Coronary chronic total occlusions (CTOs) are relatively common findings in patients with type 2 diabetes mellitus (T2DM). However, the indication for percutaneous coronary intervention (PCI) and its clinical benefit in these patients remain controversial.
MethodsA single-center retrospective cohort study with prospectively collected outcomes was carried out with CTO patients undergoing PCI in 2019 and 2020. Patients were divided into two groups according to previous T2DM diagnosis (T2DM and non-T2DM). The primary outcome was recurrence of angina and/or heart failure symptoms and secondary outcomes were myocardial infarction and all-cause mortality.
ResultsA total of 177 patients (82.5% male) were included in the analysis, with a mean age of 65±11 years. The primary outcome (total symptom recurrence) occurred in 16.6% of the sample, with no difference between groups (non-T2DM 13.6% vs. T2DM 21.2%, p=0.194) in a two-year follow-up. Angina recurrence was significantly more frequent in T2DM patients (15.2%, p=0.043). The presence of T2DM was not an independent predictor of symptom recurrence (p=0.429, HR 1.37, 95% CI 0.62–2.98). Myocardial infarction and all-cause mortality were also not different between groups (T2DM 1.5%, p=0.786 and 4.5%, p=0.352, respectively, on survival analysis). Independent predictors of all-cause mortality were left ventricular function and creatine clearance (p=0.039, HR 0.92, 95% CI 0.85–0.99 and p=0.013, HR 0.96, 95% CI 0.93–0.99, respectively).
ConclusionsT2DM did not influence outcomes in CTO patients undergoing PCI, and its presence should not be a limiting factor in deciding on CTO revascularization.
As oclusões totais crónicas (CTO) são achados comuns em doentes com diabetes tipo 2 (DM2). A indicação para revascularização percutânea (PCI) e o seu benefício clínico permanecem controversos. Este estudo foi desenhado para tentar responder a esta questão.
MétodosEstudo de coorte retrospetivo com colheita de dados prospetiva, realizada em doentes com CTO submetidos a PCI entre 2019-2020. Formados dois grupos (DM2 e não-DM2). O outcome primário foi definido como a recorrência de sintomas de angor e/ou insuficiência cardíaca e os outcomes secundários a ocorrência de enfarte do miocárdio e mortalidade por todas as causas.
ResultadosAnálise com 177 doentes, idade média de 65±11 anos e 82,5% do sexo masculino. O outcome primário ocorreu em 16,6% dos doentes, sem diferença entre os grupos (não-DM2 13,6% versus DM2 21,2%, p=0,194) em dois anos. A recorrência de angor foi significativamente maior nos doentes com DM2 (15,2%, p=0,043). A DM2 não foi um preditor independente do outcome primário (p=0,429, HR 1,37, 95% CI 0,62 a 2,98). A ocorrência de enfarte e mortalidade não mostrou diferenças entre os grupos (DM2 1,5%, p=0,786 e 4,5%, p=0,352, respetivamente – análise de sobrevivência). A função ventricular esquerda e a clearance de creatinina foram preditores independentes de mortalidade (p=0,039, HR 0,92, 95% CI 0,85 a 0,99 e p=0,013, HR 0,96, 95% CI 0,93 a 0,99, respetivamente).
ConclusõesA DM2 não influenciou os outcomes nos doentes com CTO submetidos a PCI, sendo que a sua presença não deverá ser um fator limitativo na decisão de revascularização.
Coronary chronic total occlusions (CTOs) are found in 15–25% of patients with symptoms of angina or with coronary artery disease (CAD) undergoing a coronary angiogram (CA).1,2
Data from retrospective studies and evidence from small randomized controlled trials (RCTs) show that successful recanalization of CTOs by percutaneous coronary intervention (PCI) is associated with improvements not only in quality of life, but also in angina, heart failure (HF) symptoms and left ventricular ejection fraction (LVEF), although with no statistically significant impact on survival.2–5
Nevertheless, CTO recanalization constitutes less than 5% of PCI procedures in contemporary practice, except in tertiary centers; this may be due to its greater procedural complexity, the risk of complications, and lower success rates in centers with non-specialized teams.2,6,7
Patients with diabetes have more extensive and complex CAD compared with those without, including higher rates of multivessel disease and CTO vessels, with previous studies showing an incidence of 30–40% of CTO lesions in this population.4,6 Although common, CTO PCI is performed less frequently in diabetic patients.8
Patients with diabetes and incomplete revascularization have an increased long-term risk of cardiovascular events, and CTO lesions have been shown to be a strong independent predictor of incomplete revascularization in patients undergoing PCI.7,9,10 Recent studies suggest similar technical success rates of CTO PCI in diabetic and non-diabetic patients.8,11
Research has also shown the benefits of CTO PCI in diabetic patients, mainly in symptom relief and improved quality of life, but conflicting results and a lack of well-designed RCTs contribute to some controversy involving the treatment of CTO lesions in these groups.2,4,5,8,12,14
ObjectivesThis study aimed to analyze the impact on clinical outcomes and symptom recurrence in type 2 diabetes mellitus (T2DM) patients undergoing CTO PCI. Additionally, we aimed to identify independent predictors of symptom recurrence and clinical outcomes in this group.
MethodsA single-center, retrospective cohort analysis with prospectively collected outcomes was carried out with CTO patients undergoing PCI in 2019 and 2020.
Recruitment and selectionEligibility criteria included age ≥18 years and a personal history of symptoms suggestive of ischemic heart disease in which a significant atherosclerotic vessel lesion was identified on CA and classified as a CTO (coronary lesion with thrombolysis in myocardial infarction [TIMI] anterograde flow score of 0 with chronic characteristics thought to have been present for at least three months). Patients who only underwent a diagnostic study, even if they had a CTO vessel, or had an unsuccessful procedure were excluded from the study. A previously published hybrid algorithm for CTO PCI was used for revascularization, and patients were divided into two groups according to their diabetes status (T2DM and non-T2DM).15,16 T2DM was presumed in patients with a known previous diagnosis of T2DM or with a recorded inpatient random glycated hemoglobin ≥6.5%. LVEF was estimated by the modified Simpson's biplane method. Obesity was defined as a body mass index ≥30 kg/m2, and chronic renal failure as creatinine clearance of <60 ml/m2 (Cockcroft–Gault formula). Symptoms were assessed through medical records; no scales or questionnaires were applied and no patients were contacted by telephone. The use of at least one antianginal drug, an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor-neprilysin inhibitor, 100 mg aspirin (or anticoagulation in atrial fibrillation), dual antiplatelet therapy or dual antithrombotic therapy for at least six months, and a lipid-lowering drug (statin and/or ezetimibe) were defined as optimal medical therapy (OMT). Periprocedural myocardial infarction (MI) was assessed and defined as type 4a in accordance with the fourth universal definition of MI.17 Atherosclerotic disease progression was defined as progression of atherosclerosis in the index vessel and/or in non-CTO vessels. Baseline patient demographic data, cardiovascular risk factors, and clinical, laboratory, echocardiographic and angiographic data were also recorded.
The study abides by the ethical requirements stated in the 1975 Helsinki Declaration and received ethical approval by the local hospital ethics committee.
OutcomesThe study's primary outcome was recurrence of angina and/or HF symptoms (total symptom recurrence) in a two-year follow-up. HF symptoms were defined as dyspnea and/or fatigue associated with an underlying heart condition. Secondary outcomes were defined as MI and all-cause mortality.
Statistical analysisCategorical variables were presented as frequencies and percentages, and continuous variables as means and standard deviation, or medians and interquartile range for variables with skewed distribution or a significant Shapiro–Wilk test. Comparisons between groups were performed using the chi-square test, Student's t test or Mann–Whitney test, as appropriate. Multivariate analysis was performed using logistic and Cox regression to identify predictors associated with the outcomes. A p-value <0.05 was taken to indicate statistical significance. The statistical analysis was performed using IBM SPSS Statistics, version 21.0 (IBM SPSS, Chicago, IL, USA).
ResultsBaseline patient demographics and medical historyA total of 191 patients were recruited, with 14 being excluded (12 had an unsuccessful procedure and two only underwent CA). All available characteristics for the final sample of 177 patients are summarized in Table 1. The groups were composed of 70 (39.5%) patients with T2DM and 107 (60.5%) without T2DM. The overall sample had a mean age of 64.5±11.4 years and 82.5% were male. Medical history included hypertension in 74.6% of patients, dyslipidemia in 72.9%, obesity in 18.2% and HF in 15.3%. T2DM patients were older, with a mean age of 67.9±10.1 (p=0.010), and more likely to have chronic renal failure (14.3%, p=0.011). Most patients were on OMT (83.1%, p=0.382) and antianginal drugs (98.3%, p=0.824), with no difference regarding diabetes status. Insulin replacement therapy was prescribed in 25 (35.7%) diabetic patients, and mean glycated hemoglobin was 8.2%.
Baseline characteristics of patients undergoing percutaneous coronary intervention for chronic total occlusions, compared by diabetes status.
| Non-T2DM(n=107, 60.5%) | T2DM(n=70, 39.5%) | Total(n=177) | p | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 93.0 (86.9) | 53.0 (55.7) | 146 (82.5) | |
| Female | 14.0 (13.1) | 17.0 (24.3) | 31.0 (17.5) | 0.055 |
| Age, years, mean±SD | 62.4±11.7 | 67.9±10.1 | 64.5±11.4 | 0.010 |
| Hypertension, n (%) | 77.0 (72.0) | 55.0 (78.6) | 132 (74.6) | 0.323 |
| Dyslipidemia, n (%) | 77.0 (72.0) | 52.0 (74.3) | 129 (72.9) | 0.734 |
| Smoking, n (%) | 23.0 (23.5) | 26.0 (32.9) | 49.0 (27.7) | 0.287 |
| Obesity, n (%) | 17.0 (16.0) | 15.0 (21.4) | 32.0 (18.2) | 0.364 |
| History of HF, n (%) | 12.0 (11.2) | 15.0 (21.4) | 27.0 (15.3) | 0.065 |
| Previous stroke, n (%) | 3.00 (2.80) | 5.00 (7.10) | 8.00 (4.50) | 0.174 |
| Atrial fibrillation, n (%) | 13.0 (12.1) | 9.00 (12.9) | 22.0 (12.4) | 0.889 |
| Chronic renal disease, n (%) | 4.0 (3.70) | 10.0 (14.3) | 14.0 (7.90) | 0.011 |
| Ischemic heart disease, n (%) | 60.0 (56.1) | 38.0 (52.3) | 98.0 (55.3) | 0.417 |
| Chronic lung disease, n (%) | 7.00 (6.50) | 3.00 (4.30) | 10.0 (5.60) | 0.525 |
| Clinical indication, n (%) | ||||
| ACS | 57.0 (53.3) | 35.0 (50.0) | 92.0 (52.0) | |
| Chronic coronary syndrome | 50.0 (46.7) | 35.0 (50.0) | 85.0 (48.0) | 0.670 |
| CCS score, n (%) | ||||
| I–II | 85.0 (61.4) | 48.0 (68.6) | 133 (75.1) | |
| III–IV | 22.0 (20.6) | 22.0 (31.4) | 44.0 (24.9) | 0.102 |
| OMT, n (%) | 91.0 (85.0) | 56.0 (80.0) | 147 (83.1) | 0.382 |
| Antianginal | 105 (98.1) | 69.0 (98.6) | 174 (98.3) | 0.824 |
| Aspirin or OAC | 107 (100) | 70.0 (100) | 177 (100) | – |
| DAPT-DAT 6 months | 105 (98.1) | 65.0 (92.9) | 170 (96.0) | 0.078 |
| Insulin replacement therapy, n (%) | – | 25.0 (35.7) | 25.0 (14.1) | – |
ACS: acute coronary syndrome; CCS: Canadian Cardiovascular Society; DAPT: dual antiplatelet therapy; DAT: dual antithrombotic therapy; HF: heart failure; OAC: oral anticoagulant therapy; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; SD: standard deviation; T2DM: type 2 diabetes mellitus.
The right coronary artery (RCA) was the most frequently occluded artery, and an anterograde approach was most frequently employed. Multivessel disease was present in 85.9% of patients. T2DM patients presented with lower creatinine clearance levels (69.3±27.9 ml/min, p=0.006), and contrast doses were therefore lower during PCI in these patients (225±84.8 ml, p=0.009). Ischemia or myocardial viability testing was performed in 43 (24.3%, p=0.718) patients, most frequently stress echocardiography (10.7%, p=0.212). Periprocedural MI occurred in 10 (5.6%, p=0.976) patients during CTO PCI. The rate of periprocedural complications was 5.1%, mainly vessel perforation (2.26%), but no differences were found between groups. Although LVEF was mildly reduced at baseline, ventricular function improved in both groups after intervention in a mean follow-up of 18 months (p<0.001) (Table 2).
Angiographic characteristics of chronic total occlusion patients undergoing percutaneous coronary intervention, compared by diabetes status.
| Non-T2DM(n=107, 60.5%) | T2DM(n=70, 39.5%) | Total(n=177) | P | |
|---|---|---|---|---|
| Contralateral access, n (%) | 48.0 (44.9) | 28.0 (40.0) | 76.0 (42.9) | 0.523 |
| CTO vessel | ||||
| LCA, n (%) | 60.0 (56.1) | 38.0 (54.3) | 98.0 (55.4) | |
| LAD, n (%) | 28.0 (26.2) | 18.0 (25.7) | 46.0 (26.0) | |
| LCx, n (%) | 32.0 (29.9) | 20.0 (28.6) | 52.0 (29.4) | |
| RCA, n (%) | 47.0 (43.9) | 32.0 (45.7) | 79.0 (44.6) | 0.815 |
| Multivessel disease, n (%) | 90 (84.1) | 62 (88.6) | 152 (85.9) | 0.405 |
| Approach, n (%) | ||||
| Antegrade | 93.0 (86.9) | 54.0 (91.4) | 157 (88.7) | |
| Retrograde | 14.0 (13.1) | 6.00 (8.60) | 20.0 (11.3) | 0.354 |
| J-CTO score, mean±SD | 0.74±0.71 | 0.81±0.74 | 0.79±0.73 | 0.533 |
| Radiation dose | ||||
| Air kerma, mGy, median (IQR) | 1840 (2030) | 1932 (1804) | 2043 (1784) | 0.309 |
| Kerma area product, Gycm2, median (IQR) | 120 (113) | 104 (124) | 120 (113) | 0.810 |
| Ischemia/viability test, n (%) | 27.0 (25.2) | 16.0 (22.9) | 43.0 (24.3) | 0.718 |
| Stress echocardiography | 14.0 (13.1) | 5.00 (7.10) | 19.0 (10.7) | 0.212 |
| SPECT-MPI | 3.00 (2.80) | 3.00 (4.30) | 6.00 (3.40) | 0.594 |
| Cardiac MRI | 10.0 (9.30) | 8.00 (11.4) | 18.0 (10.2) | 0.654 |
| LVEF at baseline, %, mean±SD | 47.1±10.3 | 47.2±10.7 | 47.1±10.5 | |
| LVEF after PCI, %, mean±SD | 50.6±9.53 | 52.2±9.84 | 51.2±9.73 | 0.361 |
| p<0.001 | p<0.001 | |||
| Creatinine clearance, ml/min, mean±SD | 80.6±24.5 | 69.3±27.9 | 77.1±26.6 | 0.006 |
| PCI time, min, mean±SD | 136±56.0 | 126±65.0 | 132±56.0 | 0.278 |
| Contrast volume, ml, mean±SD | 270±96.5 | 225±84.8 | 254±94.3 | 0.009 |
| Periprocedural complications, n (%) | ||||
| Total | 4.00 (3.70) | 5.00 (7.10) | 9.00 (5.10) | |
| Dissection | 2.00 (1.87) | 1.00 (1.43) | 3.00 (1.69) | |
| Perfuration | 2.00 (1.87) | 2.00 (2.85) | 4.00 (2.26) | |
| Pericardial effusion | 0.00 (0.00) | 1.00 (1.43) | 1.00 (0.56) | |
| Stroke/MI | 0.00 (0.00) | 1.00 (1.43) | 1.00 (0.56) | 0.313 |
| Periprocedural MI, n (%) | 6.00 (5.60) | 4.00 (5.70) | 10.0 (5.60) | 0.976 |
CTO: chronic total occlusion; IQR: interquartile range; J-CTO: Multicenter CTO Registry of Japan; LAD: left anterior descending artery; LCA: left coronary artery; LCx: left circumflex artery; LVEF: left ventricular ejection fraction; MI: myocardial infarction; MRI: magnetic resonance imaging; PCI: percutaneous coronary intervention; RCA: right coronary artery; SD: standard deviation; SPECT-MPI: single-photon emission computed tomography myocardial perfusion imaging; T2DM: type 2 diabetes mellitus.
The primary outcome (total symptom recurrence) occurred in 16.6% of patients, with no difference between groups (non-T2DM 13.6% vs. T2DM 21.2%, p=0.194) in two-year follow-up (mean follow-up of 18 months). Angina recurrence was significant higher in the T2DM group (15.2%, p=0.043) (Table 3).
Primary and secondary outcomes in two-year follow-up, compared by diabetes status.
| Non-T2DM(n=107, 60.5%) | T2DM(n=70, 39.5%) | Total(n=177) | p | |
|---|---|---|---|---|
| Primary outcome | ||||
| Total, n (%) | 14.0 (13.6) | 14.0 (21.2) | 28.0 (16.6) | 0.194 |
| Individual components | ||||
| Angina, n (%) | 6.00 (5.80) | 10.0 (15.2) | 16.0 (9.50) | 0.043 |
| HF symptoms, n (%) | 9.00 (8.70) | 7.00 (10.6) | 16.0 (9.50) | 0.686 |
| Secondary outcomes | ||||
| MI | 2.00 (1.90) | 1.00 (1.50) | 3.00 (1.80) | 0.838 |
| All-cause mortality | 3.00 (2.90) | 3.00 (4.50) | 6.00 (3.60) | 0.576 |
HF: heart failure; MI: myocardial infarction; T2DM: type 2 diabetes mellitus.
Of the total population, 11 (6.2%) had CTO restenosis and 22 (12.4%) had atherosclerotic disease progression, mainly in the diabetic population (p=0.030 and p<0.001, respectively). In patients with symptom recurrence (total or isolated angina), there was no difference in the restenosis rate according to diabetic status (p=0.190 and p=0.790, respectively). Nevertheless, atherosclerotic disease progression was more frequent in diabetic patients with total symptom or angina recurrence (p=0.008 and p=0.013, respectively) (Table 4).
Patients who underwent coronary angiography during follow-up, compared by diabetes status and symptom recurrence.
| Non-T2DM(n=107, 60.5%) | T2DM(n=70, 39.5%) | Total(n=177) | p | |
|---|---|---|---|---|
| CA, n (%) | 5.00 (4.70) | 24.0 (34.3) | 29.0 (16.4) | <0.001 |
| CTO restenosis, n (%) | 2.00 (1.10) | 9.00 (5.10) | 11.0 (6.20) | 0.030 |
| Disease progression, n (%) | 4.00 (3.73) | 18.0 (25.7) | 22.0 (12.4) | <0.001 |
| Total symptom recurrence, n (%) | (n=14) | (n=14) | (n=28) | |
| CTO restenosis, n (%) | 2.00 (14.2) | 5.00 (35.7) | 7.00 (25.0) | 0.190 |
| Disease progression, n (%) | 4.00 (28.6) | 11.0 (78.6) | 15.0 (53.6) | 0.008 |
| Angina recurrence | (n=6) | (n=10) | (n=16) | |
| CTO restenosis, n (%) | 2.00 (33.3) | 4.00 (40.0) | 6.00 (37.5) | 0.790 |
| Disease progression, n (%) | 3.00 (50.0) | 10.0 (100) | 13.0 (81.2) | 0.013 |
CA: coronary angiography; CTO: chronic total occlusion; T2DM: type 2 diabetes mellitus.
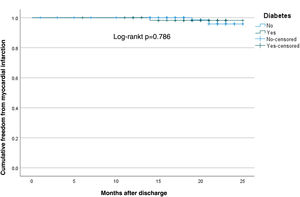
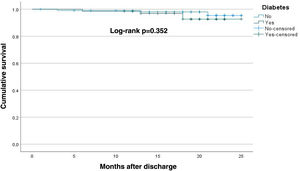
MI and all-cause mortality showed no differences between groups (T2DM 1.5%, p=0.786 and 4.5%, p=0.352, respectively, on survival analysis) (Figures 1 and 2).
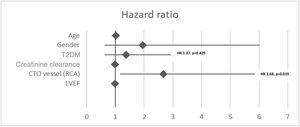
The presence of T2DM and LVEF was not independent predictors of the primary outcome (p=0.429, hazard ratio [HR] 1.37, 95% confidence interval [CI] 0.62–2.98 and p=0.737, HR 0.994, 95% CI 0.95–1.03, respectively). RCA as the CTO vessel was an independent predictor for total symptom recurrence after PCI (p=0.019, HR 2.68, 95% CI 1.17–6.14) (Figure 3).
Independent predictors of all-cause mortality were LVEF and creatine clearance (p=0.039, HR 0.92, 95% CI 0.85–0.99 and p=0.013, HR 0.96, 95% CI 0.93–0.99, respectively). The presence of T2DM was not an independent predictor of all-cause mortality (p=0.975, HR 1.03, 95% CI 0.16–6.34).
DiscussionThis study found that recanalization of CTO lesions by PCI and its benefit in terms of clinical outcomes and symptom recurrence were independent of T2DM status. However, recurrence of angina was more common in diabetics.
The sample included 40% of patients with T2DM, with a mean age of 65 years and mainly male, of whom almost three quarters had a medical history of hypertension and dyslipidemia, and a mean LVEF of 47% was observed. Sample characteristics were similar to those in other recent studies.4,8,12,13
Focusing on the procedure itself, Salisbury et al. reported that 60% of CTOs affected the RCA and an anterograde approach was adopted in 38–42% of cases. In the present study, the RCA was also the most commonly affected artery (45%), while an anterograde approach was employed more often than in Salisbury et al.’s study (89%). The rate of periprocedural complications was low, mainly vessel perforation (2%), and lower than described by Salisbury et al. (who reported perforation in 8–9% of cases).8,18
Symptom recurrence after a two-year follow-up was low (16.6%), with no difference found between groups. In addition, T2DM was not an independent predictor of symptom recurrence. Similarly, Salisbury et al. and Zhao et al. reported angina improvement of almost 20 points on the Seattle Angina Questionnaire angina frequency score and dyspnea relief during the first year after recanalization, independently of diabetes status.8,12 Notwithstanding, in the present analysis, recurrence of isolated angina was more frequent in T2DM patients (15%, p=0.043). This could be related to poorer disease control (mean glycated hemoglobin was 8.2%), which affects atherosclerotic disease progression. Even in patients with relatively controlled disease, there is evidence that progression of CAD is difficult to stop, with higher rates of angina recurrence after PCI. Although rates of stent restenosis and atherosclerotic disease progression were higher in the diabetic population, as expected, in patients with total symptom or isolated angina recurrence there was no significant difference in stent restenosis.19,20
Most patients were on OMT and taking antianginal drugs, regardless of diabetic status, which probably contributed to the low rate of symptom recurrence reported.8
Revascularization of RCA CTO and creatine clearance were independent predictors of total symptom recurrence and all-cause mortality, respectively. Patients with T2DM showed higher rates of RCA CTO (although this was not statistically significant) and significantly lower levels of creatine clearance, which may have affected outcomes, particularly angina recurrence.
We reported an improvement in LVEF from baseline to post-procedure assessment, from mildly reduced to preserved in both groups without statistical difference, which could have influenced the lower rates of HF symptoms reported.12,13
To date, only two studies have reported that successful CTO PCI relieved symptoms of angina and dyspnea regardless of diabetes diagnosis.8,12 Our study corroborates these results, and further highlights that successful CTO PCI could represent an effective strategy regardless of T2DM status. Further studies are needed to compare the outcomes of CTO PCI in T2DM patients.
LimitationsSome limitations should be acknowledged. This was an observational study, therefore the data collected are limited to medical records. Patient symptoms were not assessed by scales or questionnaires, which could increase ambiguity. Our study included only successful CTO PCI; failed CTO PCI was not addressed, which may limit the validity of the conclusions. Finally, the data in our study are from only one CTO PCI center, so our conclusions may not be generalizable to other cardiac centers.
ConclusionThe presence of T2DM did not influence total symptom recurrence or clinical outcomes in CTO patients undergoing revascularization by PCI in a two-year follow-up, despite a markedly higher recurrence of angina in this group. These results support the premise that diabetes should not be an impediment when opting for CTO PCI, which in fact may be an effective strategy in preventing symptom recurrence in this group.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to express their gratitude to all colleagues who cooperated in providing data for the current analysis.