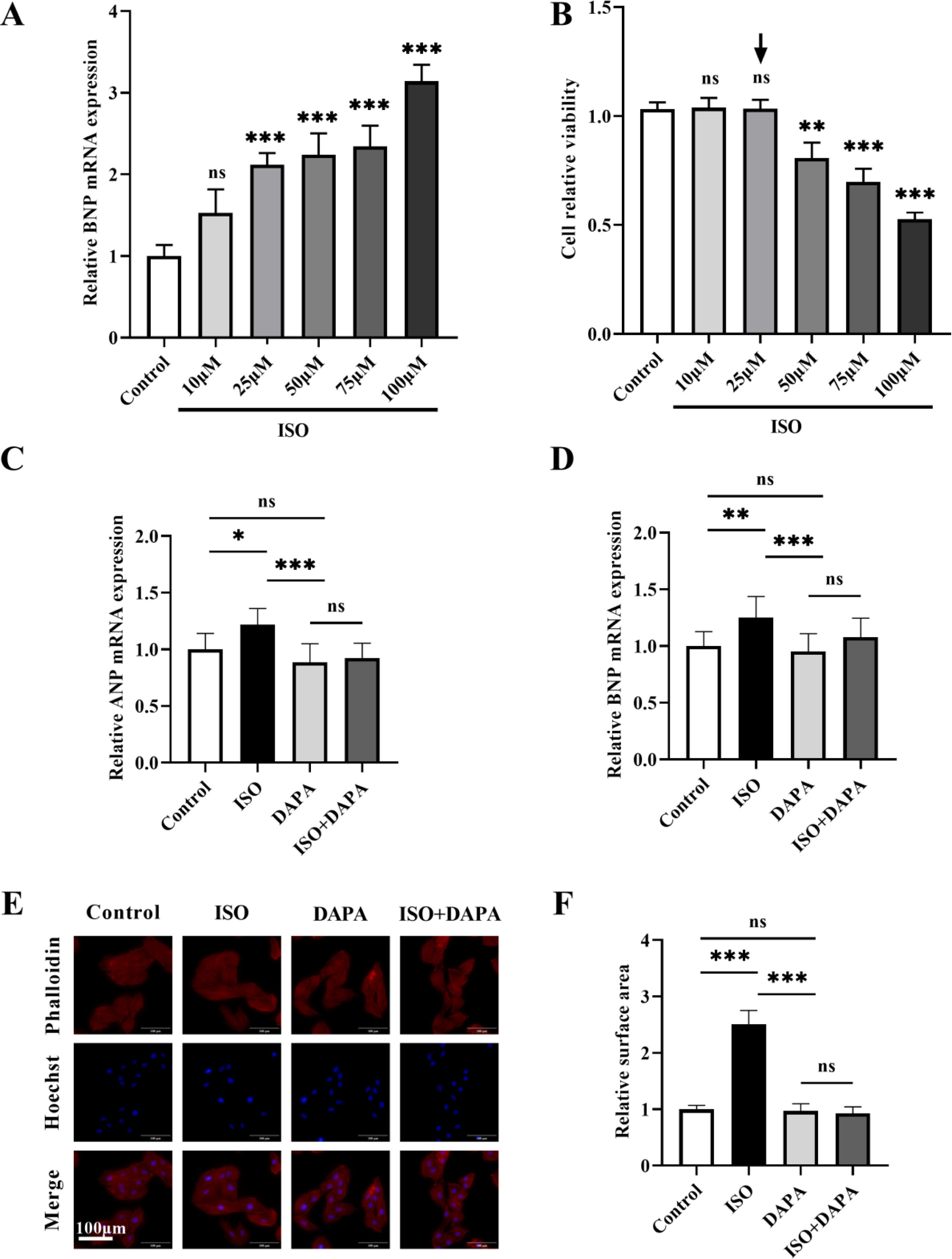
ResultsDapagliflozin attenuated isoproterenol-induced cardiac hypertrophy in vitroCardiac hypertrophy represents a critical physiopathological mechanism underlying heart failure (HF) progression we therefore employed an in vitro cardiomyocyte hypertrophy model to investigate the pharmacological effects of dapagliflozin (DAPA) on HF. A concentration gradient of isoproterenol (ISO; 0–100 μM) was used to assess BNP mRNA expression, a key hypertrophic marker, in H9c2 cells. BNP mRNA expression began to increase at 25 μM ISO, with no futher significant changes observed up to 75 μM (Figure 1A). To further determine the optimal ISO concentration, we evaluated the H9c2 cell viability using the CCK-8 assays across the ISO concentration range. Relative to the untreated control group, the cell viability decreased significantly at 50 μM (Figure 1B). We therefore selected 25 μM ISO for subsequent experiments. The DAPA concentration (10 μM) was chosen based on previous work by Yang et al.12
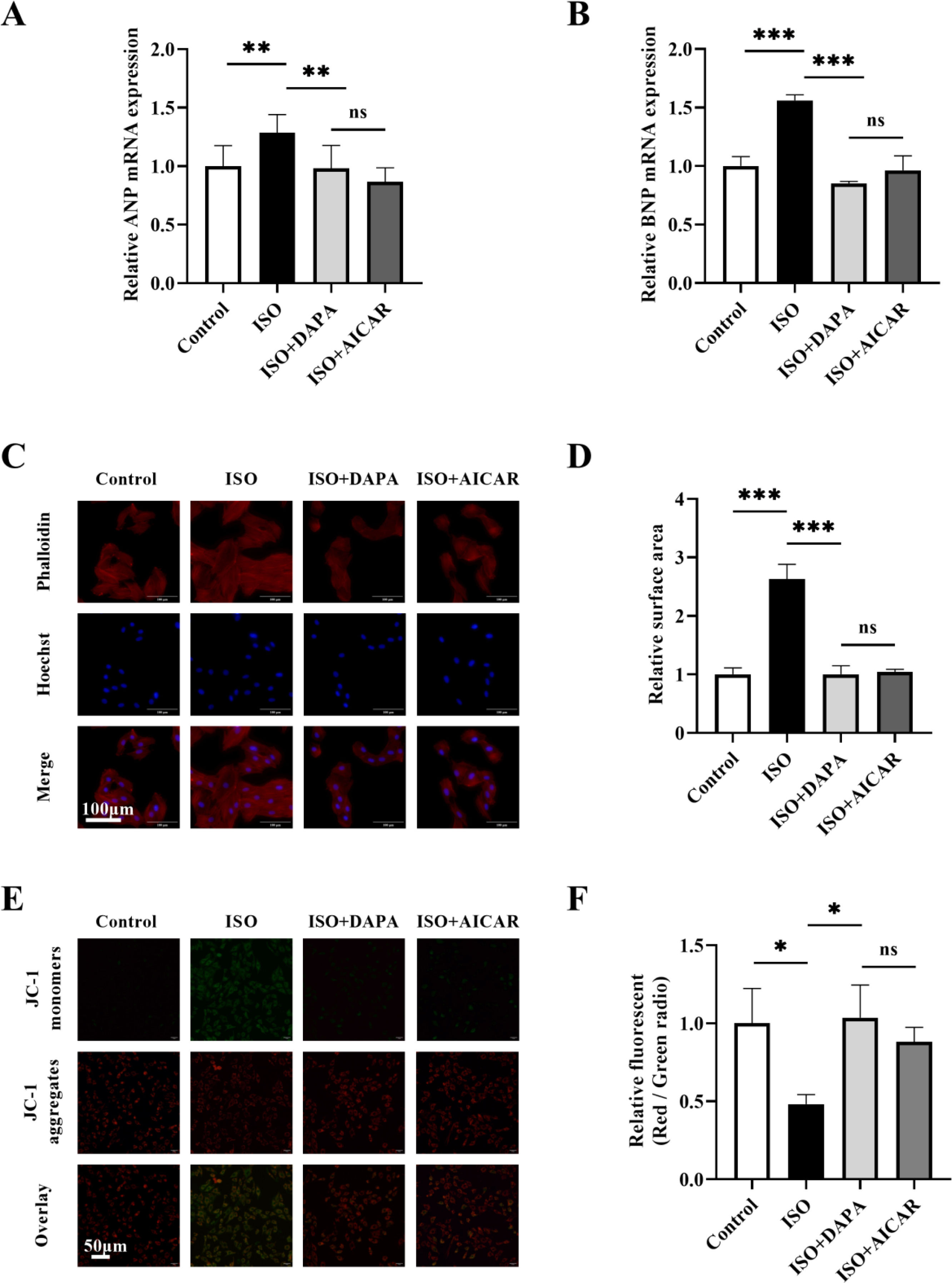
The effect of isoproterenol and dapagliflozin on hypertrophy in H9c2 cells. (A) Dose-dependent effects of isoproterenol (0–100 μM) on BNP mRNA expression in H9c2 cells. (B) CCK-8 analysis was used to detect the effect of isoproterenol (0–100 μM) on cell viability. Black arrow denotes the optimal isoproterenol concentration (25 μM) used for subsequent experiments. (C and D) mRNA expression levels of ANP and BNP were determined by quantitative real-time polymerase chain reaction. (E and F) TRITC-phalloidin staining was used to detect the cell surface area. Results were presented as mean±SD (n=3 for each group). *p<0.05, **p<0.01, ***p<0.001; ns, not significant.
To evaluate DAPA's effects on ISO-induced H9c2 hypertrophy cardiomyocytes, we assessed cell surface area and hypertrophic marker (ANP and BNP) expression. ISO treatment significantly increased both ANP and BNP expression (Figure 1C and D) and cell surface area (Figure 1E and F). DAPA alone showed no adverse effects, but co-treatment with ISO significantly attenuated ISO-induced hypertrophy in H9c2 cells (Figure 1C–F). These findings demonstrate that DAPA effectively attenuates ISO-induced cardiac hypertrophy.
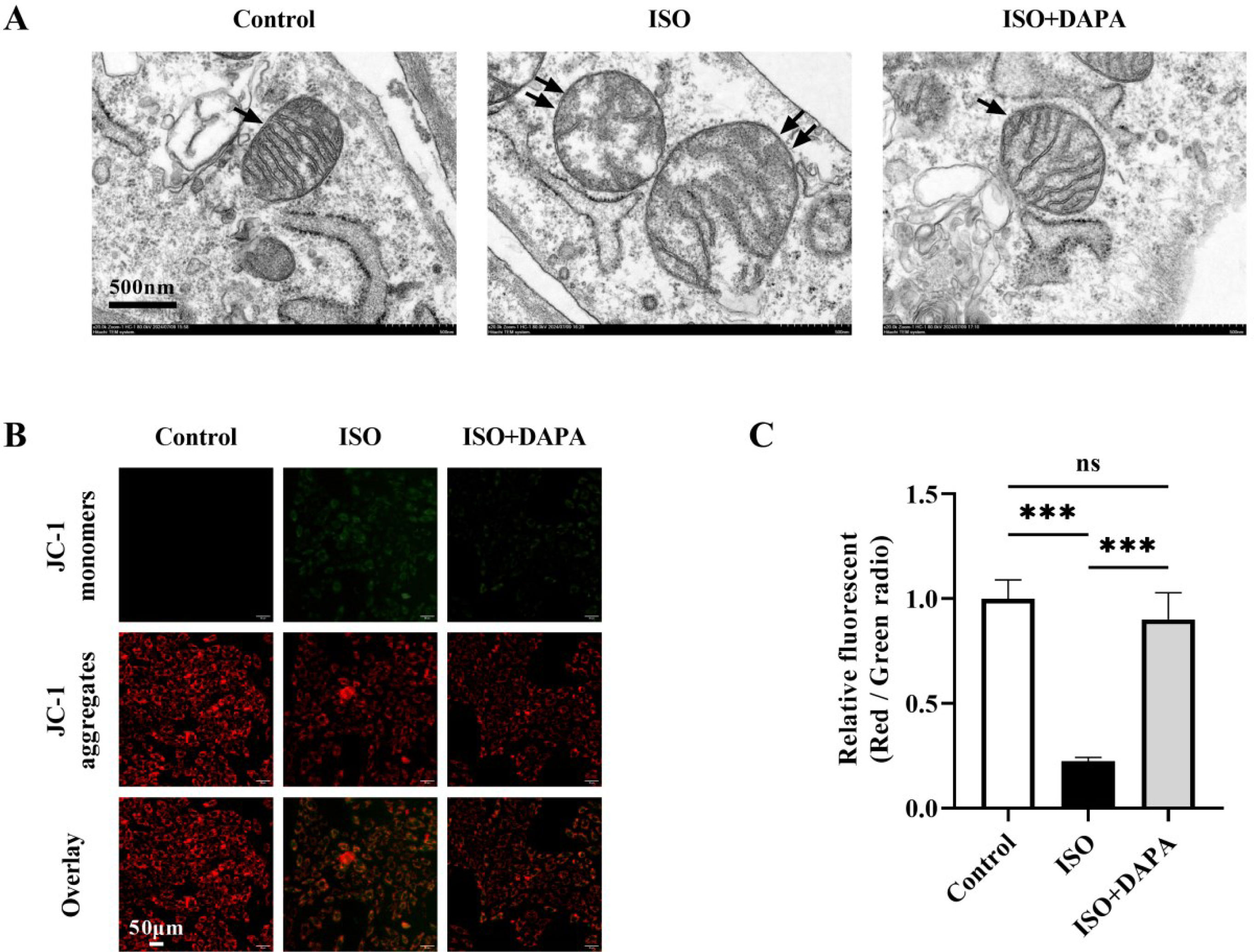
Dapagliflozin recovered mitochondrial structure and function in isoproterenol-treated H9c2 cellsGiven the established role of mitochondrial dysfunction in HF progression, we further investigated DAPA's effects on ISO-induced mitochondrial damage. Transmission electron microscopy revealed that ISO-induced mitochondrial ultrastructural abnormalities in H9c2 cells, characterized by swelling, cristae reduction, shortening and focal vacuolization (Figure 2A). Conversely, the mitochondrial ultrastructure remained intact in the DAPA-treated group (Figure 2A). Furthermore, mitochondrial membrane potential (MMP) showed a notable reduction in the JC-1 red/green fluorescence ratio in the ISO-treated group, which was reversed back to normal levels in the DAPA-treated group, as evidenced by the normalized JC-1 red/green ratio (Figure 2B and C). These data collectively indicate DAPA's protective against ISO-induced mitochondrial dysfunction, preserving both structural and functional integrity.
Dapagliflozin attenuated mitochondrial damage induced by isoproterenol in H9c2 cells. (A) Transmission electron microscopy was used to evaluate the effect of dapagliflozin on abnormal mitochondrial ultrastructure in isoproterenol-treated H9c2 cells. Single black arrow: normal mitochondria; double black arrows: damaged mitochondria. (B and C) Mitochondrial membrane potential was assessed using the JC-1 fluorescence. Results were presented as mean±SD (n=3 for each group). *p<0.05, ***p<0.001; ns, not significant.
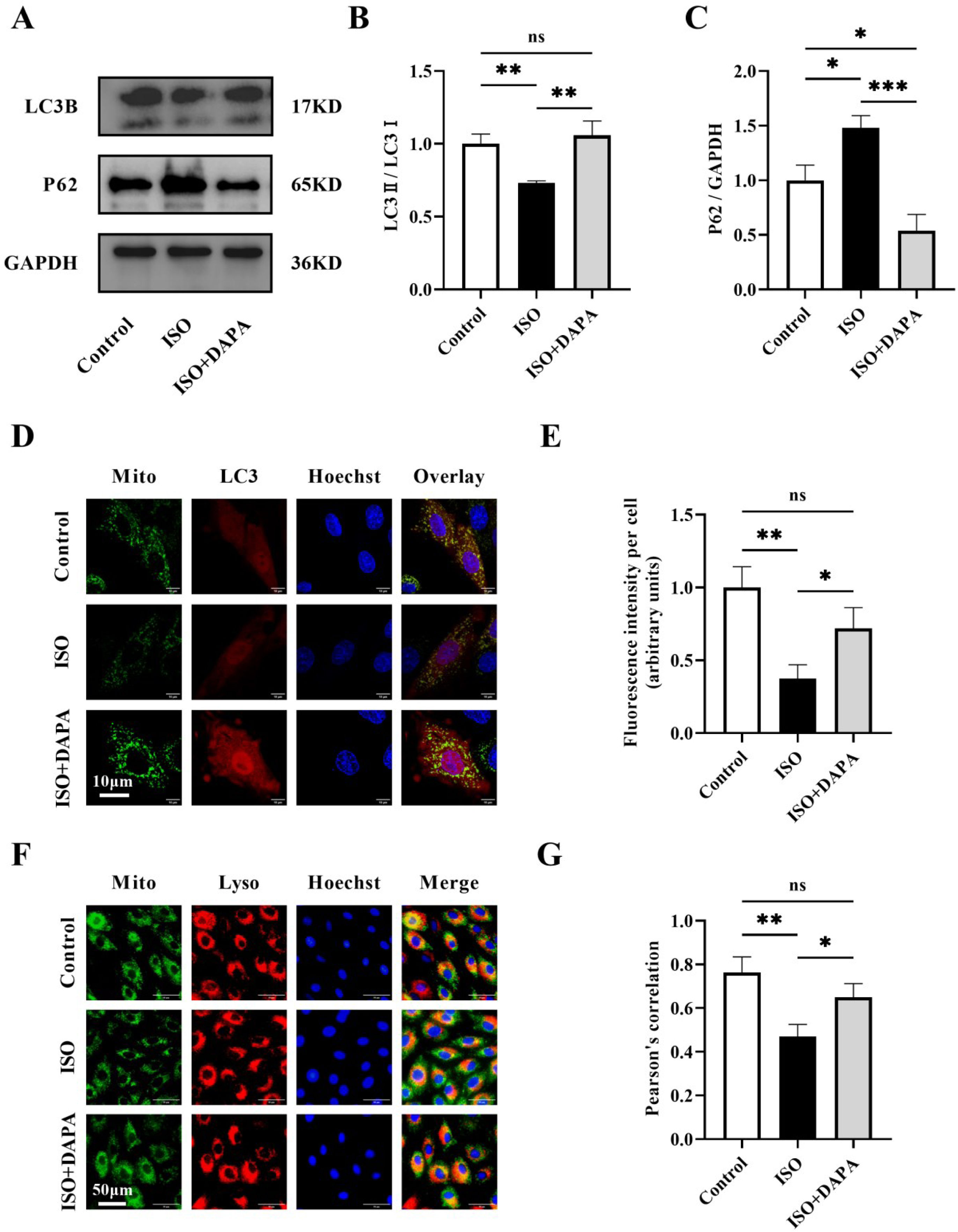
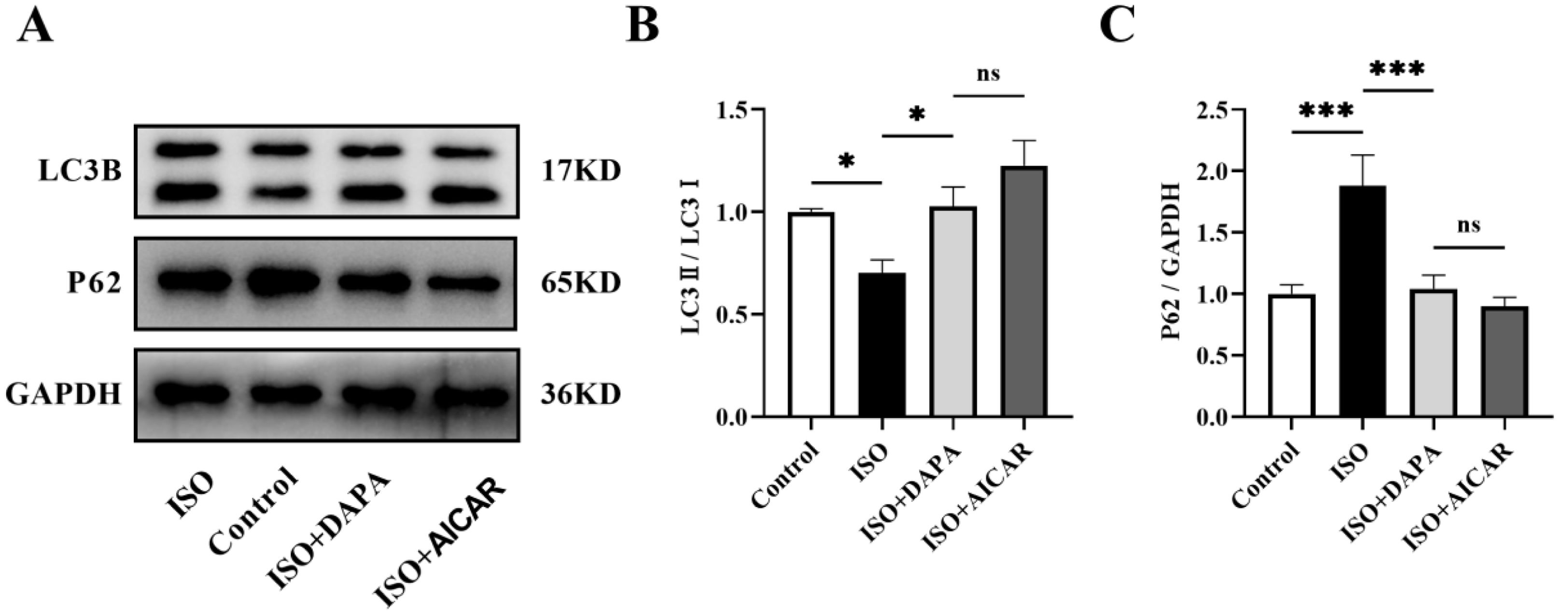
Mitophagy serves as a crucial quality control mechanism that eliminates damaged mitochondria to maintain normal mitochondrial functions. To investigate DAPA's mitophagic protection against ISO-induced damage, we examined autophagy markers (LC3II/LC3I ratio and P62) by Western blotting across treatment groups. The LC3II/LC3I ratio was reduced and P62 levels were elevated in the ISO-treated group compared to normal control cells, suggesting that ISO inhibited autophagy (Figure 3A–C). In contrast, DAPA treatment restored autophagic flux, as illustrated by an elevated LC3II/LC3I ratio and reduced P62 levels (Figure 3A–C). To further validate DAPA's mitophagic regulation, we performed mCherry-LC3 transfection combined with MitoTracker™ Green staining in H9c2 cells. DAPA treatment increased autophagosome-mitochondria co-localization (yellow puncta), indicating initial mitophagosome formation (Figure 3D and E). Furthermore, increased mitochondria-lysosome co-localization (yellow fluorescence) confirmed subsequent degradation, provides strong evidence of DAPA's mitophagy induction (Figure 3F and G). In summary, all these findings demonstrate that DAPA activates mitophagy in ISO-stimulated H9c2 cells.
Dapagliflozin promoted levels of mitophagy in isoproterenol-induced H9c2 cells. (A) Representative Western blots of LC3B and P62 in H9c2 cells. (B and C) Quantitative analysis of the expression levels of LC3B and P62. (D and E) The co-localization of autophagosomes with mitochondria was detected using confocal microscope. (F and G) Mitochondria-lysosome co-localization was assessed via fluorescence microscopy. Results were presented as mean±SD (n=3 for each group). *p<0.05, ***p<0.001; ns, not significant.
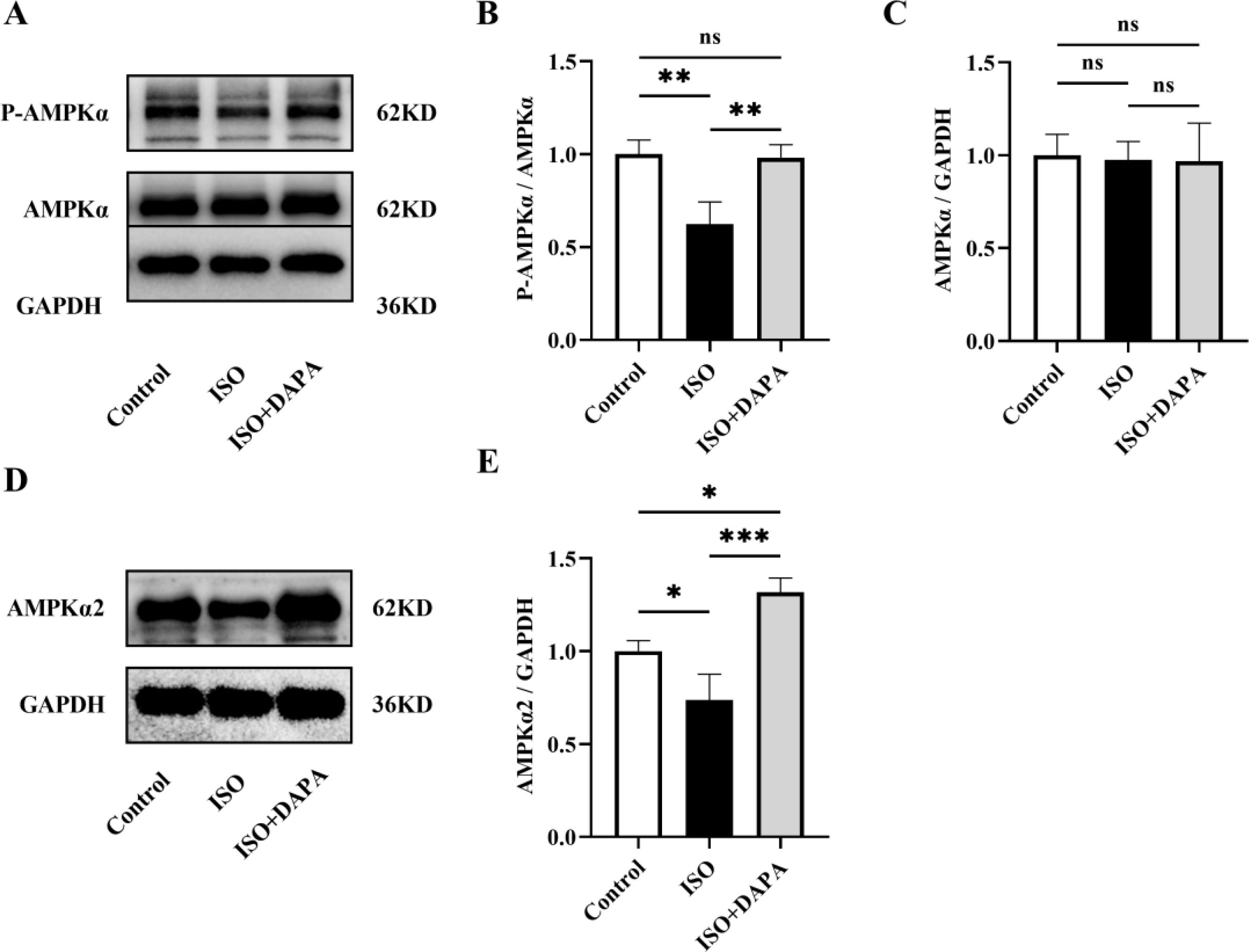
We next explored DAPA's mechanism in promoting mitophagy in ISO-treated H9c2 cells. AMP-activated protein kinase (AMPK) activation regulates various cellular processes, including mitophagy. We therefore assessed AMPKα phosphorylation by Western blotting. The p-AMPKα/AMPKα ratios were decreased in ISO-treated H9c2 cells but reversed by DAPA treatment (Figure 4A and B). Meanwhile, total AMPKα levels remained unchanged (Figure 4A and C). These results demonstrate that DAPA enhances AMPKα phosphorylation without altering its expression. We further examined the expression level of AMPKα2, the predominant cardiac AMPKα isoform. Western blotting results revealed that DAPA upregulated AMPKα2 in cells treated with ISO (Figure 4D and E). Collectively, DAPA activated AMPKα and upregulated AMPKα2 in ISO-treated H9c2 cells.
Dapagliflozin enhanced AMPKα phosphorylation and AMPKα2 expression. (A) Representative Western blots of p-AMPKα and AMPKα. (B and C) Quantitative analysis of the expression levels of p-AMPKα and AMPKα. (D) Representative Western blots of AMPKα2. (E) Quantitative analysis of the expression levels of AMPKα2. Results were presented as mean±SD (n=3 for each group). *p<0.05, **p<0.01, ***p<0.001; ns, not significant.
To investigate the role of AMPKα in DAPA's cardioprotection against ISO-induced injury, we used 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR; 500 μM), an established AMPK agonist known for its cardioprotective effects in H9c2 cells.13 Similar to DAPA, AICAR treatment significantly attenuated ISO-induced hypertrophic responses, as evidenced by reduced ANP and BNP expression (Figure 5A and B) and normalized cell surface area (Figure 5C and D). Furthermore, AICAR preserved MMP, counteracting ISO-induced depolarization (Figure 5E and F). AICAR treatment also recapitulated DAPA-mediated restoration of autophagic flux, as evidenced by normalized LC3II conversion and p62 degradation (Figure 6A–C). Collectively, these findings demonstrate that DAPA attenuates ISO-induced cardiomyocyte hypertrophy and mitochondrial dysfunction through AMPKα activation.
The AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleotide conferred cardioprotection that paralleled the therapeutic efficacy of dapagliflozin. (A and B) Hypertrophic markers ANP and BNP mRNA levels were quantified using quantitative real-time polymerase chain reaction. (C and D) Cardiomyocyte hypertrophy was assessed via TRITC-phalloidin staining of H9c2 cell surface area. (E and F) Mitochondrial membrane potential was evaluated using JC-1 fluorescence. Results were presented as mean±SD (n=3 for each group). *p<0.05, **p<0.01, ***p<0.001; ns, not significant.
The AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleotide conferred cardioprotection that paralleled the therapeutic efficacy of dapagliflozin. (A) Representative Western blots of LC3B and P62. (B and C) Quantitative analysis of the expression levels of LC3B and P62. Results were presented as mean±SD (n=3 for each group). *p<0.05, **p<0.01, ***p<0.001; ns, not significant.
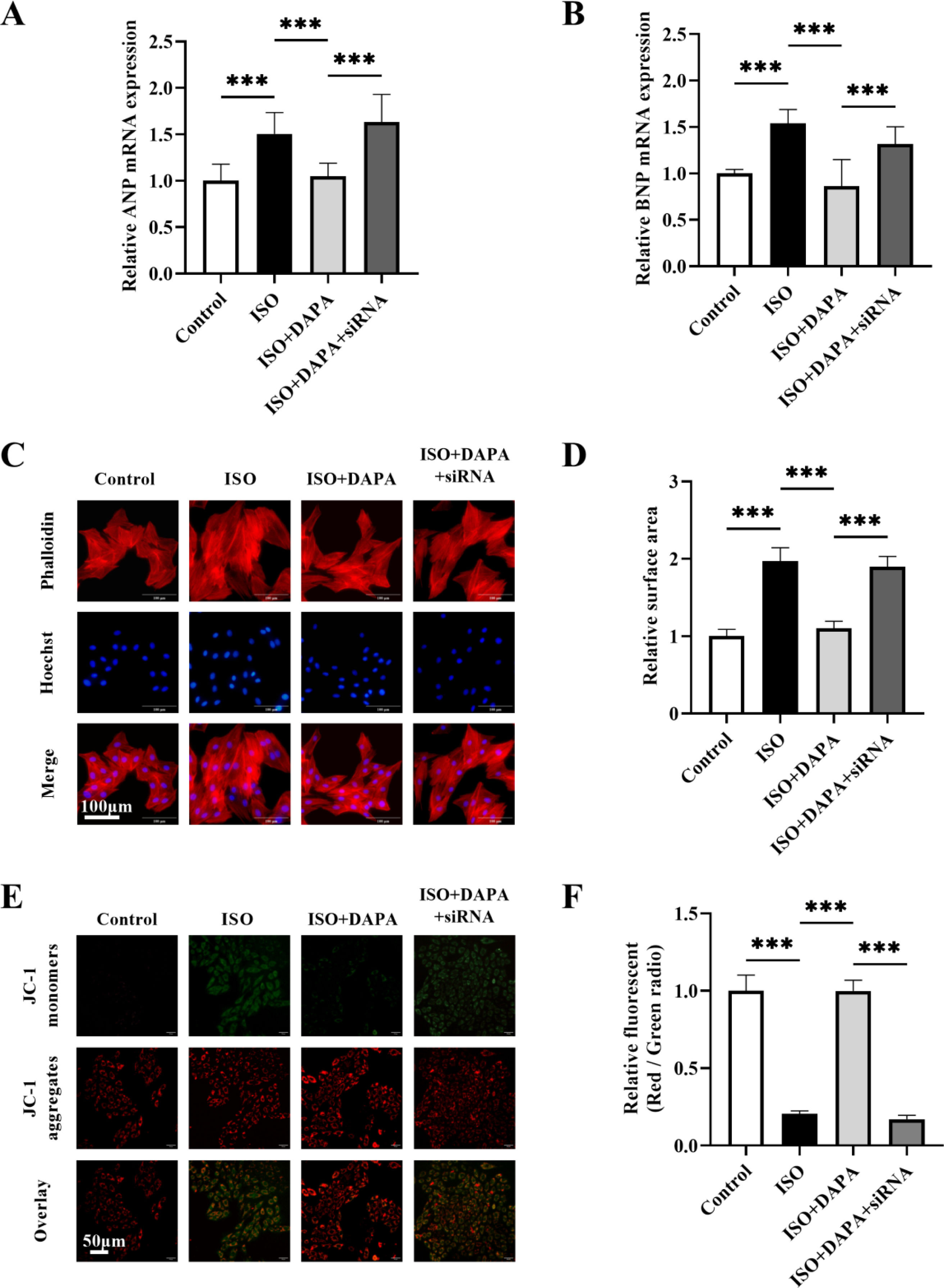
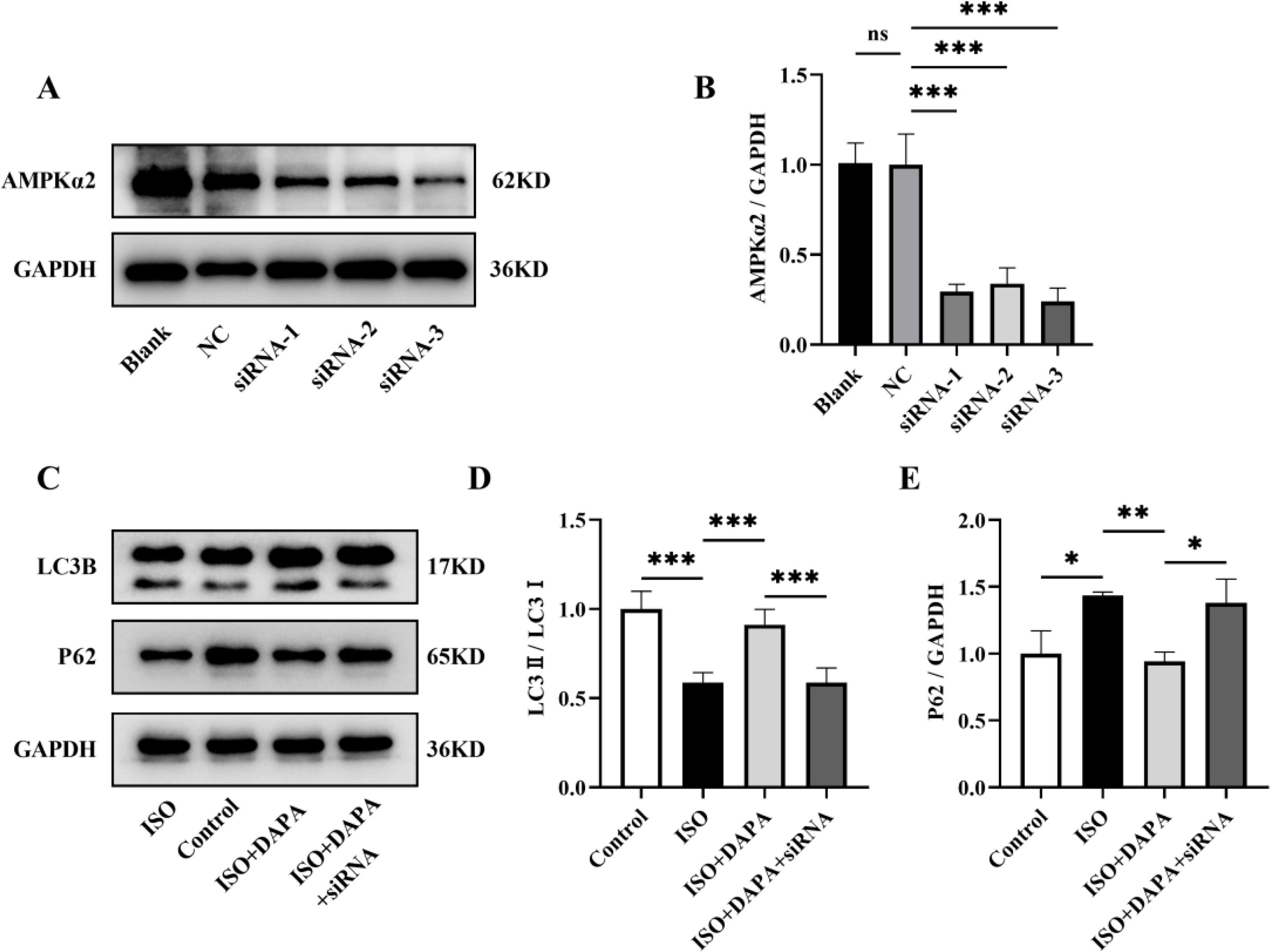
To establish AMPKα2-dependence of DAPA's effects, we knocked down AMPKα2 expression in H9c2 cells using AMPKα2 small interfering RNA (siRNA). Western blotting revealed a marked decrease in AMPKα2 levels in H9c2 cells transfected with AMPKα2 siRNA (Figure 8A and B). In comparison to the DAPA-treated group, co-treatment with AMPKα2 siRNA abolished DAPA's anti-hypertrophic effects in ISO-stimulated cells (Figure 7A–D). Meanwhile, DAPA's ameliorative effect on MMP was attenuated following AMPKα2 siRNA transfection (Figure 7E and F). Furthermore, DAPA-induced LC3 conversion and P62 degradation were both impaired by AMPKα2 deficiency (Figure 8C–E). Collectively, these results suggest that DAPA attenuates cardiac hypertrophy, promotes mitophagy and protectes mitochondrial integrity partially via the AMPKα2 pathway in ISO-stimulated cells.
AMPKα2 interference abrogated the protective effects of dapagliflozin. (A and B) The mRNA expression levels of ANP and BNP after transfection with AMPKα2 siRNA were determined by quantitative real-time polymerase chain reaction. (C and D) Cell surface area alterations in AMPKα2 siRNA-transfected H9c2 cells were detected using TRITC-phalloidin staining. (E and F) Changes in mitochondrial membrane potential following AMPKα2 siRNA interference. Results were presented as mean±SD (n=3 for each group). ***p<0.001; ns, not significant.
AMPKα2 knockdown attenuated the promotion of mitophagy by dapagliflozin. (A) Representative Western blots of AMPKα2. (B) Quantitative analysis of the expression levels of AMPKα2. (C) Representative Western blots of LC3B and P62. (D and E) Quantitative analysis of the expression levels of LC3B and P62. Results were presented as mean±SD (n=3 for each group). *p<0.05, **p<0.01, ***p<0.001; ns, not significant.
The aim of this study was to investigate dapagliflozin's (DAPA) cardioprotective effects in HF and its molecular mechanisms. In H9c2 cells, isoproterenol (ISO)-induced hypertrophy correlated by mitochondrial dysfunction, suppressed mitophagy and decreased AMP-activated protein kinase (AMPK) activity, all of which were rescued by DAPA. The AMPK agonist 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) mirrored DAPA's protective effects. In addition, AMPKα2 knockdown abolished DAPA's beneficial effects on ISO-induced hypertrophy and mitophagy regulation. In conclusion, DAPA attenuates cardiac hypertrophy via AMPKα2-dependent mitophagy activation.
Heart failure represents the end-stage manifestation of several cardiovascular diseases, with mitochondrial dysfunction being a key contributor to its complex pathophysiology. As dynamic organelles, mitochondria govern cellular energy production and metabolic homeostasis. Studies have shown that mitochondrial structure and function are altered to varying degrees in HF.14 Dysfunctional or damaged mitochondria in turn can further exacerbate HF through abnormal energy metabolism, oxidative stress and cell death pathways.15 Accumulating evidence from both animal and cellular models has consistently demonstrated that abnormalities in mitochondrial morphology and reduced MMP are prevalent in HF.16,17 Our findings are consistent with previous reports, revealing abnormalities in mitochondrial morphology, including swelling, cristae breakage and matrix vacuolization, in ISO-induced H9c2 cells, as visualized by transmission electron microscopy. JC-1 staining showed a significant reduction in MMP, indicative of mitochondrial dysfunction. To safeguard against mitochondrial damage, cardiomyocytes utilize well-coordinated quality control mechanisms that preserve mitochondrial integrity by selectively eliminating damaged mitochondria via mitophagy.18 Nevertheless, cardiac mitophagy is significantly compromised in pressure overload-induced HF models, leading to mitochondrial dysfunction and HF progression.19 Consistent mitophagy attenuation was confirmed in our ISO-induced H9c2 hypertrophy model, demonstrated by impaired autophagic flux (reduced LC3-II/I ratio and increased P62 levels), diminished autophagosome-mitochondria co-localization and impaired mitochondria-lysosome co-localization. We conclude that mitophagy plays an important role in ISO-induced cardiac hypertrophy. ISO stimulation inhibits mitophagy, reducing the clearance of damaged mitochondria and thereby exacerbating cardiomyocyte injury.
AMPK, a heterotrimeric complex, serves as a central regulator of metabolic homeostasis. Its activation depends on the phosphorylation of a threonine residue (Thr172) in the α-subunit. Upon activation, AMPK orchestrates energy homeostasis by coordinately increasing ATP production whlie decreasing its consumption.20 Beyond its canonical metabolic regulation in cardiomyocytes, AMPK modulates a variety of cellular processes, notably including mitopahgy.21 AMPK activation enhances mitophagy via both PINK1-Parkin-dependent and -independent mechanisms, with cardiovascular disease demonstrating that this upregulation attenuates disease progression by restoring physiological mitophagic flux.22–25 We assessed AMPK activity by measuring phosphorylated and total AMPKα. The study revealed that comparable total AMPKα levels across groups, but markedly reduced AMPKα phosphorylation in the ISO-treated group. We further investigated AMPKα2 isoform expression dynamics. As the predominant cardiac isoform, AMPKα2's regulatory role in mitophagy has been progressively elucidated through recent investigations. Genetic ablation of AMPKα2 induces cardiac dysfunction, fibrotic remodeling and cardiolipin abnormalities coupled with mitochondrial dysfunction.26,27 Conversely, AMPKα2 overexpression or activation promotes mitophagy and confers cardioprotection via PTEN-induced putative kinase 1 (PINK1)-, BCL2-like 13 (BCL2L13)- and mechanistic target of rapamycin (mTOR)-dependent mechanisms.7,28,29 ISO treatment significantly suppressed AMPKα2 expression, correlating with decreased mitophagy and mitochondrial dysfunction. Collectively, these data suggest that ISO-mediated mitophagy impairment occurs through downregulation of AMPKα phosphorylation, particularly involving the AMPKα2 isoform.
DAPA, a sodium-glucose cotransporter-2 inhibitor, reduces renal tubular glucose reabsorption, consequently lowering plasma glucose levels. Extensive research has shown that DAPA exerts broad protective effects against cardiovascular diseases, including HF, ischemia–reperfusion injury and myocardial infarction.30–32 Recent studies have increasingly focused on the impact of DAPA's cardioprotective properties on mitochondria.33 Previous literature has shown that DAPA treatment significantly attenuates cardiac hypertrophy by reducing oxidative stress, inhibiting mitochondrial fission and improving mitochondrial function.34 However, the role of mitophagy in DAPA's anti-hypertrophy effects remains unknown. Our findings demonstrate that DAPA significantly alleviated ISO-induced cellular hypertrophy, mitigated mitochondrial morphological damage and stabilized MMP. Quantitative assessment of autophagic flux markers (LC3-II/I ratio and P62 levels), combined with enhanced autophagosome-mitochondria and mitochondria-lysosome co-localization, conclusively demonstrated DAPA-mediated mitophagic activation. These data support the notion that DAPA's cardioprotective efficacy is partially dependent on enhanced mitophagy. AMPK serves as a master regulator of mitophagic processes.35 DAPA has been shown to prevent cardiac injury by promoting AMPKα activation in multiple studies.31,36 We validated that DAPA confers cytoprotection in ISO-stimulated H9c2 cells via AMPKα activation. Notably, AMPK agonist AICAR produced equivalent protective effects, confirming AMPKα activation as the pivotal mechanism. Furthermore, DAPA specifically upregulated AMPKα2 expression, and AMPKα2 knockdown reversed DAPA-induced protection, especially its mitophagic enhancement. These results posit AMPKα2-dependent mitophagic regulation as a crucial component of DAPA's cardioprotective effect. Current evidence indicates that AMPKα2 orchestrates cardiomyocyte mitophagy via mTOR inhibition, PINK1/Parkin pathway activation and BCL2L13 signaling, with DAPA shown to modulate the former two pathways while its potential regulation of BCL2L13 remains to be elucidated.37,38 In future studies, we will further investigate the molecular mechanism by which DAPA regulates AMPKα2 to modulate mitophagy.
ConclusionIn conclusion, dapagliflozin (DAPA) treatment ameliorated isoproterenol (ISO)-induced cardiac hypertrophy and mitochondrial dysfunction by promoting AMPKα2-mediated mitophagy. Our results demonstrate that DAPA's cardioprotective efficacy may be partly attributed to improved mitophagy. However, our study has some limitations. For example, we did not perform in vivo studies, and thus the results do not fully reflect DAPA's in vivo efficacy. Additionlly, ISO-induced hypertrophy involves complex mechanisms, and DAPA may provide benefits through other signaling pathways, warranting future studies.
CRediT authorship contribution statementConceptualization: YF, ZXZ; investigation: YF, XJ, YBZ, and YBH; writing – original draft preparation: YF; writing – review and editing: XWL; funding acquisition: ZXZ, XWL; resources: ZXZ; supervision: XWL.
FundingThis work was supported by the National Natural Science Foundation of China Grants (82170398) and the Health Commission of Shanxi Province (2023XM021).
Central figureCentral picture legend: Through systematic cellular experiments, we elucidated the cardioprotective mechanisms of dapagliflozin, with particular emphasis on its mitochondrial regulatory functions. The results suggest that dapagliflozin exerts cardioprotective effects by promoting impaired mitophagy in cardiomyocytes, potentially mediated through the AMPKα2 signaling.
Central message: Dapagliflozin (DAPA), a sodium-glucose cotransporter-2 inhibitor, exhibits cardioprotective properties through mechanisms that have not been fully elucidated. Advances in heart failure (HF) research have highlighted mitochondrial dysfunction as a pivotal contributor to HF pathogenesis. Properly regulated mitophagy can restore mitochondrial dysfunction and provide cardioprotection benefits. Although DAPA's influence on mitophagy has been investigated, its precise regulatory mechanisms in HF remain unclear. This study employed an H9c2 cell to explore DAPA's cardioprotective effects and mitophagic regulation, with particular focus on AMPKα2 signaling. Previous studies have elucidated DAPA's effects on the downstream effectors of AMPKα2, including the PTEN-induced putative kinase 1/Parkin pathway and mechanistic target of rapamycin, yet its influence on BCL2-like 13 remains undefined, representing a key direction for future research.
Conflicts of interestThe authors have no conflicts of interest to declare.
Data availabilityThe data used to support the findings of this study are available from the corresponding author upon request.