Cryoballoon ablation (CBA) for pulmonary vein isolation (PVI) has been growing as an alternative technique, not only in patients with paroxysmal atrial fibrillation (PAF) but also in persistent atrial fibrillation (AF). Cryoballoon ablation has demonstrated encouraging acute and mid-term results. However, data on long-term follow-up of CB-based PVI are scarce.
ObjectiveWe sought to examine efficacy, safety, and long-term outcomes of CBA in PAF and persistent AF in four Portuguese centers.
MethodsAll patients that were treated with the cryoballoon catheter according to routine practices with a second-generation 28-mm CB in four centers were included. This was a retrospective, non-randomized analysis. Patients were followed-up for >12 months and freedom from atrial arrhythmias (AA) was evaluated at the end of follow-up.
ResultsFour hundred and six patients (57.7±12.4 years, 66% men) participated. AF was paroxysmal in 326 patients (80.2%) and persistent in 80 (19.7%). The mean procedure time duration was 107.7±50.9 min, and the fluoroscopy time was 19.5±9.7 min. Procedural/periprocedural complications occurred in 30 cases (7.3%), being transient phrenic nerve palsy the most frequent incident (2 out of 3 complications). Anatomic variations of the PV were present in 16.1% of cases. At a mean follow-up of 22.0±15.0 months, 310 patients (76.3%) remained in stable sinus rhythm, with at least one AF episode recurrence documented in 98 cases (24.1%). The recurrence rate was 20.5% in the PAF group and 37.8% in the persistent AF group.
ConclusionIn this multicenter experience, a single CBA procedure resulted in 75.9% freedom from AF at a 22-month follow-up. This technique was demonstrated to be a safe and effective option in experienced centers for the treatment of PAF and PersAF.
Observa-se um crescimento progressivo na utilização do cateter de balão de CrioEnergia (CBC) para isolamento das veias pulmonares (IVP), não apenas em doentes com fibrilhação auricular paroxística (FAParox), mas também com fibrilhação auricular persistente (FAPers). Esta tecnologia tem apresentado resultados – a curto e a médio prazo – encorajadores. No entanto, ainda há escassez de dados sobre o acompanhamento de longo prazo do IVP baseado em CBC.
ObjetivoProcurámos examinar a eficácia, segurança e os resultados clínicos a longo prazo da ablação com CBC em doentes com FAParox e FAPers nos procedimentos realizados em quatro centros portugueses.
MétodosForam incluídos todos os doentes submetidos a ablação com CBC de 28 mm de segunda geração, em quatro centros de eletrofisiologia. Esta foi uma análise retrospetiva e não randomizada. Os doentes foram acompanhados por um período >12 meses, tendo sido avaliada a presença de taquiarrimtias auriculares (TA) nesse período, bem como a ocorrência de complicações do procedimento.
ResultadosNesta análise, foram incluídos 406 (57,7±12,4 anos, 66% homens). Nestes, observou-se que a FA era paroxística em 326 doentes (80,2%) e persistente em 80 (19,7%). O tempo médio de procedimento foi de 107,7±50,9 minutos e o tempo de fluoroscopia de 19,5±9,7 minutos. Ocorreram complicações no procedimento/periprocedimento em 30 casos (7,3%), sendo a paralisia do nervo frénico o incidente mais frequente (2 de 3 complicações). Observaram-se variações anatómicas das VPs em 16,1% dos casos. Em um seguimento médio de 22,0±15,0 meses, 310 doentes (76,3%) permaneceram em ritmo sinusal estável, sendo documentado, pelo menos um episódio de FA em 98 casos (24,1%). A taxa de recorrência foi de 20,5% no grupo FAParox e 37,8% no grupo FAPers.
ConclusãoNesta experiência multicêntrica, um único procedimento com CBC resultou em 75,9% de ausência de FA em 22 meses de acompanhamento. Essa técnica demonstrou ser uma opção segura e eficaz em centros experientes no tratamento da FAParox e FAPers.
Pulmonary vein isolation (PVI) is currently the cornerstone of invasive catheter-based treatment for patients with atrial fibrillation (AF).1 In the last two decades, catheter ablation was established as a widely accepted procedure for treating AF. Various energy sources were tested, and two main forms of energy have seen a wide diffusion: radiofrequency (RF) and cryoenergy.2 Compared with point-by-point RF ablation, cryoballoon technology has a differentiating and specific characteristic, which is the fact that it is a single-shot/single-delivery application.3 Additionally, cryoenergy has some theoretical advantages, such as improved catheter stability (due to tissue adhesion during freezing), less pain for the patient, less thrombosis risk (owing to less endothelial disruption), and the ability to create contiguous lesions more rapidly.4,5 Presently, data being accumulated from several studies comparing PVI cryoballoon ablation (CBA) with RF, have demonstrated comparable efficacy and similar safety profiles for both techniques. CBA is being associated with a trend for reduced incidence of cardiac tamponade, faster learning curve, and shorter total procedure, but higher rates of phrenic nerve palsy (mostly transient).6–10 In this context, CBA has rapidly become a widespread technology.
With the number of CBA procedures rising in Portugal,11 we considered it important to report the experience of four centers routinely using CBA, regarding safety, procedure times, and outcomes of AF ablation performed with this catheter technology.
MethodsStudy populationData concerning all patients undergoing the first PVI using CBA technology, between August 2014 and November 2020, who accomplished more than 12 months of follow-up, referred to four centers (Arrythmology Unit, Cardiology Department, Santa Marta Hospital, in Lisbon; Cardiology Department, Gaia/Espinho Hospital Center, Gaia; CUF Tejo Hospital, Heart & Vessels Center, Lisbon; CUF Porto Hospital, Heart Center, Porto) were retrospectively analyzed. The objectives of this analysis were to assess the procedural characteristics, efficacy, safety, and long-term outcomes when using a CBA catheter to treat patients with AF.
In this evaluation window, data were collected on procedures performed by seven unique operators at the mentioned centers (two centers were tertiary public hospitals, and the other two centers were private hospitals). The selection of patients to undergo the cryoablation technique was left at the discretion of the different participating centers.
Subject data were excluded from current analysis if patients were diagnosed with long-standing persistent AF (continuous AF >12 months), had a prior cardiac ablation for the treatment of atrial arrhythmia(s), had incomplete baseline data reported, or had not completed 12 months of follow-up at the time of the analysis.
All patients had symptomatic and drug-resistant (who had failed at least one antiarrhythmic drug) AF (paroxysmal or persistent for <12 months). Subjects were classified as having PAF if they had an episode(s) of AF that terminated spontaneously or with intervention within seven days of onset. Patients were classified as having persistent AF if they had a sustained episode(s) of AF that lasted longer than seven days but less than 12 months, including episodes ≥7 days that were terminated by cardioversion.1
All participants provided written informed consent for both the ablation procedure and data collection. Data were collected for each patient from clinical records and included demographic, clinical information, pharmacological therapy, date of hospitalization and discharge, presence of comorbidities, ablation characteristics, and cardiovascular events during hospitalization and long-term outcomes.
All the procedures were performed using the Arctic Front™ Advance Pro Cryoballoon (Medtronic Inc, Minneapolis, MN), and the 28-mm cryoballoon catheter. Research in this study was conducted according to the Helsinki Declaration guidelines on human research. All patients provided written informed consent.
Ablation procedure protocolPatients underwent preprocedural transthoracic echocardiography to assess left ventricular ejection fraction, left atrial dimensions, and exclusion of structural heart disease. A preprocedural computed tomography or magnetic resonance imaging (with the segmentation of the left atrium) was performed to assess left atrial anatomy and to exclude the presence of intracardiac thrombi. Additionally, if the mentioned imaging study was performed >48 hours before ablation, transesophageal echocardiography was done on the day of the procedure (for exclusion of thrombi). All antiarrhythmic medications except amiodarone were usually discontinued >5 half-lives before the ablation. Regarding oral anticoagulation, patients underwent ablation either with continued oral anticoagulation (for at least three weeks before ablation) using warfarin with a therapeutic INR (2.0–3.0) or using low-molecular-weight heparin bridging. If the patient was under direct oral anticoagulants (DOAC) the dosage was omitted in the evening before the ablation. Throughout the procedure, there was rigorous monitoring of oxygen saturation and ECG. All the procedures were carried out in conscious sedation, using propofol infusion, or under general anesthesia.
Through the right femoral vein, a deflectable decapolar catheter was inserted and positioned into the coronary sinus to guide the transseptal puncture and to pace the left atrium for electrophysiological study.
A single transseptal puncture was performed using a needle system (BRK, St. Jude Medical, St. Paul, MN, USA) and a standard transseptal sheath (SL1 8F or 8.5F, St. Jude Medical, St. Paul, MN, USA), that was later exchanged with the steerable 15F sheath (FlexCath™, 15F, Medtronic, Inc., Minneapolis, MN, USA) for the advancement of the cryoballoon.
Immediately before transseptal puncture, heparin was administered intravenously as a bolus (100 U/kg of standard heparin), followed by additional bolus to achieve an activated clotting time between 300 and 350 s during the procedure. Throughout the procedure, the FlexCath was continuously irrigated with heparinized saline. The 28-mm balloon was used for all procedures. The balloon was advanced to the antrum of each PV and different cryoballoon positions were assessed by injecting contrast into the vein distal to the balloon to ensure optimal occlusion.
Regarding energy applications, after successful PVI (240 s, aiming for a minimum temperature of less than −40°C), an additional freeze-cycle of the same duration was applied at the discretion of the operator. The application was aborted if the minimum temperature went below −60°C.
Phrenic nerve function was checked during energy application in the right PV by pacing with a diagnostic catheter at the level of the right subclavian vein and adjunctive monitoring for diaphragmatic function by observing diaphragmatic movement under fluoroscopy, by manual abdominal palpation, and concomitantly by using diaphragmatic compound motor action potential (CMAP) recording.10 The ablation was immediately stopped if phrenic nerve function was affected (either by observing a reduction in the CMAP potential amplitude or by visual or manual inspection).
The goal of CBA was to obtain electrical isolation of the four major PV. Combined common left PV was likewise ablated to achieve similar blockage. Electrical isolation was established after evaluating bidirectional block, confirmed with high-output pacing (10 V, 2.9 ms) using the Achieve™ mapping catheter (Medtronic, Inc., Minneapolis, MN, USA). If there were any residual conduction, further cryotherapy applications were delivered until isolation was established. Before withdrawing the catheter from the left atrium, operators confirmed that all PV potentials were abolished or dissociated.
After ablation, all patients remained under continuous monitoring at the cardiology ward or intensive care unit for at least 24 hours.
Postablation follow-upAfter the ablation procedure, patients were discharged on antiarrhythmic drugs at the discretion of the operator, together with either warfarin or a direct oral anticoagulant. Patients were observed for routine follow-up in the outpatient clinic one to three months after the procedure, and every three to six months (or earlier, if symptoms) during the first year post-ablation. After one year, clinical appointments were scheduled every six months. At each visit, a standard 12-lead ECG was obtained. All patients were followed up with a 24 hour Holter or external event recorder at each outpatient visit. A recurrence was considered if there were any documented episodes of AF or atrial arrhythmias lasting >30 s or sustained symptomatic episodes of rapid palpitations after a 90-day blanking period post-PVI. Antiarrhythmic drugs were continued for between three to six months after the ablation procedure and were thereafter withdrawn – except for beta-blockers – if the patients were free from arrhythmia-related symptoms. Oral anticoagulation was re-evaluated in the third month, and the decision for continued use was based on the CHA2DS2-VASc score. Clinical events occurring during the follow-up were evaluated. Success was defined as freedom from symptomatic arrhythmia and the absence of AF or any atrial tachyarrhythmia (AT) during the above-mentioned ECG monitoring.
Statistical analysisDescriptive statistics were used to summarize patient characteristics, procedural characteristics, safety, and follow-up. Continuous variables were expressed as mean±SD, and categorical data were summarized as frequencies and percentages. The baseline characteristics of the patients and outcomes were compared between the subgroups with the use of the Kruskal–Wallis test for continuous variables and the chi-square test or Fisher's exact test for dichotomous variables, when appropriate. Pearson's correlation coefficient was used to test associations between continuous variables. The Kaplan–Meier method was used to determine cumulative estimates of AF recurrence from the time of enrollment through posterior follow-up, according to the treatment group, with between-group comparisons of cumulative event rates calculated using the log-rank test. Univariate and multivariate Cox regression models were used to determine what variables were significant by themselves in predicting arrhythmia recurrence. All statistical tests were two-sided, and a p-value of <0.05 was considered to indicate statistical significance. The p-values for interaction are reported. Data were analyzed using IBM SPSS for Windows, version 28.0 (IBM SPSS Inc., Chicago, IL).
ResultsBaseline characteristicsThis non-randomized cohort consisted of 406 consecutive patients (270 men; 66.5%), which corresponded to about 25% of the total AF ablations in each center. The mean age of the cohort was 57.7±12.4 years (range 14–82 years) and the mean follow-up duration was 22.0±15.0 months. Of these, 326 (80.2%) patients had PAF, and 82 (20.1%) had PersAF. Concomitant cardiovascular diseases included essential hypertension in 165 (40.6%) patients, coronary heart disease in 16 (3.94%), and sick sinus syndrome with a permanent pacemaker in 4 (0.98%). The left ventricular ejection fraction was normal in all but 17 patients (4.18%). The left atrial diameter was 44±6 mm (range 35–62 mm). The procedure was the first ablation performed in all patients. Regarding the distribution of the patients included in the registry by centers, 147 patients were included in Gaia/Espinho Hospital Center, 182 patients in Santa Marta Hospital, 66 patients in CUF Tejo Hospital, and 11 patients in CUF Porto Hospital. Detailed baseline clinical and demographic characteristics of the study population are reported in Table 1.
Population characteristics.
| Number of patients, n | 406 |
|---|---|
| AF types, n | |
| Paroxysmal AF, n (%) | 326 (80.2%) |
| Persistent AF, n (%) | 80 (19.7%) |
| Male sex, n (%) | 270 (66.5%) |
| Mean age, years (mean±SD) | 57.7±12.4 |
| LA diameter, mm (mean±SD) | 44±6 |
| Left atrial indexed volume, mean±SD, ml/m2 | 36.6±19.7 |
| CHA2DS2-VASc (mean±SD) | 1.22±1.1 |
| Hypertension, n (%) | 165 (40.6%) |
| Hypercholesterolemia, n (%) | 103 (25.3%) |
| Diabetes mellitus, n (%) | 32 (7.8%) |
| Coronary artery disease, n (%) | 16 (3.9%) |
| Dilated cardiomyopathy, n (%) | 17 (4.1%) |
| OSAS, n (%) | 33 (8.1%) |
| Peripheral artery disease, n (%) | 4 (0.98%) |
| Mean follow-up, months (mean±SD) | 22.0±15.0 |
AF: atrial fibrillation; LA: left atrium; OSAS: obstructive sleep apnea syndrome.
Regarding anatomic variations of the PV ostia, we found that they were present in 66 (16.2%) patients. Of these anatomic variations, 47 (11.5%) patients had a left common ostium, 18 (4.4%) had a third right PV with a separate ostium (accessory right middle or upper PV draining independently into the left atrium), and 1 patient had three left PV separated ostia.
Ablation procedureAs stated previously, cryothermal energy applications were delivered to each PV (maximum duration 240 s per application), aiming for a minimum temperature of less than −40°C. The mean procedure time (from the first insertion to the withdrawal of all catheters) was 107.7±50.9min and the fluoroscopy time was 19.5±9.7 min. Isolation of all pulmonary veins was achieved in 99.2% (n=403) of patients. Radiofrequency touch up was required in three patients (0.7%), all of them undergoing cryoablation in the initial phase of the center's experience. In our study cohort, there was no documentation of atrial tachycardia or left atrial flutter, but there were two right atrial flutters during the procedure that required cavotricuspid isthmus ablation with a radiofrequency catheter.
Adverse eventsProcedural and periprocedural complications occurred in 30 patients (7.3%), the most frequent being phrenic nerve palsy (Table 2). The paralysis was observed in 20 patients (4.9%) and led to acutely stopping the application and repositioning the cryoballoon. There was an immediate recovery in all but one patient, in whom phrenic nerve palsy persisted beyond the end of the procedure, with the need for respiratory rehabilitation exercises and full recovery eight months after ablation. Other complications included four (0.98%) cases of pericardial effusion requiring draining, four (0.98%) cases of minor hemorrhage in the access site or a false aneurysm formation, one (0.25%) case of acute hemoptysis, and one (0.25%) case of transient high-degree atrioventricular block. Transfusion of blood products was not necessary in any of the cases described.
Procedural complications.
| Type of complication | Patients, n=406 | Rate, % |
|---|---|---|
| Death, TIA/stroke | 0 | 0 |
| Acute PNP | 20 | 4.9 |
| Persistent PNP at 6 months | 1 | 0.25 |
| Pericardial effusion | 4 | 0.98 |
| Femoral vascular complication | 4 | 0.98 |
| Acute hemoptysis | 1 | 0.25 |
| Transient high-degree AV block | 1 | 0.25 |
| Total adverse events | 30 | 7.3 |
AV: atrio-ventricular; PNP: phrenic nerve palsy; TIA: transient ischemic attack.
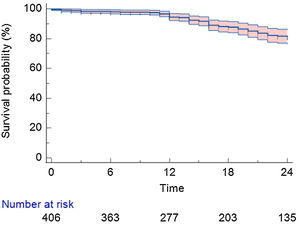
After a mean follow-up of 22.0±15.0 months, 310 (76.3%) patients remained in stable sinus rhythm, without evidence of AF recurrence, with no antiarrhythmic drugs (Figure 1). Symptomatic and clinically typical sustained episodes (without available documentation), AF, or a sustained AT (atrial tachycardia or atrial flutter – including typical and atypical atrial flutter) were documented in 98 (24.1%) patients.
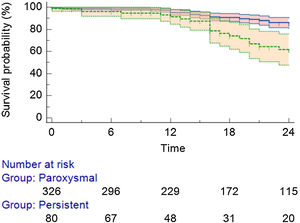
Detailing the population with AF/AT recurrence: 67 (16.5%) were from the PAF group and 31 (7.6%) from the persistent AF group (Figure 2). Regarding the specific recurrence rate inside each group, there was a 20.5% in the PAF group and a 38.7% recurrence rate in the persistent AF group (p<0.01). Twenty-seven patients (27.5% of all recurrences) received a second procedure (all cases performed with RF).
Predictors of arrhythmia recurrenceIn the univariate Cox regression analysis, the left atrial indexed volume (LAIV, ml/m2) was identified as a predictor of arrhythmia recurrence (p=0.03), left atrial diameter (mm), and the type of AF (paroxysmal vs. non-paroxysmal) were also independent predictors of AF/AT recurrence (p=0.039 and p=0.045; respectively). The remaining clinical and analytical covariates (including gender, age, body mass index, obstructive sleep apnea, heart failure, valvular, and coronary heart disease) were not considered predictors of recurrence.
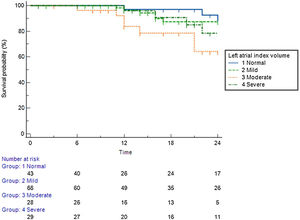
Predictive value of left atrial indexed volumePatients with a normal left atrial volume index (LAVI) (<30 ml/m2) achieved 85% arrhythmia-free survival during the long-term follow-up period (Figure 3). Patients with mildly (<35 ml/m2) and moderately (<40 ml/m2) enlarged atria achieved 79% and 75% arrhythmia-free survival, respectively. Patients with a severely dilated LA (>40 ml/m2) achieved only 64% arrhythmia-free survival.
Arrhythmia-free survival during the long-term follow-up period, according to left atrial index volume.
Left atrial index volume (LAIV): normal – <30 ml/m2; mildly dilated – <35 ml/m2; moderately dilated – <40 ml/m2; severely dilated – >40 ml/m2).12
This is the first multicenter, real-world survey report, on the experience of using the CBA technique for AF ablation in Portugal.
The 28-mm cryoballoon has been the preferred balloon size option in recent years due to a lower incidence of phrenic nerve palsy – compared to the 23-mm size – with the ability to create larger lesion formation, allowing an antral PVI.13,14
The follow-up data in this study demonstrate that PVI using a CBA is feasible and results in freedom from symptomatic AF in a large majority of patients with paroxysmal and persistent AF. Although recurrences of asymptomatic AF could not be excluded in our study, the observed number of patients free from symptomatic AF after a mean period of 22 months is favorable, as the main reason for AF ablation is to relieve patients from symptoms. Specifically, in the persistent AF group, the success rate with a single CBA procedure was 62.2%. This outcome is comparable with data from a multicenter, non-randomized trial, comparing results after a single ablation procedure, using first-generation CBA vs. RF ablation for PVI in patients with persistent AF, showing around 55% of freedom from AF/AT (both with CBA and RF).15,16 Similarly, the results of the CRYO4PERSISTENT Trial showed around 60.7% freedom from AF/AT.17
In the present series, the success rate evaluated by freedom from atrial arrhythmias was slightly superior to the 64.2% for paroxysmal AF and 36.8% for persistent AF recently reported in mid-term follow-up by Ruiz, et al.18 Also, in a recent multicenter European registry, arrhythmia-free survival after CBA for persistent AF was 64% at 24-month follow-up.19
The observed growth in the use of CBA in clinical practice is probably related to a shorter learning curve and higher reproducibility, with a less pronounced impact of center or operator experience, and comparable safety and efficacy in AF ablation (when compared with RF).19 Nevertheless, in an era in which the feasibility and safety of “fluoroless” AF ablation have been already reported,20,21 the CBA procedure still relies on a significant amount of radiation, both to the patients and to the electrophysiology team. The FIRE AND ICE trial showed that CBA was non-inferior to RF concerning efficacy or safety, it was faster than with RF, but with higher fluoroscopy times.6
As previously mentioned, cryoablation has potential advantages over RF energy such as freeze-mediated catheter adhesion.22 This characteristic may improve catheter stability as the catheter does not move relative to the myocardium during freezing. Lesions are therefore expectedly more precise, but despite this theoretical advantage, PV reconnections occur with similar frequency to point-by-point RF.23,24 An additional, important issue to highlight is that balloon-based ablation systems are of limited flexibility. The CBA catheter is an established tool for pulmonary vein isolation, but its use is restricted to that purpose. In patients with additional arrhythmias, such as typical isthmus-dependent atrial flutter or left flutter, who may require linear or focal lesions, the cryoballoon has limited use. Consequently, new cryoablation catheters that can create linear and focal transmural lesions are under investigation.25–27
In our series, the most frequently observed complication was the transient phrenic nerve palsy. Due to anatomical proximity to atrial tissue, the phrenic nerve is particularly susceptible to injury,28 making phrenic palsy the most frequent complication of CBA. With monitoring techniques, it is now possible to minimize the likelihood of a permanent phrenic nerve injury. In this regard, the challenge remains in detecting the very early absence or diminished phrenic nerve response, and stopping the application, avoiding further damage to the nerve. The monitoring of phrenic nerve integrity is achieved by high-output pacing of the right phrenic nerve from the superior vena cava, and manual palpation of the diaphragm contractions.29,30 In our patients, we additionally added monitoring of CMAP signals using diaphragmatic electromyography for the early detection of phrenic nerve injury.31–33
Considering the predictive factors of arrhythmic recurrence, it is important to highlight that the presence of an increase in atrial volume was associated with an increase in therapeutic ineffectiveness. In our cohort, we observed that there is a clear difference between patients with mild and moderate dilation and marked dilation of the LA regarding AF recurrence. Patients with mildly and moderately enlarged atria achieved 79% and 75% arrhythmia-free survival, but patients with a severely dilated LA (>40 ml/m2) achieved only 64% arrhythmia-free survival. This is in line with the information from several published studies with RF ablation in which LAVI is a useful predictor for AF occurrence and recurrence,34,35 but also in studies with cryoablation that observed that left atrial diameter is an independent predictor for AF recurrence.36–38 Regarding the probability of recurrence in a given patient, the LAVI should therefore be considered one of the most important factors in this prediction.
Study limitationsOur study is a multi-center real-life assessment, with a single-arm observational design, not a randomized controlled trial. Also, this was not a prospective study with a predefined protocol for all centers. However, all the operators used the current standard technique for PVI with the 28-mm Arctic Front Advance Pro Cryoballoon.
No continuous or long-duration monitoring for detecting arrhythmia recurrence was systematically used. Therefore, AF recurrence rate and asymptomatic episodes might have been underestimated. On the other hand, symptomatic sustained episodes, even without documentation, were considered recurrences.
ConclusionPulmonary vein isolation using a second-generation (28-mm) cryoballoon is a safe and effective option for symptomatic drug-refractory paroxysmal or persistent AF as the initial ablation procedure, with favorable long-term results and a low complication rate.
Authors’ contributionsP.S.C., M.M.O., P.F., and J.P. wrote the manuscript and provided data.
P.S.C., P.F., S.L., M.M.O., and J.P. contributed to the design and implementation of the research, to the analysis of the results, and the writing of the manuscript.
SL conducted all statistical analyses.
All authors reviewed the final manuscript.
Conflict of interestThe authors have no conflict of interest to declare.