Preterm birth and congenital heart defects are two major causes of neonatal and infant mortality. However, the relationship between them has not yet been fully clarified.
ObjectiveTo determine the prevalence and spectrum of congenital heart defects in preterms, the specific associations between categories of congenital heart defects and preterm birth and to establish the influence on outcomes.
MethodsObservational, case-control analysis that included 448 live births with congenital heart defects born between 2003 and 2017. Preterm with congenital heart defects were the case subjects and term neonates with congenital heart defects the control subjects.
ResultsOf the newborns with congenital heart defects, 23% were preterm. The odds of congenital heart defects in preterm were twofold higher than for term neonates (p<0.0001), even when considering only those with severe congenital heart defects (p=0.0002). The odds in preterm were 9.2-fold higher for abnormalities of the atria and atrial septum (p<0.0001) and two-fold higher for abnormalities of the ventricles and ventricular septum (p<0.0001) compared with term neonates. The neonatal mortality rate in the preterm group was not statistically different from that of the term group with congenital heart defects (p=0.799) or severe congenital heart defects (p=0.554).
ConclusionPreterm have more than twice as many congenital heart defects as term neonates. Although the etiology of prematurity between infants with congenital heart defects is still uncertain, our findings highlight a possible relationship between prematurity and congenital heart defects.
A prematuridade e a cardiopatia congénita são as duas principais causas de morbimortalidade neonatais. Contudo, a relação entre essas duas entidades não está totalmente esclarecida.
ObjetivoDeterminar a prevalência e espetro de cardiopatias congénitas em prematuros, a associação entre diferentes categorias de cardiopatias congénitas e a prematuridade e determinar se a prematuridade tem influência na mortalidade neonatal nos recém-nascidos com cardiopatia congénita.
MétodosEstudo observacional, caso-controlo, que incluiu 448 recém-nascidos com diagnóstico de cardiopatia congénita no período neonatal entre 2003 e 2017. Os objetos de estudo foram os prematuros com cardiopatia congénita e os controlos os recém-nascidos de termo com cardiopatia congénita.
ResultadosDos recém-nascidos com cardiopatia congénita 23% eram prematuros. O odds ratio de cardiopatia congénita em prematuros foi duas vezes maior do que em recém-nascidos de termo (p<0,0001), mesmo considerando apenas cardiopatia congénita grave (p=0,0002). O odds ratio em prematuros foi 9,2 vezes superior para defeitos auriculares ou do septo interauricular (p<0,0001) e duas vezes superior para defeitos dos ventrículos ou do septo interventricular (p<0,0001) do que em recém-nascidos de termo. Não existiram diferenças significativas na mortalidade neonatal entre prematuros e recém-nascidos de termo com cardiopatia congénita (p=0,799) ou cardiopatia congénita grave (p=0,554).
ConclusãoOs prematuros têm mais do dobro do risco de cardiopatia congénita comparativamente com os recém-nascidos de termo. Embora a etiologia da prematuridade entre recém-nascidos com cardiopatia congénita ainda seja incerta, os nossos resultados destacam uma possível relação entre prematuridade e cardiopatia congénita.
Congenital heart defects (CHD) include structural malformations of the heart and/or major vessels present at birth or persisting abnormally after birth; it is the most frequent group of major congenital anomalies with a live prevalence that varies between 4.05 and 10.4 per 1000 live births.1–3 This prevalence is constant worldwide, nevertheless, it is dependent on case definition.4
Despite substantial progress in its diagnosis and management, CHDs remain the major cause of infant mortality due to congenital anomalies, requiring multiple hospitalizations and surgical procedures.5,6 Infant mortality and morbidity associated with preterm birth have also been extensively described.
Congenital heart defects and preterm birth are, therefore, leading causes of infant mortality and morbidity, but the relationship between them remains unclear. Associations between congenital anomalies and preterm birth are known.7–10 However, there are few specific data regarding the relationship of prematurity and CHD and the association between specific categories of CHD and preterm birth. Besides, few studies have been adjusted for maternal covariates related to fetal growth.
The etiology of most non-syndromic CHD and preterm birth is uncertain. Probably it involves complex interactions between multiple environmental exposures and genetic susceptibilities. It is conceivable that both may occur independently but share common risk factors.11–13 Alternatively, preterm birth in neonates with CHD may be caused by abnormal fetal hemodynamic profiles.13 The objective of our study was to investigate the association between preterm birth and CHD while controlling other risk factors for CHD. Since CHDs are a major global health concern, it is important to identify possible risk factors associated with their development and prematurity.
Materials and methodsData sourceOur reference population comprised women residing in Central Portugal. All neonates with suspected CHD from this geographic area are referred to one of the two level-three maternal care units in Coimbra, which have the same case load (Bissaya Barreto or Daniel de Matos).
A retrospective analysis of all live-born neonates with CHD at Bissaya Barreto maternal care unit, between 2003 and 2017, was performed. Maternal, neonatal, and clinical data were collected from the medical files.
All neonates were examined daily by a physician, and neonates with heart murmurs or other clinical signs of heart disease or persistent respiratory problems were referred for a cardiology review. This study only included CHD confirmed by a pediatric cardiologist. The main outcome measure was risk of CHD in preterm neonates. Preterm was defined as a gestational age <37 weeks. Low birth weight was defined as <2500 g.
Due to a high prevalence of patent ductus arteriosus in preterm, it was excluded from the subject case group, unless there was hemodynamically significant patent ductus arteriosus in term neonates. Also neonates with physiologic pulmonary artery branch stenosis, patent foramen ovale, atrial septal defect <3 mm, trivial lesions such as aortic/pulmonary valve stenosis with a systolic pressure gradient less than 20 mmHg, isolated bicuspid aortic valve, mitral valve prolapse without regurgitation and cardiac tumors were excluded from the analysis.
To determine specific associations between CHD categories and preterm birth, an anatomical and clinical classification of CHD based on the short list of the European Pediatric Cardiac Code14 was used.
Disease severity was based on the Hoffman and Kaplan classification.4
Statistical analysisThis study is a case-control analysis of preterm neonates with CHD as our case subjects and term neonates with CHD as the control subjects. We report proportions with 95% binominal exact confidence intervals (CI). As our population had a normal distribution, we used the χ2 or Fisher exact test to assess associations between the groups. Potentially confounding variables considered included: maternal age at conception, parental consanguinity, pregestational and gestational diabetes, smoking, alcohol, and drug exposure during the first trimester of pregnancy, family history of CHD, fetal chromosomal and other genetic anomalies, fetal number (singleton or multiple), extracardiac malformations and parity.15–19
Data were analyzed using the Statistical Package for Social Sciences (SPSS version 23).
ResultsCongenital heart defects among preterm or low birth weight infantsOf the 43233 live births (preterm=4278 and term=38507), 448 infants were included in this study, revealing a prevalence of 10 cases per 1000 live births. The demographic characteristics of the groups are shown in Table 1. Of the analyzed risk factors for CHD, only extracardiac malformations were found to be statistically different (Table 2).
Demographics of preterm (PT) and term (TN) neonates with congenital heart defects (CHD).
| Case subjects – PT, n (%)a | Control subjects – TN, n (%)b | |
|---|---|---|
| Male | 54 (52%) | 158 (46%) |
| Caesarean section | 55 (53%) | 120 (35%) |
| Birth weight (g) | ||
| Mean±SD | 1702.6±716 | 3210±455 |
| Median | 1575 | 3210 |
| Mode | 1775 | 3010 |
| Gestational age (completed weeks) | ||
| Mean±SD | 31±3 | 39±1 |
| Median | 32 | 39 |
| Mode | 36 | 39 |
| 5-Minutes APGAR score | ||
| Mean±SD | 9±1 | 9±1 |
| Median | 10 | 10 |
| Mode | 10 | 10 |
SD: standard deviation.
Prevalence of specific risk factors for congenital heart defects between preterm and term neonates.
| Risk factors for congenital heart defects | Case subjects – PT, n (%)a | Control subjects – TN, n (%)b | p* |
|---|---|---|---|
| Maternal age at conception (≥40 years old) | 6 (5.8%) | 12 (3.5%) | 0.39 |
| Parental consanguinity | 0 (0%) | 0 (0%) | 1 |
| Pregestational and gestational diabetes | 8 (7.7%) | 25 (7.3%) | 0.833 |
| Smoking, alcohol and drug exposure during 1st trimester of pregnancy | 6 (5.8%) | 10 (2.9%) | 0.22 |
| Family history of CHD among first-degree relatives | 8 (7.7%) | 30 (8.7%) | 0.84 |
| Foetal chromosomal anomalies or genetic syndrome | 15 (14.4%) | 36 (10.5%) | 0.29 |
| Extracardiac malformations | 28 (26.9%) | 50 (14.5%) | 0.005 |
| Fetal Number >1 (singleton: twin) | 15 (14.4%) | 29 (8.4%) | 0.07 |
| Multiparous | 53 (50.9%) | 169 (49%) | 0.82 |
CHD: congenital heart defect.
Preterm represented 23% of the study population (n=104). In this group the prevalence of CHD was 24 cases per 1000 preterm, as opposed to nine cases per 1000 in the term neonate group, giving an odd ratio (OR) for CHD in premature infants of 2.7 (95% CI: 2.18–3.39; p<0.0001). After exclusion of cases with extracardiac malformations (n=78) the results were similar: 20% (n=76) were premature with a CHD prevalence of 18.6 cases per 1000 preterm and 7.4 cases per 1000 term neonates, giving an odd ratio for CHD in prematurity of 2.5 (95% CI: 1.97–3.24; p<0.0001).
To investigate the possibility of an ascertainment bias toward increased detection of minor malformations among preterm, we also analyzed cases with severe CHD. The prevalence of severe CHD in preterm is five cases per 1000 preterm neonates; in contrast, severe CHD was two cases per 1000 term neonates. As seen for CHD in general, the OR for severe CHD in preterm was 2.4 (95% CI: 1.52–3.92; p=0.0002) and 2.3 (95% CI: 1.31–4.08; p=0.0039) after exclusion of cases with extracardiac malformations, showing that the excess of CHD among preterm neonates cannot be explained by minor defects.
Based on birth weight, 106 infants with less than 2500 g had CHD, giving an odd ratio of 2.2 (95% CI: 1.77–2.76; p<0.0001) for CHD in low birth weight infants.
Spectrum of congenital heart defectsVentricular septal defects (VSD) were the most common lesion in both groups, representing 46% of the CHD in preterm and 57% in term neonates. Considering severe cases of CHD, the most prevalent diagnoses in the preterm population were complete atrioventricular septal defect (CAVSD) (4%), tetralogy of Fallot (TOF) (3%) and pulmonary valve stenosis (PS) (3%). In comparison, the most prevalent severe CHD in the term neonates’ group were dextro-transposition of the great arteries (TGA) (5%), coarctation of the aorta (COA) (5%) and TOF (3%).
The odds of preterm neonates were 9.2-fold higher for abnormalities of the atria and the atrial septum (95% CI: 5.8–14.4; p<0.0001) and 2-fold higher for abnormalities of the ventricles and the ventricular septum (95% CI: 1.5–2.8; p<0.0001) compared with the term neonates’ group. The results were similar after exclusion of cases with extracardiac malformations: odd ratio for abnormalities of the atria and the atrial septum in preterm was 8.9 (95% CI: 5.4–14.9; p<0.0001) and 1.6 for abnormalities of the ventricles and the ventricular septum (95% CI: 1.1–2.4; p=0.0069).
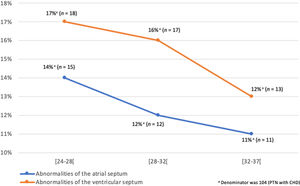
There was no significant difference (95% CI: 0.5–2.1; p=0.89) in the type of VSD found in the two groups; muscular VSD accounted for 73% (n=35) of all VSD in the preterm group and 70% (n=140) in the term group being the most frequent type. Due to the high prevalence of atrial/ventricular septum abnormalities in preterm neonates, we did an evaluation stratified by gestational age that showed that these defects are more prevalent as the prematurity is more marked (Figure 1). The risk of other categories of CHD did not vary significantly between preterm and term neonates (Table 3). Over the 15 years of study, there were no significant changes in the prevalence of the categories of CHD analyzed.
Prevalence of specific groups of congenital heart defects between preterm and term neonates, considering the totality of the study population or among infants without extracardiac malformations.
| Group of congenital heart defectsa | Case subjects – PT, n (%) | Control subjects – TN, n (%) | p* | OR (95% CI)b | ||||
|---|---|---|---|---|---|---|---|---|
| Totale | WMf | Totalg | WMh | Total | WM | Totalc | WMd | |
| Abnormalities of position and connection of heart | 3 (2.9%) | 2 (2.6%) | 24 (6.9%) | 17 (5.9%) | 0.87 | 0.95 | 1.1 [0.3–3.7] | 1.0 [0.2–4.5] |
| Tetralogy of Fallot and variants | 4 (3.9%) | 3 (3.9%) | 12 (3.5%) | 9 (3.1%) | 0.06 | 0.10 | 2.9 [0.9–9.2] | 2.9 [0.8–10.9] |
| Abnormalities of great veins | 1 (0.9%) | 1 (1.3%) | 3 (0.9%) | 2 (0.7%) | 0.34 | 0.22 | 2.9 [0.3–28.4] | 4.5 [0.4–49.2] |
| Abnormalities of atria and atrial septum | 38 (36.5%) | 30 (39.0%) | 37 (10.8%) | 30 (10.4%) | <0.0001 | <0.0001 | 9.2 [5.8–14.4] | 8.9 [5.4–14.9] |
| Abnormalities of atrioventricular valves and atrioventricular septal defect | 4 (3.8%) | 1 (1.3%) | 9 (2.6%) | 6 (2.1%) | 0.71 | 0.02 | 1.5 [0.2–12.3] | 3.9 [1.2–12.8] |
| Abnormalities of ventricles and ventricular septum | 48 (46.1%) | 37 (48.7%) | 205 (59.6%) | 188 (65.1%) | <0.0001 | 0.0017 | 2.1 [1.5–2.9] | 1.8 [1.2–2.5] |
| Abnormalities of ventricular and arterial valves and great arteries | 8 (7.7%) | 6 (7.9%) | 39 (11.3%) | 30 (10.4%) | 0.12 | 0.19 | 1.8 [0.9–3.9] | 1.3 [0.7–4.3] |
| Abnormalities of coronary arteries, arterial duct and pericardium; arteriovenous fistulae | 1 (0.9%) | 1 (1.3%) | 10i (2.9%) | 7 (2.4%) | 0.91 | 0.83 | 0.89 [0.1–6.9] | 1.3 [0.2–10.3] |
Anatomic and clinical classification of congenital heart defects based on the short list of the European Pediatric Cardiac Code.
OR: odds ratio. For the case subjects OR calculation, the numerator was the preterm (PT) with a specific CHD for the exposed with a positive outcome and the term neonate (TN) with the same specific CHD as the control with a positive outcome; denominator was the total number of PT (n=4278) excluding the PT with the specific CHD (n=4278 – PT with a specific CHD) for the exposed group with a negative outcome and the total number of TN (n=38507) excluding the TN with the specific CHD (n=38507 – TN with a specific CHD) for the control group with a negative outcome.
Without malformations: extracardiac malformations were excluded. For the case subjects OR calculation, the numerator was the PT with specific CHD and no extracardiac malformations for the exposed with a positive outcome and the TN with the same specific CHD and no extracardiac malformations as the control with a positive outcome; denominator was the total number of PT with no extracardiac malformations (n=4382) excluding the PT with the specific CHD (n=4382 – PT with a specific CHD and no extracardiac malformations) for the exposed group with a negative outcome, and the total number of TN with no extracardiac malformations (n=38851) excluding the TN with the specific CHD (n=38851 – TN with a specific CHD and no extracardiac malformations) for the control group with a negative outcome.
Prematurity and low birth weight impacted the therapeutic guidance of patients with large VSD (n=3) and CAVSD (n=1) in heart failure despite optimized medical therapy. These patients had gestational ages between 31 and 33 weeks, birth weights between 1395 and 1765 g and underwent pulmonary artery banding when they reached 3 kg, before undergoing complete correction some months later. The acute and late postoperative period was uneventful. Our institution's general neonatal mortality rate between 2003 and 2017 was 2%, of which 1.9% related to prematurity.
The neonatal mortality rate for term neonates with CHD (17 (5.1%)) and severe CHD (15 (14.1%)) was similar to that found in the preterm group with CHD (6 (5.8%)) and severe CHD (three (12%)). Therefore, there were no statistically significant differences in neonatal mortality rate between cases and controls with CHD (p=0.799) or severe CHD (p=0.554).
In the group of preterm neonates, two died due to their cardiovascular malformations. They were near term with severe CHD, namely a 36-weeks gestational age TOF case in the setting of ventricular tachycardia with severe systolic dysfunction, and a 35-week gestational age case with truncus arteriosus, bilaterally hypoplastic pulmonary arteries and VACTREL association, in which the parents opted for conservative treatment. Other deaths in preterm neonates were related to extracardiac causes – four extreme preterm infants with large ASD/VSD and gestational ages between 26 and 28 weeks from necrotizing enterocolitis, sepsis or central nervous system injury; one CAVSD and 34 weeks from stroke; one large ASD and 35 weeks with severe respiratory failure from myotonic dystrophy.
In contrast, only one term neonate with CHD died from extracardiac complications caused by muscular spinal atrophy type 1; others were related to CHD. In all five hypoplastic left heart syndrome cases, parents declined intervention postnatally and opted for comfort care treatment. Four deaths occurred in the postoperative period:
- •
coronary artery complications during an arterial switch procedure for TGA
- •
necrotizing enterocolitis after end-to-end anastomosis and reconstruction of the aortic arch in CoAo with hypoplastic aortic arch
- •
two modified Blalock-Taussig shunt thrombosis for pulmonary atresia with intact septum.
Five deaths occurred during cardiac catheterization:
- •
two prolonged Raskind procedures with severe hemodynamic instability for TGA and tricuspid atresia
- •
two percutaneous balloon valvuloplasty in critical aortic and pulmonary valve stenosis, both complicated by severe and irreversible bradycardia during balloon inflation
- •
one right ventricle outflow tract perforation with cardiac tamponade during an attempt for pulmonary valve perforation in pulmonary atresia with intact septum.
This study showed that preterm and low birth weight neonates (<2500 g birth weight) have more than twice the prevalence of CHD as term neonates, findings similar to previous studies.5,11,13,20,21 The odd ratio for CHD in prematurity was 2.7 (or 2.5 excluding extracardiac malformations) even when considering only the severe CHD group, showing that the excess of CHD among preterm cannot be explained by minor malformations.
The only statistically significant variable between the study groups related to a higher risk for CHD was the presence of extracardiac malformations. However, the exclusion of this variable had no significant impact on the results. Furthermore, 68% of new-borns with CHD born between 2003 and 2017 were identified prenatally. As we do not recommend premature delivery, it is unlikely that prenatal diagnosis had any significant influence on gestational age at delivery. Therefore, the twofold increase in the overall risk of CHD was probably due to spontaneous preterm birth.
The spectrum of CHD among preterm and term neonates is inconsistent between series. Laas et al. and Matthiesen et al. reported that the category of anomalies of the ventricular outflow tract, mainly affecting the right ventricle, had the highest risk of PTB.5,21 Tanner et al. reported that preterms had a higher prevalence of pulmonary atresia with VSD, CAVSD, COA, TOF and PS.11 Our results were similar to those reported by Godfrey et al., a small study of a single neonatal unit, where septal defects were the prevalent anomalies.11
Over the 15 years of study, there were no significant changes in the prevalence of CHD categories, probably because the recent advances in diagnostic methods had more impact in detecting mild CHD, and these kinds of alterations were not included in this study.
Although the study covered many years, the CIs for some rare and more complex CHD were large, indicating the limited precision of some of our estimates, due to relatively small numbers of these lesions.
Surprisingly, there were no statistically significant differences in the neonatal mortality rate between preterm and term neonates with CHD (p=0.799) or severe CHD (p=0.554). A possible explanation for these findings is that the additional effect of CHD on neonatal mortality rates, particularly severe CHD, is more pronounced in term and near term infants, for whom mortality rates are otherwise low, which puts preterm and term neonates on the same severity level. This suggests that, as the severity of CHD increases, the relative effect of preterm birth decreases.11,12 The cause of death in most term neonates with CHD was cardiac, in contrast to the preterm group in which their death was mainly due to complications related to prematurity, highlighting the higher impact of CHD on the outcomes of term neonates.
In the term neonate group, mortality decreased over the years, with 80% of deaths reported between 2003 and 2007; however, mortality did not decrease significantly in the preterm neonate group. Thus, although survival among premature infants has improved, this shows that the technical evolution over the years had more impact in overcoming cardiac lesions than complications related to prematurity.
Previous studies have found that a 1–2 week increase in gestational age increases an infant's odds of survival over time. This is probably due to increased organ maturation, mainly lung maturation, and the increased likelihood of successful surgery to correct the heart defect.11,23–25 However, unlike our analysis, these results did not take into consideration neonatal mortality. In the neonatal period, the CHD factor is probably more significant than the comorbidities associated with prematurity, which will have more weight later in life, contributing to the increase in long-term morbidity and mortality. Nevertheless, prematurity impacted the treatment strategy chosen for low birth weight large VSD and CAVSD, where they underwent a staged correction, but the follow-up was uneventful.
As this is a retrospective study, there are limitations, some related to incomplete medical records. For instance, we adjusted our estimates for several known risks of CHD, however, maternal drug intake could not be completely clarified, as well as other risk factors, such as maternal illnesses and obesity, which can also increase the risk of CHD.15–19
Due to our sample size, we were unable to examine the risk of neonatal mortality for extremely (<28 weeks) preterm infants. In addition, we were unable to assess in detail the extent to which differences in neonatal mortality between the two groups were due to the CHD spectrum.
The data could not be truly representative of the CHD profile in our community, as it was collected in a single tertiary Center. Second, only 40–50% of CHD are diagnosed in the first week and 50–60% in the first month of life.4 As our study included only cases diagnosed during the prenatal and neonatal periods, a significant number of CHD cases could have been lost, especially in term neonates who usually have a shorter hospital stay. In addition, to minimize the ascertainment bias, we excluded mild cardiac defects, however, it may also have been a source of selection bias. Despite the possibility of selection bias, the prevalence of live birth CHD in our study was higher than the average prevalence of live births CHD in Europe.2,3
The excess of CHD among preterm is intriguing, but unclear. One possible explanation is that CHD and preterm births share risk factors or a common cause.5 CHDs have a multifactorial etiology, involving a wide range of genetic and environmental risk factors.15,18 Thus, prematurity may be causally related to the same factor(s) that precipitated CHD.4 Las et al. conjectured that a deterioration in the genetic program of fetal development might cause both CHD and preterm births.
Alternatively, preterm birth in neonates with CHD can be caused by abnormal fetal hemodynamics.13 However, most cardiovascular malformations have little effect on the developing fetus and become hemodynamically significant only after birth. There is also no evidence that CHD is associated with any impairment of placental function that may precipitate premature birth or cause stillbirth.11,21
Max Godfrey et al. speculated that minor septal defects spontaneously close in utero, so fewer defects might be apparent in mature neonates.22 This theory is consistent with our results since abnormalities of the atria/ventricles and the atrial/ventricular septum were the only CHD significantly higher in preterm compared to term neonates. Furthermore, as we demonstrate in Figure 1, septal defects are more prevalent as the prematurity is more marked. Also, as usually seen in term neonates, muscular VSD was the most frequent type, probably because defects in the muscular septum are supposed to close as a result of growth and hypertrophy of the surrounding muscular septum over time, and prematurity does not allow for completion of the embryological process of septal development.26,27 It is also possible that CHD may be independently associated with preterm birth or low birth weight.
Ultimately, the relationship between CHD and preterm neonates can have significant implications on prenatal care, prevention and risk stratification, and also on postnatal care. Following prenatal diagnosis of CHD, women can benefit from more frequent and detailed ultrasounds to follow-up fetal growth and other manifestations conducive to premature labor.13
ConclusionWe found an increased risk of CHD, mainly septal defects, in preterm neonates, although the etiology of prematurity between neonates with CHD is still uncertain. Further research on the association between CHD and preterm birth is needed to identify common pathways and shared risk factors to help prevent these two major contributors to infant morbidity and mortality.
FundingThis research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interestNone declared.