Echocardiography (echo) is the primary non-invasive imaging modality for the assessment of congenital heart disease (CHD). Computed tomography angiography (CTA) also has potential to examine the anatomy of complex heart anomalies as well as extracardiac involvement.
ObjectivesThe aim of this study is to determine the impact of new CTA technology in the diagnosis of CHD and to compare echo and CTA in terms of diagnostic accuracy.
MethodsForty-five patients who underwent preoperative echo and CTA assessment in the intensive care unit were included in this study. The results were assessed for three main types of CHD (cardiac malformations, cardiac-major vessel connections and major vessels). The main groups were also divided into subgroups according to surgical features in order to assess them more objectively. Imaging methods were compared for diagnostic accuracy, sensitivity and specificity, while surgical findings were accepted as the gold standard.
ResultsPatients’ median age and weight were two months (three days-eight years) and 12 kg (2.5-60 kg), respectively. In 45 operated cases, 205 subgroup malformations were assessed. Diagnostic accuracy was significantly greater in echo (echo vs. CTA: 98.4% and 96.2% [chi-square=6.4, p=0.011]). During surgery, 84 cardiac malformations (echo vs. CTA: 97.4% and 95.1% [chi-square=4.9, p=0.03]), 47 cardiac-major vessel connections (echo vs. CTA: 98.3% and 95.4% [chi-square=7.5, p=0.03]), and 74 major vessel malformations (echo vs. CTA: 96% and 98% [chi-square=1.8, p=0.48]) were confirmed.
ConclusionEchocardiography and CTA are imaging methods with high diagnostic accuracy in children with CHD. The use of echocardiography together with CTA, especially for the visualization of extracardiac anatomy, provides additional information for clinicians.
A ecocardiografia é a principal modalidade de imagem não invasiva na avaliação das cardiopatias congénitas (CC). A angiotomografia computorizada (ATC) tem também potencial para examinar as anomalias morfológicas cardíacas complexas, assim como do envolvimento extracardíaco.
ObjetivosDeterminar o impacto de avanços tecnológicos da ATC no diagnóstico de CC e comparar a ecocardiografia e a ATC em termos de precisão diagnóstica.
MétodosForam incluídos neste estudo 45 doentes submetidos a avaliação por ECO e por ATC pré operatória (n=45) em unidade de cuidados intensivos. Os resultados foram avaliados nos três principais tipos de grupos de CC (malformações cardíacas, conexões vasculares anómalas em nível cardíaco e malformações dos grandes vasos). Estes grupos foram também divididos em subgrupos de acordo com as características das cirurgias, de modo a serem avaliados mais objetivamente. Os métodos de imagem foram comparados para precisão diagnóstica, sensibilidade e especificidade, enquanto os resultados cirúrgicos foram aceites como gold standard.
ResultadosA média da idade e do peso dos doentes foi de dois meses (3 dias-8 anos) e 12 kgt (2,5-60 kg), respetivamente. Em 45 casos operados, foram avaliadas 205 malformações de subgrupos. A precisão diagnóstica foi significativamente superior na ECO (ECO versus ATC; 98,4% & 96,2%, (X2=6,4, p=0,011). Durante as 84 intervenções, foram confirmadas 84 malformações cardíacas (ECO versus ATC; 97,4% & 95,1% (X2=4,9, p=0,03), 47 conexões vasculares anómalas em nível cardíaco (ECO versus ATC; 98,3 & 95,4% (X2=7,5, p=0,03), 74 malformações dos grandes vasos (ECHO versus ATC; 96% & 98% (X2=1,8, p=0,48).
ConclusãoA ecocardiografia e a ATC são métodos de imagem com elevado rigor diagnóstico em crianças com CC. A utilização da ecocardiografia juntamente com a ATC, especialmente na avaliação da anatomia extracardíaca, fornece informação adicional para os clínicos.
Congenital heart disease (CHD) is a relatively common condition with an incidence of 4-10 per 1000 live births. CHDs are a heterogeneous group of diseases that include a wide spectrum of conditions and sub-conditions, for which the treatment approach varies widely. Timely and accurate diagnosis and treatment are important in order to increase patients’ chances of survival.1,2
Echocardiography (echo) is the main imaging method used in the diagnosis of CHD. Echo is a powerful technological tool that enables direct and detailed visualization of cardiac structures and also reveals hemodynamic status. It is widely used due to the rapidity of the procedure, applicability at the bedside, targeted use (heart and large vessels), absence of radiation exposure, and early recognition of findings. However, operator dependence and inability to show non-cardiac vascular tissues are the most important disadvantages of this modality.1,2
Multidetector-row computed tomography angiography (CTA) allows noninvasive imaging of the heart and coronary arteries. In recent years, there have been significant advances in computed tomography (CT) hardware, software, and machine learning, which have expanded its clinical utility for cardiovascular imaging in patients with complex CHD. This modality is noninvasive, with high spatial resolution and powerful three-dimensional (3D) post-processing image reconstruction. It thus provides excellent anatomic information that can replace echocardiography and cardiac catheterization, particularly in the assessment of extracardiac vessels and coronary arteries.3–5 Older CT scanners, using a retrospective electrocardiogram (ECG)-gated scan mode, have been reported to expose CHD patients to effective radiation doses of up to 28 mSv per cardiac scan.6 More recently, innovations such as prospective ECG gating, ECG-controlled tube current modulation, high-pitch helical scanning, lower tube potentials, wider detector coverage, and iterative reconstruction techniques have dramatically lowered radiation exposure. Newer CT scanner platforms also allow rapid image acquisition that decreases the need for sedation or anesthesia. With dual-source scan technology and wide detector coverage, studies can be obtained in a single heartbeat without requiring a breathhold for most indications.6,7
There are studies in the literature comparing echo and old-generation 64-slice CTA for assessing surgical anatomy.8 However, there have been a limited number of studies comparing echo with new generation CTA.9,10 In the present study, our aim was to determine the diagnostic accuracy of echo and 320-row multidetector CTA in cases of CHD and to assess the efficacy of these two methods for detecting morphological findings during surgery.
MethodsThis study was conducted on patients under eight years of age admitted to the pediatric intensive care unit and undergoing echocardiography and 320-row multidetector CTA procedures who subsequently underwent cardiac surgery for CHD between August 1, 2019 and December 31, 2019.
The study was designed in accordance with the Helsinki Declaration after obtaining permission from the hospital's institutional review board (no. 2020-46). A study form was created for each case including age, gender, weight, transthoracic echo and 320-row multidetector CTA results, and cardiac anatomy data from surgery.
Echocardiographic assessments were performed using a GE Vivid S5 cardiac ultrasound system (General Electric Vingmed, Horten, Norway) with a 3-MHz or 6-MHz probe. Standard pediatric echo views were recorded including parasternal (long- and short-axis), apical (4- and 5-chamber), subcostal and suprasternal views. Cardiac morphology was assessed in the direction of blood flow within the framework of the segmental approach. Atrial situs, venoatrial connections (systemic and pulmonary venous return), atrioventricular (AV) connections, ventricles, ventricular-great artery connections, spatial position of the great arteries, intracardiac defects and extracardiac vascular anomalies were reviewed as the main components of this approach. The echo images were analyzed by two experienced cardiologists (EO, ICT) who had at least 10 years of clinical practice.
Multidetector CTA examinations were performed with a 320-row multidetector scanner (Aquilion ONE, Toshiba Medical Systems, Otawara, Japan) with a gantry rotation time of 350 ms and temporal resolution of 175 ms. A non-ECG gated protocol with a pitch factor of 3 was used, and every scan in every patient was obtained using the z-axis CARE dose modulation technique. The voltage and tube current were adjusted to the patient's weight (80 kV dosage was used for patients weighing <20 kg, and 100 kV for those weighing 20-80 kg; tube current was 10 mA/kg for patients weighing <9 kg, and 5 mA for each additional kg).10 The imaging data were obtained during an intravenous injection of 1-1.5 ml/kg of the contrast agent iohexol at a rate of 1-3 ml/s for children and manual intravenous injection of the drug in newborns or those under one year old (Omnipaque 300 mgI/ml; GE Healthcare, Milwaukee, WI). Contrast agent was removed with 4-15 ml saline according to the patient's weight. The scanning delay was determined using a bolus-tracking technique. All the patients were in sinus rhythm, and none needed beta-blockers despite heart rates of more than 80 beats/min. Uncooperative children and newborns were sedated with ketamine and/or midazolam. A central venous line (n=30) or peripheral line (n=15) was used. All CTA acquisitions were obtained with patients breathing freely.
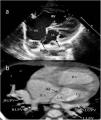
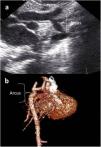
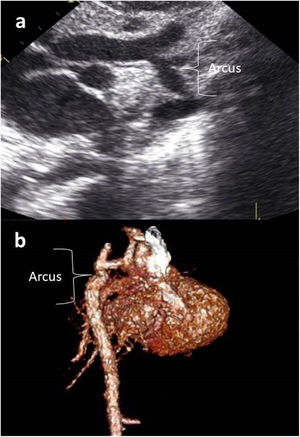
Images were reconstructed to 0.6 mm in thickness and to a reconstruction interval with a 25f kernel filter; they were processed on a separate workstation (Vitrea, Vital Images Inc., Minnetonka, MN) with multiplanar reformatting, maximum-intensity projection, and volume rendering (Figures 1 and 2). Multidetector CT images were prospectively reviewed by two radiologists with 15 (AT) and five (CT) years experience in cardiovascular imaging, and decisions were made by consensus.
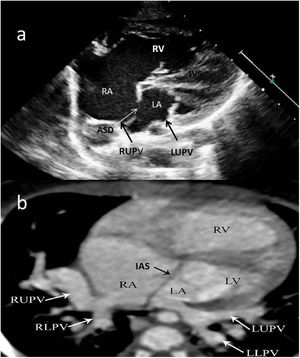
Two-year-old female patient with septum primum malposition and partial anomalous pulmonary venous return. (a) Modified 4-chamber view on echocardiographic examination; (b) computed tomography images in axial view. ASD: atrial septal defect; IAS; interatrial septum; IVS: interventricular septum; LA; left atrium; LLPV: left lower pulmonary vein; LUPV; left upper pulmonary vein; LV: left ventricle; RA; right atrium; RLPV: right lower pulmonary vein; RUPV: right upper pulmonary vein; RV: right ventricle.
Patients were divided into three main CHD groups, as follows:
Intracardiac malformationsAtrial septal defect (ASD), ventricular septal defect (VSD), atria, ventricles, right ventricular outflow tract (RVOT), tricuspid valve and mitral valve.
Ventriculoarterial connectionsAortic root and coronary artery, pulmonary valve, aortic valve, double outlet ventricle, spatial position of the great arteries.
Great vesselsAortic root dilatation, coarctation of the aorta, interrupted aortic arch, pulmonary artery dilatation, pulmonary stenosis or atresia, major aortopulmonary collateral artery (MAPCA), pulmonary vein anomaly, patent ductus arteriosus (PDA), persistent left superior vena cava, inferior vena cava (IVC) anomalies, double aortic arch.
These groups were divided into subgroups according to the features of the surgical operation and the cardiac and non-cardiac regions assessed by the surgeon. All echo and CTA findings were compared with the morphological anatomy determined during the operation.
Information on radiation doses was obtained from the CTA system. For examination purposes, the estimated effective radiation doses (in mSv) were calculated by multiplying the dose-length product (mGy·cm) by a conversion coefficient (mSV·mGy 1·cm-1) corrected for the patient's age. This was obtained from previously published literature (0.039 for <4 months of age, 0.026 for 4-12 months, and 0.018 for 1-7 years).10,11
Statistical analysisThe distribution of study variables was classified and descriptive results were obtained using SPSS for Windows version 15. Descriptive scores were expressed as median and percentile. Echo and CTA results were compared in terms of diagnostic sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) by accepting surgical findings as the gold standard. The accuracy of the two methods was assessed using McNemar's test, the chi-square test and the kappa statistic. A p-value <0.05 was considered statistically significant.
ResultsForty-five patients (30 boys and 15 girls) were included in the study. The patients’ median age was two months (range three days-eight years) and median weight was 12 kg (range 2.5-60 kg). The results were assessed in terms of 205 malformations and 1045 surgical fields.
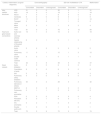
Detailed results of echo and CTA (intracardiac structures, relationships between heart and great vessels, great vessels) according to the main headings of concordant diagnosis, discordant diagnosis and unrecognized diagnosis are shown in Table 1. On echo, the diagnosis was unrecognized in patients with mitral valve pathology (n=1), aortic root and coronary artery abnormality (n=3), atrium (n=1), RVOT (n=1), spatial relationship of the great arteries (n=1), aortic valve (n=2), pulmonary valve (n=1), double ventricle (n=1), pulmonary stenosis or pulmonary atresia (n=2), MAPCA (n=2), pulmonary vein anomaly (n=1), PDA (n=2), persistent left superior vena cava (SVC) (n=1), and IVC (n=1). On CTA, the diagnosis was unrecognized in patients with VSD (n=1), ASD (n=1), ventricle (n=1), RVOT (n=4), tricuspid valve pathology (n=2), mitral valve pathology (n=3), aortic root and coronary arteries (n=4), spatial relationship of the great arteries (n=3), aortic valve (n=2), pulmonary valve (n=3), double ventricular structure (n=1), pulmonary stenosis or pulmonary atresia (n=3) and persistent left SVC (n=1).
Correlation of surgical diagnosis with preoperative echocardiography and computed tomography angiography (surgery considered as gold standard).
| Cardiac malformation (surgical diagnosis) | Echocardiography | 320-row multidetector CTA | Malformation | |||||
|---|---|---|---|---|---|---|---|---|
| Concordant | Discordant | Unrecognized | Concordant | Discordant | Unrecognized | |||
| Intra- cardiac structures | VSD | 31 | 0 | 0 | 30 | 0 | 1 | 31 |
| ASD | 18 | 0 | 0 | 19 | 2 | 1 | 18 | |
| Atrium | 7 | 0 | 1 | 8 | 0 | 0 | 8 | |
| Ventricles | 9 | 0 | 0 | 8 | 0 | 1 | 9 | |
| RVOT | 11 | 0 | 1 | 9 | 1 | 4 | 12 | |
| Tricuspid valve | 2 | 0 | 0 | 1 | 1 | 2 | 2 | |
| Mitral valve | 3 | 0 | 1 | 1 | 0 | 3 | 4 | |
| Total | 81 | 0 | 3 | 76 | 4 | 12 | 84 | |
| Heart and great vessel connections | Aortic root and coronary arteries | 16 | 1 | 3 | 16 | 2 | 4 | 18 |
| Spatial relationship of the great arteries | 6 | 0 | 1 | 5 | 1 | 3 | 7 | |
| Aortic valve | 5 | 2 | 2 | 3 | 0 | 2 | 5 | |
| Pulmonary valve | 11 | 0 | 1 | 10 | 1 | 3 | 12 | |
| Double outlet ventricle | 4 | 0 | 1 | 5 | 1 | 1 | 5 | |
| Total | 42 | 3 | 8 | 39 | 5 | 13 | 47 | |
| Great vessels | Aortic dilatation | 6 | 0 | 0 | 6 | 0 | 0 | 6 |
| Coarctation of aorta | 8 | 0 | 0 | 8 | 0 | 0 | 8 | |
| IAA | 5 | 2 | 0 | 3 | 0 | 0 | 3 | |
| Pulmonary artery dilatation | 4 | 0 | 0 | 4 | 0 | 0 | 4 | |
| Pulmonary stenosis or atresia | 30 | 0 | 2 | 29 | 0 | 3 | 32 | |
| MAPCA | 2 | 0 | 2 | 4 | 0 | 0 | 4 | |
| Pulmonary vein anomalies | 3 | 1 | 1 | 3 | 0 | 0 | 3 | |
| PDA | 2 | 0 | 2 | 4 | 0 | 0 | 4 | |
| Persistent left SVC | 6 | 0 | 1 | 6 | 0 | 1 | 7 | |
| IVC anomalies | 3 | 2 | 1 | 2 | 0 | 0 | 2 | |
| Double aortic arch | 1 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Total | 70 | 5 | 9 | 70 | 0 | 4 | 74 | |
ASD: atrial septal defect; CTA: computed tomography angiography; Echo: echocardiography; IAA: interrupted aortic arch; IVC: inferior vena cava; MAPCA: major aortopulmonary collateral artery; PDA: patent ductus arteriosus; RVOT: right ventricular outflow tract; SVC: superior vena cava; VSD: ventricular septal defect.
The results of the assessment revealed 200 true positive, 828 true negative, five false positive and 12 false negative results on transthoracic echo (Table 2). Sensitivity, specificity, PPV, NPV and diagnostic accuracy were 94.3%, 99.4%, 97.5%, 98.5% and 98.4%, respectively (Table 3). In the same surgical areas, 320-row multidetector CTA showed 196 true positive, 814 true negative, 12 false positive and 23 false negative results (Table 2). Sensitivity, specificity, PPV, NPV and diagnostic accuracy were 89.4%, 98.5%, 94.2%, 97.2% and 96.2%, respectively (Table 3).
Sensitivity and specificity of echocardiography and computed tomography angiography.
| Method | Sensitivity | Specificity | PPV | NPV | AUC | Accuracy | Uniformity |
|---|---|---|---|---|---|---|---|
| Echo | 94.3% | 99.4% | 97.50% | 98.5% | 0.983 | 98.40%a | 0.950b |
| CTA | 89.4% | 98.50% | 94.2% | 97.2v | 0.966 | 96.20%a |
AUC: area under receiver operating characteristic curve; CTA: computed tomography angiography; Echo: echocardiography; PPV: positive predicative value, NPV: negative predicative values.
When the sensitivity of echo and CTA is compared in terms of diagnostic accuracy, although significantly higher in echo, both methods effectively and reliably detected cardiac defects identified during surgery (echo vs. CTA: 98.4% and 96.2% [chi-square=6.4, p=0.011]). The predictive value and accuracy of echo and CTA are shown in Table 4. During surgery, 84 cardiac malformations were detected, and both imaging methods predicted the results with statistical significance. However, intracardiac malformations were more accurately identified by echo than by CTA (echo vs. CTA: 97.4% and 95.1% [chi-square=4.9, p=0.034]).
Predictive value and accuracy of echocardiography and computed tomography angiography.
| Predictive value and accuracy for intracardiac malformations with echo and CTA | |||||
|---|---|---|---|---|---|
| Diagnosis | Surgical diagnosis | Chi-square | p | ||
| + | - | ||||
| Echo | + | 80 | 3 | 4.978 | 0.034 |
| - | 5 | 228 | |||
| CTA | + | 80 | 6 | ||
| - | 10 | 236 | |||
| Echo vs. CTA: accuracy (97.4% and 95.1%) | |||||
| Predictive value and accuracy for heart and great vessel connections with echo and CTA | |||||
|---|---|---|---|---|---|
| Diagnosis | Surgical diagnosis | Chi-square | p | ||
| + | - | ||||
| Echo | + | 29 | 1 | 7.580 | 0.028 |
| - | 4 | 266 | |||
| CTA | + | 26 | 3 | ||
| - | 8 | 203 | |||
| Echo vs. CTA: accuracy (98.3% and 95.4%) | |||||
| Predictive value and accuracy for great vessels with echo and CTA | |||||
|---|---|---|---|---|---|
| Diagnosis | Surgical diagnosis | Chi-square | p | ||
| + | - | ||||
| Echo | + | 72 | 3 | 1.800 | 0.480 |
| - | 8 | 198 | |||
| CTA | + | 74 | 2 | ||
| - | 5 | 280 | |||
| Echo vs. CTA accuracy: 96% and 98.0% | |||||
CTA: computed tomography angiography; echo: echocardiography.
The predictive value and accuracy of echo and CTA for identifying cardiovascular connections are shown in Table 4. During surgery, cardiovascular connection anomalies in the great arteries were detected in 47 patients, and both imaging methods predicted the results with statistical significance. However, echo predicted cardiovascular connection anomalies more accurately than CTA (echo vs. CTA: 98.3% and 95.4% (chi-square=7.5, p=0.03).
Table 4 also shows the predictive value and accuracy of echo and CTA for identifying malformations of the great vessels. During surgery, 74 major great vessel malformations were detected. There was no significant difference in predicting great vessel malformations between the two methods (echo vs. CTA: 96% and 98% (chi-square=1.8, p=0.48).
The overall mean effective radiation dose for 45 patients was 0.74 mSv (range 0.15-1.38 mSv) and 0.58 mSv (range 0.12-0.80) in the 32 patients aged under one year.
DiscussionIn this study, the effectiveness of transthoracic echo and 320-row multidetector CTA in predicting surgical findings in patients with CHD was compared. Both methods were highly sensitive and specific in identifying anatomy confirmed during surgery, and additionally CTA was useful as a complementary imaging method along with echo in the assessment of extracardiac vascular structures.
CHD is common in all age groups and roughly half of cases consist of complex CHD. Imaging techniques such as transthoracic echo, CTA, magnetic resonance angiography, heart catheterization and angiography are used in the diagnosis and management of subgroups of those diseases, some of which may be quite complex.2,3
Traditionally, echo is the first-line imaging method used to assess cardiac function, anatomical diagnosis and hemodynamic status in pediatric cases. This is due to a variety of factors such as low cost, portability, widespread availability and low radiation exposure. In patients with CHD in particular, echo plays a key role in assessing morphology before, during and after surgery.2,12–14
In the literature, various studies state that transthoracic echo shows high sensitivity and specificity in determining surgical findings. Bu et al.15 reported that the sensitivity of transthoracic echo was 90.6% and its specificity was 99.8% in their study of 35 CHD patients with ages ranging between three days and 74 months. Mei et al. reported a diagnostic accuracy of 88.1% in their study assessing 99 malformations in 39 cases with echo, which was confirmed by surgical findings.16 Alghamdi et al. found an insignificant difference between echo and surgical findings in only seven (1.8%) among 392 cases, which led to minor changes in the surgical strategy.13 In a study by Marek et al. involving 2788 cases, the diagnostic accuracy was 96% for transthoracic echo.17 In our study, sensitivity was 94.3% and specificity was 99.4%, in accordance with the literature. The diagnostic accuracy of echo was 98.4%.
New-generation multidetector CTA is increasingly used in the assessment of children with complex CHD. The accurate identification of anatomic details, possibility of 3D assessment, need for small volumes of contrast material to show all vascular structures, better visualization of the coronary arteries and their branches, assessment of non-cardiac structures, very short shooting time (a few seconds) and relatively low radiation exposure, are the chief advantages of the high spatial resolution of CTA. In addition, it has been suggested in several studies that CTA dramatically reduces the need for diagnostic heart catheterization and angiography, which was considered a gold standard in the diagnosis of CHD.18,19 The main disadvantages of the technique are lack of hemodynamic information, exposure to ionizing radiation, and problems associated with application of contrast materials. Nevertheless, technological advances have led to easy application and increased diagnostic accuracy in children with CHD.10,18–21
In a study using 64-slice spiral CTA, the scan was found to be 91% accurate.6 In another study with similar diagnoses and age distribution, for which a 128-slice spiral CTA technique was used, the accuracy was reported as 95%.22 An accuracy of 97.3% was reported in another study using 256-slice spiral CTA.23 In our study, which used 320-row multidetector CTA, the accuracy was 96.2% for the entire group. Assessment of different anatomical regions and the lack of ECG gating may have led to the lower accuracy in our study in comparison to others.
An important finding of the present study was that despite its high diagnostic accuracy, sensitivity and specificity, the predictive power of 320-row multidetector CTA for detecting anomalies of intracardiac structures and heart-great vessel connections was less than that of transthoracic echo. Nevertheless, 320-row multidetector CTA appeared to be as effective as transthoracic echo in the diagnosis of extracardiac vascular malformations (accuracy for CTA 98.0%, 96% for transthoracic echo). The latter finding was also consistent with previous studies. For instance, Li and al. reported the diagnostic accuracy for extracardiac vascular anomalies as 98.6% for CTA and 96% for transthoracic echo.24 In another study, Bu et al. reported sensitivity of 92% and 68%, respectively15 On the other hand, in their study comparing CTA and echo, Cheng and al. demonstrated similar sensitivity and specificity.23
In our study, some cases were misdiagnosed or undiagnosed by both imaging methods. This issue has also been highlighted in other studies in the literature. Koplay et al. stated that they encountered two false positive and one false negative diagnoses among 105 cases.25 In their study involving 30 cases with assessment of 205 surgical areas, Li et al. concluded that with CTA, 2.4% of the cases were misdiagnosed and 6.8% were undiagnosed, while these figures were 1.4% and 3.9%, respectively, for echo.24
Certain aspects of CTA may explain this difference, such as that it can show only static images, provides insufficient data on valvular structures, and cannot detect hemodynamic changes, in contrast to the ability of echo to dynamically detect shunts, regurgitation and intracardiac structures.
The main limitation of this analysis was that the study was carried out in a single center with a small number of patients. With more cases available, more precise results could be obtained concerning sensitivity and specificity. In addition, the difficulty in classification might have affected the results due to the complex and heterogeneous properties of patients with CHD. Examinations were assessed thoroughly under the guidance of experienced pediatric cardiologists and radiologists in order to prevent errors and misdiagnosis as much as possible.
In conclusion, echo and CTA are imaging methods with high levels of diagnostic accuracy in patients with CHD. Echo is the preferred method for diagnosing a wide range of conditions, especially intracardiac anomalies. The use of CTA, on the other hand, provides additional benefit for demonstration of extracardiac anatomy.
Financial supportNone.
Conflicts of interestThe authors have no conflicts of interest to declare.