Heart disease and cancer are the two leading causes of morbidity and mortality worldwide. Advances in cancer screening and management have led to longer survival and better quality of life. Despite this progress, many cancer patients experience cardiovascular complications during and after cancer treatment. This study describes the experience of a cardio-oncology program at tertiary academic hospital.
MethodsIn this retrospective observational study, cancer patients referred to the CHULN cardio-oncology consultation (COC) between January 2016 and December of 2019 were included. Data collected included: patient demographics, cancer type, reason for referral, cardiovascular risk factors, cardiac and oncologic treatments and clinical outcomes.
ResultsA total of 520 patients (mean age: 65 ± 14 years; 65% women) were referred to the COC. The main reasons for referral were suspected heart failure (26%), pre-high risk chemotherapy assessment (20%) and decreased LVEF (15%). Pre-existing cardiovascular risk factors were common (79%) and 309 (59%) were taking cardiac medications. The most common type of malignancy was breast cancer (216, 41%) followed by gastrointestinal (139, 27%). More than half received anthracycline-based regimens (303, 58%). Most patients (401; 77%) successfully completed cancer therapy. At the time of last data collection, the majority of patients were alive (430, 83%). Cardiac-related mortality was observed in 16%.
ConclusionsThe close collaboration between cardiology and oncology teams and timely cardiac monitoring was the key to the majority of patients to completing their prescribed cancer therapy.
As doenças cardiovasculares e o cancro são as principais causas de morbilidade e mortalidade em todo o mundo. Avanços na triagem e tratamento do cancro têm permitido uma sobrevivência mais longa e uma melhor qualidade de vida. Apesar deste progresso, muitos pacientes com cancro apresentam complicações cardiovasculares durante e após o tratamento oncológico. Este estudo descreve a experiência de um programa de cardio-oncologia num hospital universitário terciário.
MétodosNeste estudo observacional retrospetivo, foram incluídos pacientes com cancro referenciados para consulta de cardio-oncologia (CCO) do CHULN entre janeiro de 2016 e dezembro de 2019. Os dados coletados foram: dados demográficos, tipo de cancro, motivo de referenciação, fatores de risco cardiovasculares (FRCV), terapêutica cardíaca e oncológica e desfechos clínicos.
ResultadosForam referenciados para a CCO, 520 pacientes (idade média: 65 ± 14 anos; 65% mulheres). Os principais motivos de referenciação foram: suspeita de insuficiência cardíaca (26%), avaliação pré-quimioterapia de alto risco (20%) e diminuição da FEVE (15%). CVRF pré-existentes foram frequentes (79%) e 309 (59%) estavam com terapêutica cardiovascular. O tipo de cancro mais frequente foi o cancro da mama (216; 41%), seguido pelo cancro gastrointestinal (139; 27%). Mais da metade dos pacientes tez terapêutica oncológica baseada em antraciclinas (303, 58%). A maioria dos pacientes (401; 77%) completou com sucesso a terapêutica oncológica. Na altura da última coleta de dados a maioria dos pacientes estava viva (430, 83%). A mortalidade relacionada com o coração foi observada em 16%.
ConclusõesA estreita colaboração entre as equipas de cardiologia e oncologia e a monitorização cardíaca atempada foi decisiva para que a maioria dos pacientes concluísse o tratamento oncológico.
Cardiovascular diseases (CVD) and cancer are the leading causes of death in the developed world. Improvements in early detection and major advances in cancer treatments have led to an increasing number of survivors worldwide. However, cancer patients, including survivors, have a high risk for CVD that can be explained by multiple factors: cancer treatment (old and new drugs, chest radiotherapy) toxicity; shared cardiovascular risk factors (CVRF), such as age, genetics, obesity, smoking and lifestyle; increased age with higher burden of pre-existing CVD. Conversely, certain CVD conditions, such as heart failure and myocardial infarction, have been linked to an increased risk of cancer as well.
The damage caused to the cardiovascular (CV) system affects the full scope of cardiovascular structures and disease types, with manifestations of symptomatic or asymptomatic left ventricular dysfunction, hypertension (HT), arrhythmias, prolonged QT interval, thromboembolism and myocardial ischemia, that often result in discontinuation of cancer therapy, thereby compromising patient outcomes. In cancer patients, CVD is the most frequent non-cancer cause of death.1
With the growing awareness of these CV complications in cancer patients and survivors, the demand for a multidisciplinary approach has led to the development of cardio-oncology structured programs aimed at caring for these patients, particularly in large, academic, tertiary institutions.
In 2016, we established the first cardio-oncology dedicated program in Portugal, at the University Hospital Santa Maria in Lisbon (CHULN). The program comprises three components: (1) cardio-oncology consultation (COC); (2) pre and post-graduate education program; (3) clinical and translational research program.
The COC program was promoted through a dedicated website (https://cardio-oncologia.medicina.ulisboa.pt), as well as lectures at oncology hospitals, oncology meetings, cardio-oncology conferences and post-graduate courses.
The aim of the COC is to streamline the referral of cancer patients to specialized cardiac care, in order to maximize the potential of cancer treatment, minimize CV toxicity and improve overall outcomes. The referral criteria include cardiac therapy optimization and/or CVD prevention prior to cancer treatments and treatment of CV complications which may arise during and/or after those.
In this observational study, we report the first four-years’ experience of a multidisciplinary COC in a tertiary academic hospital.
MethodsThe COC in the cardiology department at CHULN was set up with three dedicated cardiologists, two nurses and one sonographer.
The protocol created with the departments of oncology, hemato-oncology and radio-oncology included both referral criteria and a referral sheet detailing relevant clinical information, cancer therapies and main clinical issues requiring evaluation.
The first appointment operated under the “single-act consultation” model; the consultation, the electrocardiogram and the echocardiogram were performed on the same day for all patients, enabling a quick response to be given to the referring doctor. At this appointment, patients also completed out a quality-of-life survey conducted by a nurse and receive a brochure explaining, in tangible language, what the COC is, outlining potentially cardiotoxic cancer treatments and their manifestations. Subsequent appointments and additional cardiac examinations, including laboratory work-up with cardiac biomarkers, are scheduled by the attending physician, according to each patient's risk stratification and cancer therapies.
Transthoracic echocardiography (TTE) was the main diagnostic modality used for the serial cardiac evaluation. The studies were acquired on a Vivid S70 GE Healthcare and the images were transferred to the Echo PAC workstation (version 202) for offline analysis. Echocardiographic evaluation and monitoring followed the recommendations of the European2 and American Societies of Cardiology and Oncology.3,4
Myocardial toxicity was defined, according to the position paper of the European Society of Cardiology,5,6 as a reduction in left ventricular ejection fraction (LVEF) of at least 10% absolute points to a value <50%. For patients with available baseline strain measurements, a relative percentage reduction in global longitudinal strain >15% from baseline was considered abnormal.7,8
All patients referred to the COC between January 2016 and December 2019 were included in this retrospective observational study. Referrals were mainly from the Departments of Oncology and Hemato-Oncology. For data collection, CHULN electronic medical records were consulted. Demographic data, type of cancer, staging, biomarkers, reason for referral, timing of consultation and cancer therapy were included. Cardiovascular risk factors (HT, diabetes, smoking, obesity, dyslipidemia, etc.), previous history of cardiovascular disease, medication and cardiovascular interventions, cardiovascular examinations, especially echocardiographic (LVEF and strain when available) and electrocardiographic assessment were recorded and collected.
Cardiotoxicity monitoring occurred in three steps: (1) prior to cancer treatment; (2) in high risk patients; (3) during cancer treatment, to detect and treat cardiovascular complications promptly; and after cancer treatment.
Regarding mortality, if the cause of death was not entirely objective, the following criteria were used: when there was clear evidence of severe worsening CV disease impacting prognosis during the last COC visits, death was deemed as cardiac-related; otherwise, it was classified as cancer-related.
ResultsA total of 520 cancer patients were referred between January 2016 and December of 2019 to our COC. This resulted in a total of 1447 patient visits. The average time from referral to first consultation was 13.3 days.
The baseline characteristics of the patients are listed in Table 1. The mean age was 65±14 years (range: 22–101 years); women accounted for 65%.
Baseline patient characteristics.
| Variable | Value |
|---|---|
| Patients (n) | 520 |
| Age (mean ± SD) | 65±14 |
| Women (n; %) | 336 (65) |
| Men (n; %) | 184 (35) |
| Reason for referral (n; %) | |
| Decreased LVEF (<50%) | 80 (15) |
| Symptoms/signs evaluation | 135 (26) |
| Pre-chemotherapy assessment | 102 (20) |
| Arrythmias | 61 (12) |
| Coronary artery disease | 68 (13) |
| Other reasons | 74 (14) |
| Patient-related risk factors (n; %) | |
| Hypertension | 145 (28) |
| Dyslipidemia | 135 (26) |
| Smoking | 94 (18) |
| Diabetes | 72 (12) |
| Obesity | 62 (12) |
LVEF: left ventricle ejection fraction; SD: standard deviation.
The most common reasons for referral to the COC were suspected heart failure (135, 26%), pre-high risk chemotherapy assessment (102, 20%) and decreased LVEF (80, 15%), regardless of symptoms. Less common reasons included known or suspected coronary disease and arrhythmias.
Pre-existing CVRFs were common (79%) and patients had an average of two risk factors, the most common being HT (28%) and dyslipidemia (26%). Baseline ECG showed atrial fibrillation in 53 (10%) patients.
The results of baseline cardiac evaluation with TTE are depicted in Table 2. Mean baseline LVEF in the study population was 61%. The majority had no changes (267; 51%) or minor findings in 75 (15%): mild valvular regurgitation and/or valvular fibrosis. The remaining findings were as follows: 80 cases with decreased LVEF (15%); 61 (12%) with left atrial enlargement; 15 (3%) with moderate pericardial effusion; 12 with segmental wall motion changes (hypo/akinesis), all with previous myocardial infarction; six with moderate or severe asymptomatic aortic stenosis; four with cardiac masses (left atrial myxoma: 2; right atrial large thrombus: 2).
Baseline echocardiogram evaluation.
| Parameter | n=520 |
|---|---|
| LVEF <50% n (%) | 80 (15) |
| Pericardial effusion n (%) | 15 (3) |
| Hypo/akynesis n (%) | 12 (2) |
| Aortic stenosis n (%) | 6 (1) |
| Cardiac tumors n (%) | 4 (1) |
| LA enlargement n (%) | 61 (12) |
| “Minor” alterations n (%) | 75 (15) |
| Normal n (%) | 267 (51) |
LA: left atrial; LVEF: left ventricular ejection fraction
At the time of first COC, 309 (59%) were taking cardiac medications: 60 were treated with beta-blockers (BB), 135 with angiotensin-converting-enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARBs), 80 with combination of both BB and ACEI/ARBs, 26 with calcium channel blockers (CCB) and eight were on anticoagulation drugs.
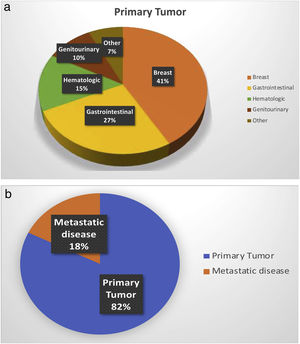
The most common type of malignancy was breast cancer (216; 41%) followed by gastrointestinal (139; 27%), genitourinary (51; 10%); hematologic (76; 15%) and others (38; 7%). Ninety-two (18%) had metastatic cancer (Figure 1).
More than half of patients received anthracycline based regimens (303, 58%): first line therapy (67; 13%); anthracyclines and radiotherapy (135; 26%); anthracyclines, radiotherapy and HER2 inhibitors, trastuzumab or pertuzumab (43; 8%); and anthracyclines, radiotherapy and HER2 inhibitors combo (58; 11%) even though not simultaneously.
Thirteen patients were exposed to chest radiotherapy alone (3%), all of whom had breast cancer. Two hundred and four patients (39%) received non-anthracycline therapy that included drugs like 5-Fluorouracile, capecitabine, tyrosine kinase inhibitors, angiogenesis inhibitors and immunotherapy (Table 3).
In December 2019, most patients (401; 77%) had successfully completed their cancer therapy. Treatment was ongoing in 36 patients.
Cancer therapy was discontinued for cardiac reasons in 83 (16%). The main reason was LV dysfunction in 70 patients (asymptomatic or symptomatic), related to anthracyclines and/or HER2 inhibitors, mostly in breast cancer patients (persistent LV dysfunction in 57 and transient in 13). Other reasons for therapy discontinuation were angina pectoris related to 5-FU/capecitabine in four patients and arterial HT secondary to VEGF inhibitors in nine patients.
During or after cancer therapy, cardiac medication was optimized or initiated in 148 patients, mainly beta-blockers and ACEI. Additionally, a CRT-D was implanted in three patients with heart failure; percutaneous coronary intervention was performed in five patients; atrial fibrillation catheter ablation was performed in eight patients; two patients with severe valvular aortic stenosis had transcatheter aortic valve implantation and the two patients with myxoma underwent surgical tumor resection.
At the time of data collection, most patients were alive (430, 83%), and 90 patients (17%) had died. Genito-urinary and gastro-intestinal malignancies had the highest mortality — 25% and 24%, respectively. The majority of deaths were cancer-related (76; 84%). The remainder (14; 16%) were cardiac-related, all of which were attributed to heart failure due to cardiotoxicity induced by anthracycline-based therapy.
DiscussionIn this four-year retrospective observational study, we report the outcome of 520 cancer patients with existing/potential CV issues referred to our COC. Our population had a high incidence of CVRF (79%). The main reason for referral was suspected heart failure (26%), followed by pre-high risk chemotherapy assessment in 20%.
The baseline echocardiogram showed abnormal findings in 167 (32%) patients. At the time of first COC, 59% were already taking cardiac medications either for risk factor control or for previous CVD.
Although primary prevention strategies to minimize cardiac toxicity due to cancer-related therapies have shown only a modest benefit on LV function in small size randomized trials,9–11 the use of BB and ACEI is recommended in high risk populations.12
Al Kindi et al.,13 in a “big data” analysis, found that patients diagnosed with common cancers have an unexpectedly higher prevalence of CVD than the general population, which also varies according to cancer etiology. Notwithstanding, only half of the patients with cancer and CVD are treated with guideline-directed medical therapy. In addition, patients with preexisting CVD are more susceptible to cardiotoxicity and other cardiac events, either becoming ineligible for treatment with certain agents or requiring dose reductions and/or premature discontinuation.
In our experience, the COC program had an immediate impact on patient care especially since most patients (77%) referred to COC were able to complete their prescribed cancer therapy. Additionally, it enabled optimization of cancer treatment when needed, providing cardiovascular treatment during or after cancer therapy in as many as 168 patients (32%).
At the time of data collection 90 patients had died: 76 due to cancer progression and 14 (16%) due to heart failure related to anthracycline-based therapy.
Recently, three observational studies reported their experience with COC. Kappel et al.,14 reported their seven-year experience with the first multidisciplinary COC in Canada at the Ottawa Hospital. In a population similar to ours — high prevalence of CVRF, baseline cardiac medication, cancer type and treatment — the outcomes were similar either in terms of rate of cancer therapy completion or mortality due to cardiac causes. They concluded that the implementation of COC had many benefits such as prompt access to a cardiologist that could risk-stratify, diagnose and manage cancer patients with CVD. Sadler et al.15 reported their experience of the first 25 months of operation at the COC at the Cleveland Clinic Florida. In 476 patients with a high incidence of CVRF and pre-existing CVD, their cardiology care enabled the vast majority to complete cancer treatments. They came to the conclusion that this kind of population benefited from early screening and cardioprotective strategies. Costa et al.16 reported their 10-year experience with a multidisciplinary COC in an academic tertiary hospital similar to ours. Their referred patients also had a high prevalence of CVRF such as HT, dyslipidemia and smoking. Although the types of cancer differed from our population, in both studies LV dysfunction was the most common manifestation of cardiotoxicity, which was present in 57% of their cohort and in 16% of our patients. This difference could be explained by a longer follow-up in the Brazilian survey. We can also speculate whether the fact that 59% of our patients were already on cardiac therapy at baseline contributed to a higher rate of cancer treatment completion, and therefore a better outcome. The preventive administration of BB, ACEI or ARB to avoid cardiotoxicity is the subject of ongoing research. There is a meta-analysis supporting their prophylactic use in cancer patients.17
Two important prospective studies have been recently published. Pareek et al.18 reported their five-year experience of patients referred to a COC at Royal Brompton Hospital in the United Kingdom. This prospective study of 535 patients focused specifically on those with reduced LVEF, used a new classification of cancer therapy-induced myocardial damage, based on the risk of clinical events (Brompton Hospital myocardial toxicity). Their results showed that the implementation of standardized protocols can achieve optimization of the cardiovascular status of patients at high baseline risk or with established myocardial toxicity, leading to higher rates of cancer treatment continuation. The Cardiotox Registry19 prospectively studied 865 patients scheduled for anticancer therapy with moderate/high risk of cardiotoxicity. Four degrees of progressive myocardial damage/dysfunction were considered: normal, mild, moderate and severe, according to LVEF. After a median follow-up of 24 months, they concluded that the overall prognosis was directly related to their degree of cardiotoxicity, as severe cardiotoxicity was strongly related with all-cause mortality. And while non-severe forms did not have an impact on prognosis, a close follow-up was nonetheless required, as heart failure treatment and even discontinuation of chemotherapy were often required.
In summary, with close collaboration between cardiology and oncology teams, rigorous cardiac monitoring and timely cardiovascular therapy initiation or optimization were possible, enabling the majority of patients to maintain and complete their prescribed cancer therapy.20,21
Identifying patients at high risk of CV complications associated with cancer treatments, as well as implementing early detection, prevention and treatment strategies are paramount in improving cardiac care. Regular review of COC referral patterns should be implemented based on updated standardized protocols.22
In the near future, we aim to move from a traditionally reactive model to an even more proactive model for the prevention of cardiovascular toxicities related to cancer therapies. Risk mitigation strategies in the general population can also be applied in cardio-oncology. The concept of cardio-oncology rehabilitation represents a paradigm shift to initiate preventive efforts, particularly in cancer patients at high risk for cardiac dysfunction.23,24
Study limitationsOur retrospective observational study reflects the clinical experience of a single center, and therefore may not reflect the practices of others. The development of cardio-oncology services needs to be tailored to each institution's organizational needs.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors are grateful to nurses Anabela Matos and Luciano Claro for their contribution to the data collection for this study.