The coronary sinus Reducer (CSR) device has emerged as a complementary therapy in patients with severe angina refractory to optimal medical therapy and not amenable to revascularization. Our aim was to assess the safety and efficacy of the CSR in a real-world setting.
MethodsTwenty-six patients with refractory angina (RA) and evidence of myocardial ischemia attributable to the left coronary artery considered unsuitable for revascularization were treated with the CSR at two centers between May 2017 and July 2019. Safety endpoints were procedural success and complications. Efficacy endpoints, assessed at six-month follow-up, were reduction in CCS class, improvement in quality of life (QoL) assessed using the short version of the Seattle Angina Questionnaire (SAQ-7), and reduction in antianginal therapy.
ResultsTwenty-three patients had end-stage coronary artery disease without revascularization targets and three had microvascular disease without epicardial stenosis. Procedural success was achieved in 23 patients, with two device/procedure-related complications and one anatomically-related failure to deliver the device. A total of 24 patients had the device implanted and entered the efficacy analysis. Eighteen patients (75.0%) had a reduction of at least one CCS class, 41.7% had a reduction of at least two classes, and 16.7% became asymptomatic, with a mean reduction in CCS class of 1.3±0.2 (p=0.001) at six-month follow-up. All SAQ-7 domains improved, notably physical limitation (p=0.001), angina frequency (p=0.005) and QoL (p=0.006). There was a mean reduction in anti-ischemic drugs from 3.4±1.1 to 2.9±1.2 (p=0.010).
ConclusionIn this real-world, multicenter experience, implantation of the CSR was associated with improvement in angina and QoL in patients with RA.
O dispositivo de redução do seio coronário (DRSC) constitui uma terapêutica complementar em doentes com angina refratária à terapêutica médica e não passíveis de revascularização. O objectivo era avaliar a segurança e eficácia do DRSC numa coorte do mundo real.
MétodosVinte e seis doentes com angina refratária, evidência de isquemia miocárdica atribuível à artéria coronária esquerda e considerados inadequados para revascularização foram tratados com o DRSC em dois centros terciários portugueses entre maio de 2017 e julho de 2019. Os endpoints de segurança foram o sucesso do procedimento e complicações. Os endpoints de eficácia, avaliados aos seis meses de follow-up, foram uma redução na classe de angina da CCS, melhoria na qualidade de vida avaliada pela versão abreviada do Seattle Angina Questionnaire (SAQ-7) e redução na terapêutica antianginosa.
ResultadosVinte e três doentes tinham doença coronária sem alvos de revascularização e três, doença microvascular sem estenoses epicárdicas. O sucesso do procedimento foi alcançado em 23 doentes, com duas complicações relacionadas com dispositivo/procedimento e uma falha na entrega do dispositivo. No total, 24 doentes implantaram o dispositivo e entraram na análise de eficácia. Dezoito doentes (75,0%) sofreram uma redução de pelo menos uma classe CCS, 41,7% sofreram uma redução de pelo menos duas classes e 16,7% tornaram-se assintomáticos, com uma redução média da classe CCS de 1,3±0,2 (p=0,001), seis meses de follow-up. Verificou-se uma melhoria de todos os domínios do SAQ-7, nomeadamente limitação física (p=0,001), frequência de angina (p=0,005) e qualidade de vida (p=0,006). Verificou-se uma redução média de fármacos antianginosos de 3,4±1,1 para 2,9±1,2 (p=0,010).
ConclusãoNesta experiência multicêntrica do mundo real, a implantação do DRSC foi associada à melhoria das queixas anginosas e da qualidade de vida em doentes com angina refratária não passível de revascularização.
The coronary sinus (CS) Reducer® device (Neovasc Inc., Richmond BC, Canada) has come to the fore in the treatment of refractory angina (RA) due to the growing evidence supporting its efficacy in angina relief and improved quality of life (QoL).1–4
Despite optimal combinations of guideline-directed anti-ischemic therapies and myocardial revascularization, a substantial proportion (20–40%) of patients with obstructive coronary artery disease (CAD) continue to experience an unacceptably high symptomatic burden.5,6 These patients are classified as having RA.7
No precise estimates of the real incidence and prevalence of RA are currently available; however, it is believed to be a growing entity, due to the aging of the population, the increasing complexity of CAD presenting to the catheterization laboratory, and the presence of multiple comorbidities, all of which limit therapeutic options.
The prognosis of patients with RA is within the range of chronic coronary syndromes, but RA is associated with poorer QoL and increased healthcare and associated social costs (admissions, outpatient visits, diagnostic investigations and absenteeism from work),8,9 making symptom relief and QoL improvement the main therapeutic aims in this population.
The Reducer is an hourglass-shaped, stainless steel, balloon-expandable device implanted percutaneously via the jugular vein to create a focal narrowing in the lumen of the CS in order to generate a pressure gradient, which is thought to decrease microvascular resistance and improve perfusion to the more ischemic territories of the left coronary artery, alleviating the symptoms of angina.
A single randomized sham-controlled clinical trial (COSIRA)10 and several small11–18 and one large real-world registry (RESOURCE)19 have demonstrated encouraging results with regard to the Reducer's efficacy and safety. Based on the available data, the 2019 European guidelines on chronic coronary syndromes stated that the Reducer device could be considered for the treatment of patients with angina refractory to medical and interventional therapies (class of recommendation IIb, level of evidence B).20
The present study reports the initial experience of two tertiary centers with the Reducer device for the treatment of RA.
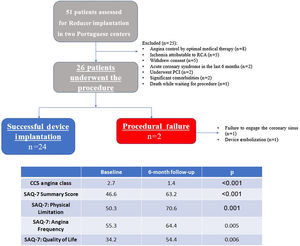
MethodsPopulationThis was a prospective, single-arm, non-blinded registry of 51 consecutive patients referred for Reducer implantation, of whom 26 underwent the procedure and entered the analysis, between May 2017 and July 2019 in two Portuguese tertiary centers.
Inclusion criteria were angina (at least Canadian Cardiovascular Society [CCS] class 2) despite maximally tolerated pharmacological therapy and not amenable for revascularization, and ischemia documented by myocardial stress single-photon emission computed tomography (SPECT) or myocardial stress magnetic resonance imaging (MRI) attributable to the left coronary artery regardless of the presence of obstructive epicardial CAD.
Exclusion criteria were ischemia related exclusively to the right coronary artery, severely depressed left ventricular ejection fraction (LVEF) (<35%), symptoms predominantly of heart failure, presence of a cardiac resynchronization therapy device, recent acute coronary syndrome (<3 months), recent revascularization (<6 months), right heart disease, severe valvular heart disease, mean right atrial pressure >15 mmHg, or any other clinical context that would preclude clinical benefit or antithrombotic therapy.
Each patient with RA who was referred for Reducer implantation by their attending cardiologist was assessed by one of the study investigators regarding their eligibility for the procedure, namely the existence of revascularization targets and candidacy for alternative procedures like percutaneous coronary intervention (PCI) for chronic total occlusions; documented ischemia in the left coronary artery territory by SPECT, cardiac MRI or stress echocardiography; and whether antianginal therapy was titrated to its maximum tolerated dose and metabolic risk factors were controlled.
ProcedureBriefly, right atrial pressure measurement and CS retrograde venography were performed to assess hemodynamic and anatomical suitability for Reducer implantation, using a multipurpose catheter via the right internal jugular vein. The device was then implanted, under 5000 U of unfractionated heparin, through a 9 F delivery guide catheter over a 0.035″ wire at the proximal portion of the CS (2–3 cm distal to the ostium, avoiding collaterals), with an intentional overexpansion of 10–20%. A final venography was performed to assess device position and complications. A detailed description of the procedure can be found elsewhere.9,10,14
Dual antiplatelet therapy with aspirin and clopidogrel was recommended for at least one month, and ideally six months. If oral anticoagulant therapy was prescribed, additional clopidogrel was mandated for one month.
Follow-upAll data regarding the procedure, in-hospital outcomes and follow-up were recorded in a dedicated database. All patients had at least one pre-implantation consultation with optimization of pharmacological therapy followed by clinical reassessment on the day of the procedure, at one month post procedure (by telephone) and at three and six months (in person). Symptomatic status and therapeutic appropriateness were assessed at each appointment.
EndpointsThe primary safety endpoint was successful implantation of the Reducer device at the intended site in the CS without complications. Secondary safety endpoints were periprocedural and six-month procedure- or device-related complications including device success (defined as successful implantation at the intended site), CS perforation, CS dissection, cardiac tamponade, migration, arrhythmias, vascular complications, myocardial infarction and death.
The primary efficacy endpoint was CCS class change, and the secondary endpoints were improvement in QoL assessed by score on the short version of the Seattle Angina Questionnaire (SAQ-7) and reduction in pharmacological antianginal therapy between baseline and six months after the procedure.
Statistical analysisCategorical variables were expressed as proportions and compared using the chi-square test. Normality of continuous variables was assessed by the Shapiro-Wilk test. Continuous variables with normal distribution were expressed as mean±standard deviation and compared using the paired-samples t test, while non-normally distributed variables were expressed as median [interquartile range] and compared using the Wilcoxon signed test. All comparisons are between baseline and six-month follow-up.
A p-value <0.05 was considered statistically significant. IBM SPSS Statistics version 21 was used for the statistical analysis.
ResultsBaseline characteristicsFifty-one consecutive patients with angina considered to be refractory were assessed as possible candidates for Reducer device implantation. Of this initial cohort, eight patients had symptomatic control after optimization of medical treatment, two underwent percutaneous revascularization, five had ischemia limited to the right coronary artery, five refused to proceed, two were considered to have significant comorbidity that would preclude clinical benefit, one died and two suffered an acute coronary syndrome while waiting for the procedure. A total of 26 patients thus proceeded to Reducer implantation and were analyzed (Figure 1).
Detailed baseline population characteristics are described in Table 1. The mean age was 71±7 years and 20 (77%) were male. Obstructive CAD judged not amenable to further revascularization was present in 23 (88%) patients and three patients had microvascular angina (defined by the presence of anginal symptoms and reversible ischemia on stress SPECT and MRI in the absence of angiographically significant CAD). All patients with obstructive CAD (n=23) had already had at least one coronary intervention: 18 (69%) had previous PCI, 16 (62%) had previous coronary artery bypass graft surgery (CABG), and 12 (46%) had both. The main reasons precluding coronary revascularization in this population (most patients had more than one reason for no further revascularization) were chronic total occlusions (54%), long diffuse CAD (58%), multiple coronary tandem lesions (54%), poor distal targets (39%), last remaining patent coronary artery (23%), and microvascular disease (12%).
Baseline characteristics of patients referred for Reducer implantation (n=26).
| Age, years | 71.8±7.2 |
| Male gender | 20 (76.9%) |
| BMI, kg/m2 | 30.3±2.2 |
| Hypertension | 24 (92.3%) |
| Dyslipidemia | 23 (88.9%) |
| Diabetes | 14 (53.8%) |
| Current or former smoker | 14 (53.8%) |
| Family history of premature CAD | 2 (7.7%) |
| Cerebrovascular disease | 6 (23.1%) |
| PAD | 6 (23.1%) |
| Heart failure | 7 (26.9%) |
| AF | 3 (11.5%) |
| CKD | 8 (30.8%) |
| COPD | 1 (3.8%) |
| 3-vessel CAD | 20 (76.9%) |
| Left main disease | 5 (19.2%) |
| Microvascular disease | 3 (11.5%) |
| Non-severe valvular heart disease | 3 (11.5%) |
| Previous MI | 16 (61.5%) |
| Previous PCI | 18 (69.2%) |
| Previous CABG | 16 (61.5%) |
| Previous PCI and CABG | 12 (46.2%) |
| CIED | 3 (11.5%) |
| CCS angina class | 2.7±0.5 |
| LVEF, % | 53.6±9.8 |
| Serum hemoglobin, g/dl | 12.9±1–7 |
| Serum creatinine, mg/dl | 1.3±0.6 |
| NT-proBNP, pg/ml | 271.1±286.9 |
| Non-invasive ischemia test | |
| Dobutamine stress echocardiography | 3 (11.5%) |
| SPECT scan | 19 (73.2%) |
| Cardiac MRI | 4 (15.3%) |
| SAQ-7 | 46.6±18.0 |
| Physical limitation | 50.3±15.2 |
| Angina frequency | 55.3±21.4 |
| QoL | 34.2±23.2 |
| Dual antiplatelet therapy | 11 (42.3%) |
| High-intensity statin | 24 (92.3%) |
| No. of antianginal drugs | |
| Beta-blockers | 23 (88.4%) |
| Calcium channel antagonists | 18 (69.2%) |
| Long-acting nitrates | 17 (65.4%) |
| Ranolazine | 12 (46.2%) |
| Ivabradine | 5 (19.2%) |
| Nicorandil | 6 (23.1%) |
Values are mean±SD, n (%), or median (interquartile range).
AF: atrial fibrillation; BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CCS: Canadian Cardiovascular Society; CIED: cardiac implantable electronic device; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; MI: myocardial infarction; MRI: magnetic resonance imaging; NT-proBNP: N-terminal pro-brain natriuretic peptide; PAD: peripheral artery disease; PCI: percutaneous coronary intervention; QoL: quality of life; SAQ-7: short form of the Seattle Angina Questionnaire; SPECT: single-photon emission computed tomography.
CAD risk factors were highly prevalent and did not differ between patients with obstructive CAD and microvascular disease. Eight patients (31%) had moderate-to-severe chronic kidney disease (glomerular filtration rate 15–60 ml/min/1.73 m2), 16 (62%) had a history of previous myocardial infarction and median LVEF was 55% [44–66%].
Mean baseline CCS class was 2.7±0.47, with 18 (69%) in CCS 2 and eight (31%) in CCS 3.
The mean baseline SAQ-7 scores were 50.3±15.2 points for physical limitation, 55.3±21.4 for angina frequency, and 34.2±23.2 for quality-of-life domains. The mean baseline modified SAQ-7 score in both centers was 46.6±18.0.
All patients were treated with statins and at least one antiplatelet drug. The mean number of anti-ischemic drugs at baseline was 3.3±1.1. Twenty-three patients (88.4%) were on beta-blockers, 18 (69.2%) on calcium channel blockers, six (23.1%) on nicorandil, 12 (46.2%) on ranolazin, 17 (65.4%) on long-acting nitrates, and five (19.2%) on ivabradin.
Procedure-related characteristicsThe right jugular vein was used as the access site in all patients. The median duration of the procedure was 58 [46–95] min and mean balloon inflation pressure was 4.1±0.9 atm. Twenty-four (92.3%) were discharged on the same day. Median fluoroscopy time was 17.8±11.1 min and median radiation dose was 1918.3±4021.7 mGy.
Safety outcomesThe primary safety outcome of successful device deployment at the intended site without complications was achieved in 23 patients (88.5%), with two unsuccessful attempts and one isolated procedure-related complication. The unsuccessful implantations were due to one failure to cannulate the CS and one device migration. In the former, a challenging CS anatomy prevented selective engagement despite the use of several catheters and techniques.
Regarding the secondary safety outcomes, device success was achieved in 24 patients (92.3%). There were two complications: one device migration to the superior vena cava after full stent expansion, requiring surgical retrieval; and one wire-related perforation of a distal branch of the CS with cardiac tamponade requiring pericardiocentesis. These patients were discharged at 24 h and 48 h, respectively, with an otherwise uneventful hospital stay.
Efficacy outcomesThe mean follow-up was 22.9±8.3 months, all patients having completed six-month follow-up. The two patients in whom implantation failed remained under maximally tolerated antianginal therapy and were referred to a cardiac rehabilitation program. No symptomatic improvement was noted thereafter. The following results refer to the 24 patients in whom implantation was successful.
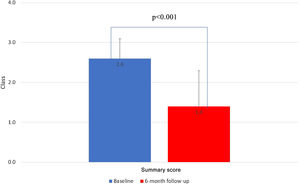
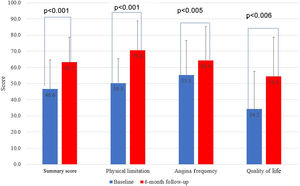
Mean CCS class improvement was 1.3±0.2, from 2.7±0.5 to 1.4±0.9 (p<0.001). Eighteen patients (75%) showed improvement of at least one CCS class, 10 (42%) presented a reduction of two CCS classes and in six (25%) patients the CCS class remained unchanged (Figure 2). All scales of the SAQ-7 showed a significant improvement after Reducer implantation. The physical limitation score improved from 50.3 to 70.6 (p=0.001), the angina frequency score improved from 55.3 to 64.4 (p=0.005), and the QoL score improved from 34.2 to 54.4 (p=0.006). This translated into a mean SAQ-7 summary score improvement from 46.6 to 63.2 (p<0.001) (Figure 3).
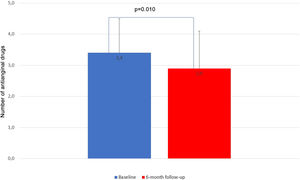
Thirteen patients (54%) discontinued or had the dose reduced of at least one anti-ischemic drug. The mean number of anti-ischemic drugs fell from 3.4±1.1 to 2.9±1.2 (p=0.010) (Figure 4).
During the six-month post-procedure time frame, none of the patients suffered any major cardiovascular event.
DiscussionIn the present real-world two-center experience, implantation of the Reducer device showed acceptable safety and was effective in symptom relief and QoL improvement, in a heterogeneous RA cohort including patients with obstructive CAD and microvascular disease.
Regarding safety, there were two severe procedure-related complication (8%), one cardiac tamponade (4%) and one device migration (4%) that were resolved immediately. In the largest real-world registry published to date (RESOURCE), the overall complication rate was 5.7% in 663 attempted procedures; device migration occurred in 2.3% and CS perforation occurred in three patients (0.5%). Twenty-four (93%) patients in our experience eventually received a device correctly positioned at the intended site in the CS, slightly below the 96.7% rate of implantation success (defined as leaving the device in place) reported in the RESOURCE study,19 but within the range of other smaller registries (90–100%).12,13 The differences in implantation success and complication rates can be considered normal for the early stage of the learning curve of a new technique in a small sample.
Regarding efficacy, a significant clinical benefit was observed, reflected in reduced CCS class, improvement in QoL (assessed by the SAQ-7), and reductions in the number and/or dose of anti-ischemic drugs at six months.
There was a reduction of at least one and two CCS classes in 77% and 54% of the patients, respectively, which is in line with the reduction in CCS class in previously published registries and a sham-controlled trial (67.5–81% for a reduction of one CCS class and 20–45% for two classes).10–19
Previous studies assessed the Reducer's impact on QoL through the Seattle Angina Questionnaire. We applied the short version of this questionnaire, the SAQ-7, which has been shown to improve clinical care by providing a simpler and more efficient mechanism to assess patients’ health status.21 The procedure's clinical benefit is supported by significant improvements in all dimensions of the SAQ-7. The absolute changes in SAQ-7 scores between baseline and six months post-procedure is of the same order of magnitude as in previous reports.10,13,15 However, the mean scores for each domain are higher at both baseline and six months in our series; this may be explained by differences in patients’ sociocultural background influencing the individual's subjective interpretation of symptoms, perception of the disease and overall QoL, and also by the fact that these patients were included in an intensive program at the angina clinic that included pragmatic rehabilitation, teaching of symptom control strategies and improvement of self-confidence accompanied by timely medical therapy optimization.
Compared to other series, the mean number of anti-ischemic drugs at baseline (3.4±1.1) was higher, which may also explain the greater proportion of patients (54%) who had their dose reduced and/or these drugs discontinued and the larger reduction in the mean number of anti-ischemic drugs (0.54±0.5).13,15
Six patients (25%) had no clinical response to Reducer implantation, which is within the range of previously reported rates of non-responders (18–29%).10,13,15–17 Several mechanisms may be responsible for this constant rate of non-responders, including ischemia in the right coronary artery territory, well-developed alternative venous drainage systems, progression of CAD, non-anginal pain, and right heart disease.
Three patients did not have obstructive CAD, but had typical angina and documented reversible ischemia in the left coronary artery territory. Two of these patients improved with Reducer implantation, with the clinical effect not differing from those with obstructive CAD. This effect has been noted in other series and in a small study including only patients with microvascular disease.22
LimitationsOur study suffers from the inherent limitations of a single-arm, open-label study assessing subjective endpoints, prone to bias from both investigators and patients regarding reporting and interpretation of symptoms, especially in a setting in which the placebo effect can be as high as 40%.10 Nevertheless, our findings were very similar to the results of the treatment arm of the COSIRA trial and other observational data, suggesting a consistent effect. The small sample size may to some extent have limited the power of the statistical analysis (due to type II error), nevertheless given the magnitude of the differences in the endpoints between baseline and follow-up this seems unlikely. The lack of functional capacity assessment and of echocardiographic and objective quantification of ischemia are two other limitations worth mentioning, as other studies have documented reductions in ischemic territories and improvements in cardiopulmonary fitness and LVEF after Reducer implantation.23–27 As depression is commonly present in CAD patients, a depression assessment questionnaire should have been used, as this comorbidity may affect treatment response.28 Finally, there is a lack of data regarding the best antithrombotic therapy modality after Reducer device implantation and whether this could influence clinical outcomes.
ConclusionIn this real-world multicenter initial experience of selected patients with severe forms of RA, not amenable to revascularization, resistant to appropriate titration of medical therapy and assessed by experienced multidisciplinary teams including interventional cardiology, cardiac surgery, clinical cardiology, rehabilitation and psychotherapy, complementary implantation of the Reducer device was useful in reducing angina and improving QoL with acceptable safety. Therefore, CS Reducer implantation should be reserved for this niche of highly selected patients.
FundingNone declared.
Conflicts of interestThe authors have no conflicts of interest to declare.