Current guidelines recommend not routinely testing patients with chest pain and low pretest probability (PTP <15%) of obstructive coronary artery disease (CAD), but envisage the use of risk modifiers, such as coronary artery calcium score (CACS), to refine patient selection for testing. We aimed to assess the cost-effectiveness (CE) of three different testing strategies in this population: (A) defer testing; (B) perform CACS, withholding further testing if CACS=0, and proceeding to coronary CT angiography (CCTA) if CACS>0; (C) CCTA in all.
MethodsWe developed a CE model using data from a two-center cross-sectional study of 1385 patients with non-acute chest pain and PTP <15% undergoing CACS followed by CCTA. Key input data included the prevalence of obstructive CAD on CCTA (10.3%), the proportion with CACS=0 (57%), and the negative predictive value of CACS for obstructive CAD on CCTA (98.1%).
ResultsNot testing would correctly classify 89.7% of cases and at a cost of €121433 per 1000 patients. Using CACS as a gatekeeper for CCTA would correctly diagnose 98.9% of cases and cost €247116/1000 patients. Employing first-line CCTA would correctly classify all patients, at a cost of €271007/1000 diagnosed patients. The added cost for an additional correct diagnosis was €1366 for CACS±CCTA vs. no testing, and €2172 for CCTA vs. CACS±CCTA.
ConclusionsCACS as a gatekeeper for further testing is cost-effective between a threshold of €1366 and €2172 per additional correct diagnosis. CCTA yields the most correct diagnoses and is cost-effective above a threshold of €2172.
As recomendações atuais sugerem não testar por rotina doentes com dor torácica e baixa probabilidade pré-teste (PPT < 15%) de doença arterial coronária (DAC) obstrutiva. No entanto, propõem a utilização de modificadores de risco, como o score de cálcio coronário (ScCa). O objetivo deste trabalho foi avaliar o custo-efetividade (CE) de três estratégias de diagnóstico de DAC nesta população: (A) não testar; (B) realizar ScCa, evitando exames adicionais se ScCa=0, e procedendo a angiotomografia computorizada (angioTC) coronária se ScCa>0; (C) angioTC como primeira linha.
MétodosDesenvolvemos um modelo de CE com base num estudo transversal em dois centros, incluindo 1385 doentes com dor torácica estável e PPT<15% submetidos a ScCa seguido de angioTC coronária. As principais variáveis incluíram a prevalência de DAC obstrutiva na angioTC (10,3%), a proporção com ScCa=0 (57%) e o valor preditivo negativo do ScCa para DAC obstrutiva na angioTC (98,1%).
ResultadosNão testar diagnosticaria corretamente 89,7% dos casos, a um custo de €121.433 por 1.000 doentes. Usar o ScCa como gatekeeper permitiria classificar corretamente 98,9% e custaria €247.116/1.000 doentes. A angioTC como primeira linha diagnosticaria todos os doentes, a um custo de €271.007/1.000 doentes. O custo de um diagnóstico correto adicional foi de €1.366 para ScCa±angioTC versus não testar e €2.172 para angioTC versus ScCa±angioTC.
ConclusõesA estratégia usando ScCa como gatekeeper é custo-efetiva entre um limiar de €1.366 e €2.172 por diagnóstico correto adicional. A angioTC coronária produz a maior taxa de diagnósticos corretos e é custo-efetiva acima de um limiar de €2.172.
Contemporary testing patterns for coronary artery disease (CAD) result in a relatively low prevalence of obstructive disease in patients undergoing diagnostic evaluation.1,2 As a result, increased attention has been drawn to the appropriate identification of low-risk patients not requiring additional testing.3 The assessment of the clinical pretest probability (PTP) of obstructive CAD according to the patient's sex, age, and symptom typicality has long been recommended to guide downstream testing.4 Nonetheless, consensus on which PTP threshold should be employed to select low-risk patients and on the use of risk modifiers to improve risk classification is still lacking. The 2019 European Society of Cardiology guidelines on the management of chronic coronary syndromes recommend not routinely testing patients with a low PTP of obstructive CAD (<15%), but envisage the use of risk modifiers, such as the coronary artery calcium score (CACS), to refine patient selection for further investigations.5 The 2021 North American chest pain recommendations advocate similar guidance on deferring testing, despite CAC scoring being considered a reasonable alternative (Class IIa recommendation, level of evidence B).6 Conversely, the 2016 National Institute for Health and Care Excellence (NICE) guidelines recommend first-line coronary computed tomography (CCTA) for all patients with typical and atypical chest pain, regardless of the clinical PTP.7
In an era where healthcare systems are exposed to significant economic pressure, cost-effectiveness (CE) analyses have become increasingly important in the decision-making process. While the use of newer technologies has brought a wide range of clinical advantages, its application is counterbalanced by an escalation of costs. The estimated cost of CAD in Europe has been steadily increasing over time, reaching approximately €59 billion in 2015.8 Therefore, determining the CE of each of the recommended strategies for obstructive CAD diagnosis in low-risk patients is paramount.
ObjectivesThe aim of this study was to assess the CE of three different testing strategies in the approach to symptomatic patients with low PTP of obstructive CAD: (A) defer testing; (B) performing CACS, withholding further testing if CACS=0 and proceeding to coronary CT angiography (CCTA) if CACS>0; and (C) performing CCTA in all.
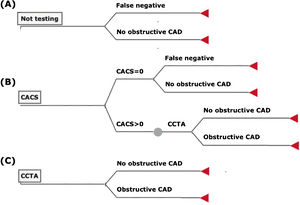
MethodsCost-effectiveness modelWe developed a decision tree model to test the CE of three different strategies: (A) defer testing; (B) perform CACS, withholding further testing if CACS=0 and proceeding to CCTA if CACS>0; and (C) perform first-line CCTA in all cases, without prior CACS. In this simulation, CCTA was defined as the gold standard, yielding 100% diagnostic accuracy for obstructive CAD, and no non-diagnostic results. Nonetheless, to better reflect real-world clinical practice and costs, we considered that all patients with obstructive CAD on CCTA would ultimately undergo a confirmatory invasive coronary angiography (ICA) (Figure 1). Bayesian inference was then used to estimate the costs and effectiveness of each strategy according to the test characteristics.9
Decision tree model for patients presenting with chest pain and low clinical pretest probability of suspected CAD (<15%): not testing (A), CACS as a gatekeeper for CCTA (B), and first-line CCTA (C). In strategy (B), all patients with CACS>0 would undergo CCTA to confirm or exclude obstructive CAD. The cost of a diagnostic ICA was later added to all patients with obstructive CAD on CCTA.
The base case population derives from a cross-sectional study performed at two tertiary centers in Lisbon, Portugal.10 Briefly, after excluding those with non-diagnostic results, a total of 1385 patients with non-acute chest pain and PTP <15%, who underwent CACS immediately followed by CCTA, were included.10 The PTP of obstructive CAD was estimated for each patient using the Juarez-Orozco tabular method endorsed by current societal guidelines.5,6
All scans were performed using an ECG-gated acquisition on dual-source 64-slice and 192-slice computed tomography scanners (Somatom Definition® and Somatom Force® Siemens Healthineers, Erlangen, Germany) in accordance with Society for Cardiovascular Computed Tomography guidelines.11 CACS was assessed on a non-contrast acquisition using the Agatston scoring method.12 Obstructive CAD was defined as the presence of maximal diameter stenosis ≥50% in any major epicardial vessel.
The baseline characteristics of the base case population are detailed in Supplementary Table 1. Key input data included the proportion of patients with obstructive CAD on CCTA (10.3%), the proportion of those with CACS=0 (57%), and the negative predictive value of CACS for obstructive CAD on CCTA (98.1%).
Comparison of cost-effectivenessComparison between each testing strategy was performed from the perspective of the society, including all attributable costs regardless of the payer. The CE of each strategy was defined as the cost per correct diagnosis (inclusion or exclusion of obstructive CAD). Accordingly, a decrease in that cost indicates improved CE. Mean costs, effectiveness (i.e., diagnostic performance), and cost-effectiveness ratios were derived. The incremental cost-effectiveness ratio (i.e., the added cost per additional correct diagnosis; ICER) was calculated for each strategy relative to the previous less expensive one, after excluding those subjected to strong dominance (less effective and more costly) and extended dominance (less effective with a higher ICER).
Definition of costThe overall cost of a diagnostic strategy includes both direct and indirect costs. Direct costs were calculated according to the current price list from the Portuguese National Health Service and are depicted in Table 1.13 In the CACS±CCTA strategy, a cost of €88.70 was assumed for patients undergoing CACS alone, whereas a cost of €299.40 was considered for those who also underwent CCTA (sum of the prices of CACS and CCTA). The indirect cost included (i) the cost associated with incidental findings during CACS and CCTA; (ii) the cost related to complications of ICA; and (iii) the cost of false negative tests:
Price of tests included in the different diagnostic strategies (values for Portugal).
| Code13 | Designation | Price (€) |
|---|---|---|
| 16062 | Coronary artery calcium scoring | €88.70 |
| 16063 | Coronary CT angiography | €210.70 |
| 40820 | Left catheterization with selective coronary angiography | €531.17 |
| 16060 | Chest CT without intravenous contrast | €74.70 |
CAC scoring and CCTA may uncover incidental non-cardiac findings of uncertain significance that warrant follow-up examination, most frequently lung nodules.14 Accordingly, we have assumed that 7% of patients undergoing those testing strategies would require a follow-up non-contrast chest CT scan, at an additional cost of €74.70.13,14
Because in this simulation all patients with a diagnosis of obstructive CAD on CCTA would undergo ICA, we assumed that the rate of major cardiovascular adverse complications (death, myocardial infarction, or stroke) of this procedure would be 0.083%, as reported in the literature.15 According to a previous study for the Portuguese reality, the cost of a typical ICA complication was estimated at €4300.16 The costs of complications related to noninvasive tests were not included in this simulation, as severe complications are extremely rare and would only marginally increase costs. Furthermore, costs regarding potential exposure to ionizing radiation were not included as this was a short-term CE study, and because the clinical impact of the radiation dosages used in these imaging tests is still uncertain.17
The costs of false negatives are remarkably difficult to estimate and include the cost of additional testing and of added risk for cardiovascular events (due to inappropriate management of CAD).16 In previous studies, these costs have been variably estimated as two to six times the cost of a false-positive.18–21 As such, a false negative was conservatively set at three times the cost of a false-positive test (€1178.96). In the defer testing strategy, the costs of false negatives were the only ones considered.
Sub-group and sensitivity analysesIt is known that for the same age and chest pain characteristics, women have a lower PTP of obstructive CAD and a lower likelihood of having calcified atherosclerotic plaques compared to men.5,22 Accordingly, we performed sex-specific CE analyses, to test whether the cost-effectiveness of each strategy would vary among both sexes.
Additionally, to test the robustness of our model and to assess the extent to which the results depended on some of the variables under study, several sensitivity analyses were performed. Calculations were repeated using new assumptions, such as varying the cost of both CACS and CCTA from −50% to +50%, the cost of a false negative result from two to five times the cost of a false-positive, the prevalence of obstructive CAD from 5% to 14%, and the proportion of patients with noncalcified obstructive CAD from 0.5% to 5%. The fundamental definitions of comparative cost-effectiveness analysis can be found elsewhere.23
ResultsThe baseline characteristics of the population included in the model have been previously described (Supplementary Table 1). The results of the main CE analysis for the diagnosis of obstructive CAD are summarized in Table 2. Not testing (strategy A) would correctly classify 89.7% of cases at a cost of €121433 per 1000 patients, exclusively due to the costs imputed to false negatives. Using CACS as a gatekeeper for CCTA (strategy B) would correctly diagnose 98.9% of cases and cost €247116 per 1000 patients. In this strategy, €184951 would be attributable to the costs of imaging tests, including incidental findings, €49196 to ICA in patients with obstructive CAD on CCTA, and the remaining €12969 to false negatives. Employing stand-alone CCTA as the first-line test (strategy C) would correctly classify all patients, at a cost of €271007 for 1000 diagnosed patients.
Results of cost-effectiveness of the diagnostic strategies for 1000 patients.
| Testing strategy | Costs (€ per 1000 patients) | Correct diagnoses (%) | False negatives (%) | ICER (€/correct diagnosis) | |
|---|---|---|---|---|---|
| Defer testing | Total costs | €121433 | 89.7% | 10.3% | – |
| CACS | 0 | ||||
| CCTA | 0 | ||||
| Incidental findings | 0 | ||||
| ICA | 0 | ||||
| ICA complications | 0 | ||||
| False-negatives | 121433 | ||||
| CACS±CCTA | Total costs | €247116 | 98.9% | 1.1% | €1366 |
| CACS | 88700 | ||||
| CCTA | 91022 | ||||
| Incidental findings | 5229 | ||||
| ICA | 48868 | ||||
| ICA complications | 328 | ||||
| False-negatives | 12969 | ||||
| CCTA | Total costs | €271007 | 100.0% | 0.0% | €2172 |
| CACS | 0 | ||||
| CCTA | 210700 | ||||
| Incidental findings | 5229 | ||||
| ICA | 54711 | ||||
| ICA complications | 368 | ||||
| False negatives | 0 | ||||
Overall, the cost per correct diagnosis (average cost-effectiveness ratio; ACER) was €135, €250, and €271, for strategies A, B, and C, respectively. When comparing the different testing methods, the added cost for an additional correct diagnosis (ICER) was €1366 for CACS±CCTA vs. not testing, and €2172 for stand-alone CCTA vs. the strategy using CACS as a gatekeeper.
Sub-group and sensitivity analysesIn our base case population, the prevalence of obstructive CAD was 12.5% for men and 9.7% for women and the proportion of patients with CACS=0 was 56.7% and 57.0%, respectively. The likelihood of having obstructive CAD without any coronary artery calcification was numerically higher for women compared to men (2.1% vs. 1.2%, p=0.46), yielding an overall diagnostic accuracy of the strategy using CACS±CCTA of 98.8% and 99.3%, respectively (Table 3). The ICER for strategy B vs. A was €943 in men and €1527 in women, whereas the ICER for strategy C vs. B was €3450 and €1972, respectively (Table 3).
Results of cost-effectiveness of the diagnostic strategies for 1000 patients according to patient's sex.
| Testing strategy | Cost (per 1000 patients) | Correct diagnoses (%) | False-negatives (%) | ICER | |
|---|---|---|---|---|---|
| Male sex | Defer testing | €147370 | 87.5% | 12.5% | - |
| CACS±CCTA | €258621 | 99.3% | 0.7% | €943 | |
| CCTA | €282771 | 100.0% | 0.0% | €3450 | |
| Female sex | Defer testing | €114358 | 90.3% | 9.7% | - |
| CACS±CCTA | €244130 | 98.8% | 1.2% | €1527 | |
| CCTA | €267799 | 100.0% | 0.0% | €1972 | |
The results of the sensitivity analyses are depicted in Table 4. Overall, increasing the cost of CAC scoring by 50% or decreasing the price of a CCTA by 50% would reclassify the CACS±CCTA strategy as non-cost-effective (strong dominance of stand-alone CCTA). First-line CCTA would also be the most cost-effective approach should the rate of false negatives in the strategy using CACS as a gatekeeper (i.e., obstructive CAD with CACS=0) increase to 5%. For a prevalence of obstructive CAD of 5%, not testing would be cost-effective at a CET of €3674.
Results of sensitivity analyses.
| ICER | ||
|---|---|---|
| CACS±CCTA vs. No testing | CCTA vs. CACS±CCTA | |
| Cost of CACS | ||
| €44.35 (−50% from base case) | €884 | €6204 |
| €133.05 (+50% from base case) | Dominated* | €1452 |
| Cost of CCTA | ||
| €105.35 (−50% from base case) | Dominated* | €745 |
| €316.05 (+50% from base case) | €1545 | €7296 |
| Cost of false-negative | ||
| 2×cost of false-positive | €1582 | €2388 |
| 4×cost of false-positive | €1150 | €1956 |
| 5×cost of false-positive | €934 | €1740 |
| Prevalence of obstructive CAD | ||
| 5% | Dominated** | €3674 |
| 14% | €789 | €2140 |
| Prevalence of obstructive CAD and CACS=0 | ||
| 0.5% | €1243 | €5551 |
| 5% | Dominated* | €1452 |
In recent years, there has been an increasing awareness of the need to consider the economic efficiency of medical decisions. Cost-effectiveness analyses can guide stakeholders in utilizing limited healthcare resources more efficiently, including latest-generation diagnostic technologies, often more costly than their alternatives.16,24
In this study, we have assessed the cost-effectiveness of three approaches recommended in different guidelines for the diagnosis of obstructive CAD in patients with chest pain and PTP <15%.5–7 The main results of our analysis can be summarized as follows:
- i.
Not testing should be disfavored unless the cost-effectiveness threshold is below €1366, as it would result in a rate of missed diagnoses of about 10%;
- ii.
CACS as a gatekeeper for CCTA is the best strategy if the society is willing to pay up to €2172 for an additional correct diagnosis. With this approach, the use of CCTA could decrease by roughly 50%, at the expense of approximately 1% of patients being misdiagnosed as not having obstructive CAD (false negatives).
- iii.
For a cost-effectiveness threshold (i.e., willingness to pay for an additional correct diagnosis; CET) above €2172, first-line CCTA would be the best method, yielding the highest diagnostic accuracy.
The burden of non-acute chest pain in healthcare services remains remarkably high, with patients with a low pretest probability of CAD accounting for a large proportion.25 Whether, on the one hand, effective diagnostic strategies are necessary to detect CAD and eventually inform about secondary preventive measures, the low diagnostic yield of testing in this setting can result in a high rate of false-positive results, in an increased number of incidental findings of unknown significance, and in higher costs for the society.2,4 Along with the overall good prognosis of patients with low PTP, these arguments have been the rationale for recommendations to defer testing in this subset of patients.5 Notwithstanding this, it has been previously shown that CCTA referrals in patients with PTP < 15% can account for over 60% of all referrals due to suspected CAD, possibly because many are not willing to accept the diagnostic uncertainty of not testing.10
The selection of a diagnostic method, beyond its diagnostic accuracy, should also consider costs. As opposed to long-term CE studies – using quality-adjusted life years (QALYs) as a measure of outcome – a universally accepted CET to provide guidance on adopting or dismissing a certain diagnostic strategy does not exist. Of note, a previous study on the cost-effectiveness of different diagnostic strategies for CAD in Portugal identified a CET of at least €1010 for the Portuguese society,16 corresponding to the added costs of stress single photon emission computed tomography (SPECT) followed by ICA, one of the most used diagnostic pathways in our country. In that study, at a willingness to pay of €1000, CACS±CCTA has been shown to be cost-effective for the detection of obstructive CAD in symptomatic patients with a PTP <20–30%.16 No further studies have explored the cost-effectiveness of CACS as a gatekeeper in an all-comer population with suspected CAD, and to the best of our knowledge, our study is the first to access it specifically in symptomatic patients with a low PTP of obstructive CAD. On the other hand, in the setting of primary prevention in asymptomatic patients, CACS has been shown to be cost-effective in guiding the initiation of statin therapy.26 Although cost-effectiveness studies can inform clinical decision-making, the best strategy will ultimately depend on society's willingness to pay for an additional correct diagnosis and on the number of missed diagnoses stakeholders are willing to accept.
Sub-group and sensitivity analysesWe have found that the CETs of each diagnostic method vary among both sexes. Using CACS as a gatekeeper for CCTA was the most cost-effective strategy between a willingness to pay for additional correct diagnosis of €1527 to €1972 for women and €943 to €3450 for men. The variable rate of false negatives from using this approach can help explain this difference. In our study, despite the prevalence of obstructive CAD being lower in women, and the proportion of patients without coronary artery calcifications being somewhat similar, female patients were approximately twice as likely to have obstructive CAD with CACS=0. It should be noted however that data from prior studies on plaque composition have shown heterogeneous findings for women. The CONFIRM registry, including over 5600 patients, reported a higher prevalence of plaques of every type in men than in women.27 In contrast, other studies, including one with 1050 matched patients found a higher proportion of non-calcified plaques in females.28,29
Of the scenarios assessed in the sensitivity analyses, the influence of the prevalence of obstructive CAD is particularly important. For very low probabilities of obstructive CAD (prevalence <5%), not testing would be a cost-effective approach for a wide margin of CET. In this context, CACS±CCTA would not be cost-effective, as it is extendedly dominated by the strategy using first line CCTA. Of note, the results remained similar when varying the cost of a false negative from two to five times the cost of a false-positive.
LimitationsSome limitations of this study should be acknowledged. Firstly, our definition of obstructive CAD was based on CCTA and therefore we did not consider the costs of false negative CCTA results (having ICA as the reference). However, provided that the negative predictive value of CCTA in patients with low PTP ranges from 97% to 99%, the additional costs would be neglectable.30 Secondly, as false-positive results on CCTA are far more frequent in this setting, the proportion of missed diagnoses as well as the costs resulting from not testing are likely overestimated.4 Nevertheless, to reflect better real-world clinical practice, we considered that all patients with obstructive CAD on CCTA would undergo ICA. Thirdly, as this was a short-term CE analysis, we did not evaluate the impact of each strategy on subsequent therapeutic adjustments and their potential impact on quality-adjusted life years, even though there is currently evidence that using CACS/CCTA to guide therapy may improve clinical outcomes.31 Likewise, the economic impact of detecting non-obstructive CAD was not ascertained. Finally, the results of this CE study refer to patients with low PTP and to the costs currently practiced in the Portuguese National Health System and may not be applicable to other settings.
ConclusionsNot testing patients with low PTP of obstructive CAD should be disfavored unless the CET is below €1366 per correct diagnosis. First-line CCTA yields the highest rate of correct diagnoses and is cost-effective above CET over €2172 per additional correct diagnosis. Using CACS as a gatekeeper for further testing is cost-effective between these thresholds, which are wider for men than for women. These findings may inform decisions on testing, but the most suitable strategy will ultimately depend on the costs and amount of missed diagnoses stakeholders are willing to accept.
Conflicts of interestThe authors have no conflicts of interest to declare.