Chronic thromboembolic pulmonary hypertension (CTEPH) is part of group 4 of the pulmonary hypertension (PH) classification and generally affects more than a third of patients referred to PH centers. It is a three-compartment disease involving proximal (lobar-to-segmental) and distal (subsegmental) pulmonary arteries that are obstructed by persistent fibrothrombotic material, and precapillary pulmonary arteries that can be affected as in pulmonary arterial hypertension. It is a rare complication of pulmonary embolism (PE), with an incidence of around 3% in PE survivors. The observed incidence of CTEPH in the general population is around six cases per million but could be three times higher than this, as estimated from PE incidence. However, a previous venous thromboembolic episode is not always documented. With advances in multimodality imaging and therapeutic management, survival for CTEPH has improved for both operable and inoperable patients. Advanced imaging with pulmonary angiography helps distinguish proximal from distal obstructive disease. However, right heart catheterization is of utmost importance to establish the diagnosis and hemodynamic severity of PH. The therapeutic strategy relies on a stepwise approach, starting with an operability assessment. Pulmonary endarterectomy (PEA), also known as pulmonary thromboendarterectomy, is the first-line treatment for operable patients. Growing experience and advances in surgical technique have enabled expansion of the distal limits of PEA and significant improvements in perioperative and mid- to long-term mortality. In patients who are inoperable or who have persistent/recurrent PH after PEA, medical therapy and/or balloon pulmonary angioplasty (BPA) are effective treatment options with favorable outcomes that are increasingly used. All treatment decisions should be made with a multidisciplinary team that includes a PEA surgeon, a BPA expert, and a chest radiologist.
A hipertensão pulmonar tromboembólica crónica (CTEPH) pertence ao grupo 4 da classificação da hipertensão pulmonar (PH) e geralmente afeta mais de um terço dos doentes referenciados a centros de PH. É uma doença tricompartimental que envolve as artérias pulmonares proximais (lobares e segmentares) e distais (subsegmentares), que são obstruídas por material fibro-trombótico persistente, bem como as artérias pulmonares pré-capilares, que são afetadas de forma semelhante à observada na hipertensão arterial pulmonar. É considerada uma complicação rara do tromboembolismo pulmonar (PE), com uma incidência de cerca de 3% nos sobreviventes de PE. A incidência da CTEPH na população geral ronda os seis casos por milhão, mas poderá ser três vezes superior, de acordo com a incidência de PE. Contudo, nem sempre é documentado um episódio prévio de tromboembolismo venoso.
Com os avanços na imagiologia multimodal e na gestão terapêutica, a sobrevivência dos doentes com CTEPH tem aumentado em doentes operáveis e inoperáveis. Técnicas de imagem avançada com angiografia pulmonar auxiliam na distinção entre doença obstrutiva proximal e distal. No entanto, o cateterismo do coração direito é de importância fulcral no estabelecimento do diagnóstico e da gravidade hemodinâmica da PH. A estratégia terapêutica centra-se numa abordagem faseada que começa na avaliação da operabilidade. A tromboendarterectomia pulmonar (PEA) é o tratamento de primeira linha em doentes operáveis. O aumento da experiência e os avanços na técnica cirúrgica têm permitido a expansão dos limites distais da PEA e a melhoria significativa da mortalidade peri-operatória e a médio e longo prazo. Em doentes inoperáveis ou com CTEPH persistente/recorrente após PEA, a terapêutica médica e/ou a angioplastia pulmonar por balão (BPA) são opções terapêuticas eficazes, com resultados favoráveis e que têm sido cada vez mais utilizadas. Todas as decisões terapêuticas devem ser tomadas em equipa multidisciplinar que inclui um cirurgião de PEA, um expert de BPA e um radiologista torácico.
Chronic thromboembolic pulmonary hypertension (CTEPH) is in group 4 of the pulmonary hypertension (PH) clinical classification, alongside other forms of vascular obstruction.1,2 This life-threatening condition is defined by the presence of a precapillary hemodynamic phenotype resulting from incomplete resolution and fibrotic transformation of thromboembolic deposits in the pulmonary arteries after at least three months of effective anticoagulation. In the absence of PH at rest, the term ‘chronic thromboembolic pulmonary disease (CTEPD) is preferred.3 Although previously thought to be a direct result of persistent intravascular obstruction from chronic blood clots after acute pulmonary embolism (PE), there is growing evidence that the pathophysiology of CTEPH is more complex, with concomitant small-vessel remodeling in both obstructed and non-obstructed territories,4,5 indicating a multicompartmental disease that can involve the proximal (lobar-to-segmental) and distal (subsegmental) pulmonary arteries with obstructive chronic fibrothrombotic material, and the precapillary bed (microvasculature), with a non-thrombotic vasculopathy as in pulmonary arterial hypertension (PAH). Combined data from advanced imaging and hemodynamics can distinguish proximal from distal obstructive disease and establish the degree of microvasculopathy.
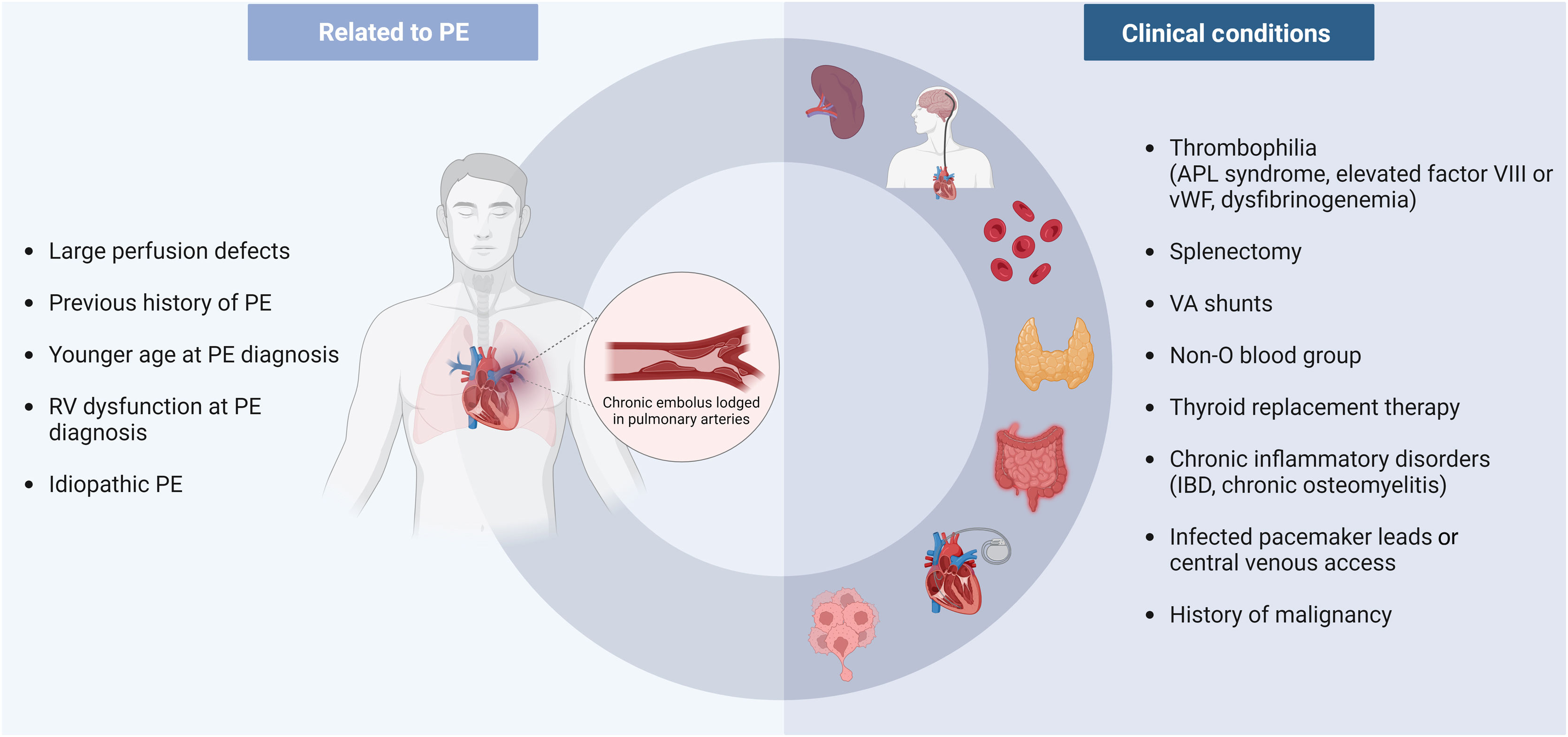
Patients often present with non-specific symptoms reflecting systemic congestion and low cardiac output (CO). Recent guidelines2 recommend systematic follow-up assessment of residual symptoms after acute PE and provide information on anatomical and functional imaging manifestations of chronic disease that support a diagnosis of CTEPD /CTEPH. Appropriate integration of the clinical context is mandatory, since numerous conditions such as malignancies, splenectomy, implanted devices, inflammatory diseases, antiphospholipid syndrome, and thyroid hormone replacement therapy are known risk factors for the development of CTEPH.
All treatment decisions should be made in the context of a multidisciplinary CTEPH team. The aim of this article, which takes recent advances into account, is to provide a comprehensive and up-to-date review of CTEPH, mainly focusing on its pathogenesis, diagnostic approach, and treatment strategies in all the compartments of the disease.
EpidemiologyCTEPH is classically considered a consequence of acute PE. Some studies report that 50–75% of patients have a previous history of PE.6–8 Persistent perfusion defects are present in about 30–50% of patients after acute PE,9,10 although fewer will present with CTEPH symptoms.9 However, the true incidence of CTEPH has not been clearly established. A meta-analysis of 16 studies11 reported figures ranging from 0.4% to 6.2%, with an overall weighted pooled incidence of 2.3%. In the four studies that focused on consecutive survivors after three months of anticoagulation, the pooled incidence was 3.2%. When analyzing all-comers studies (i.e. all consecutive patients with symptomatic pulmonary embolism and no exclusion criteria), the incidence was found to be only 0.56%, a number that probably best reflects the incidence of CTEPH on a population level, in the authors’ opinion.11 Other national registries have also reported a lower incidence compared with the pooled analysis.12,13
In fact, the reported incidence of CTEPH may be overestimated. In the aforementioned studies, the CTEPH diagnosis was established only a few months after pulmonary embolism (PE), in contrast to what is generally seen. It is therefore possible that the reported incidences were a mix of incident and prevalent cases, due to patients having pre-existing, undiagnosed CTEPH. In one of these studies,14 of the seven CTEPH diagnoses confirmed by right heart catheterization (RHC), five patients presented with markedly elevated pulmonary artery (PA) systolic pressure (PASP) (62–102 mmHg) at the time of the index PE. This would not be compatible with a first PE, because a non-adapted right ventricle could not generate such high pressures. After reviewing the initial computed tomography (CT) pulmonary angiography (CTPA), radiologists confirmed the presence of CTEPH signs in these five patients, which changed the cumulative incidence from 4.8% to 1.5%. Conversely, CTEPH may also be underdiagnosed because of its non-specific presentation, variable rates of previous PE, the expertise required to read CTPA exams, and the infrequent use of ventilation/perfusion (V/Q) scans.15,16 A recent review article17 stated that in the US, even on a conservative estimate, around 1500–3000 cases of CTEPH should be diagnosed each year; however, considerably fewer cases are observed, with only 0.9 PEA procedures performed annually per million adults.18
Risk factorsAfter an episode of acute PE, significant resolution of thrombus occurs in the majority of patients who are treated with anticoagulation.4,5 However, for poorly understood reasons, a small minority will continue to have organized residual clots in the pulmonary vessel walls and will subsequently develop CTEPH.4,5
Several clinical conditions may increase the risk for developing CTEPH after an acute PE (Figure 1). Previous history of PE, larger perfusion defects, idiopathic etiology and younger age at PE diagnosis seem to increase the risk of CTEPH, as shown in a prospective study with consecutive patients after acute PE.19 In a score developed for prediction of CTEPH, development of symptoms >14 days before PE diagnosis, right ventricular (RV) dysfunction at the time of presentation, and idiopathic PE were all independent predictors for CTEPH.20
Thrombophilic disorders increase the risk for PE. It therefore seems logical that conditions which predispose to a hypercoagulable state would lead to a higher risk of CTEPH. However, classical inherited thrombophilias such as antithrombin deficiency, protein C/S deficiency and factor II or V mutations are not associated with CTEPH.21 By contrast, antiphospholipid antibodies have been identified in 20% of CTEPH patients, and in higher titers than in idiopathic PAH patients, underlining its role in the pathogenesis of CTEPH.21 Other elevated prothrombotic factors have been identified in CTEPH patients, including factor VIII and von Willebrand factor.22,23 Fibrinogen polymorphisms have also been reported in some CTEPH patients.24–26
A case–control study comparing consecutive CTEPH patients with patients after acute PE identified splenectomy, ventriculoatrial shunts, and chronic inflammatory disorders such as inflammatory bowel disease and chronic osteomyelitis as risk factors for CTEPH.27 This was later reinforced by a retrospective study comparing CTEPH patients with non-thrombotic PH patients, which identified other risk factors such as infected pacemaker leads, non-O blood group, antiphospholipid antibodies, thyroid replacement therapy and history of malignancy.28 More recently, Jevnikar et al. demonstrated an association between totally implantable central venous access systems and CTEPH.29 Although the evidence was deemed insufficient to consider it as a risk factor, the authors speculated that the higher risk of catheter-related thrombosis, infection and inflammation might indirectly increase the risk for CTEPH.29
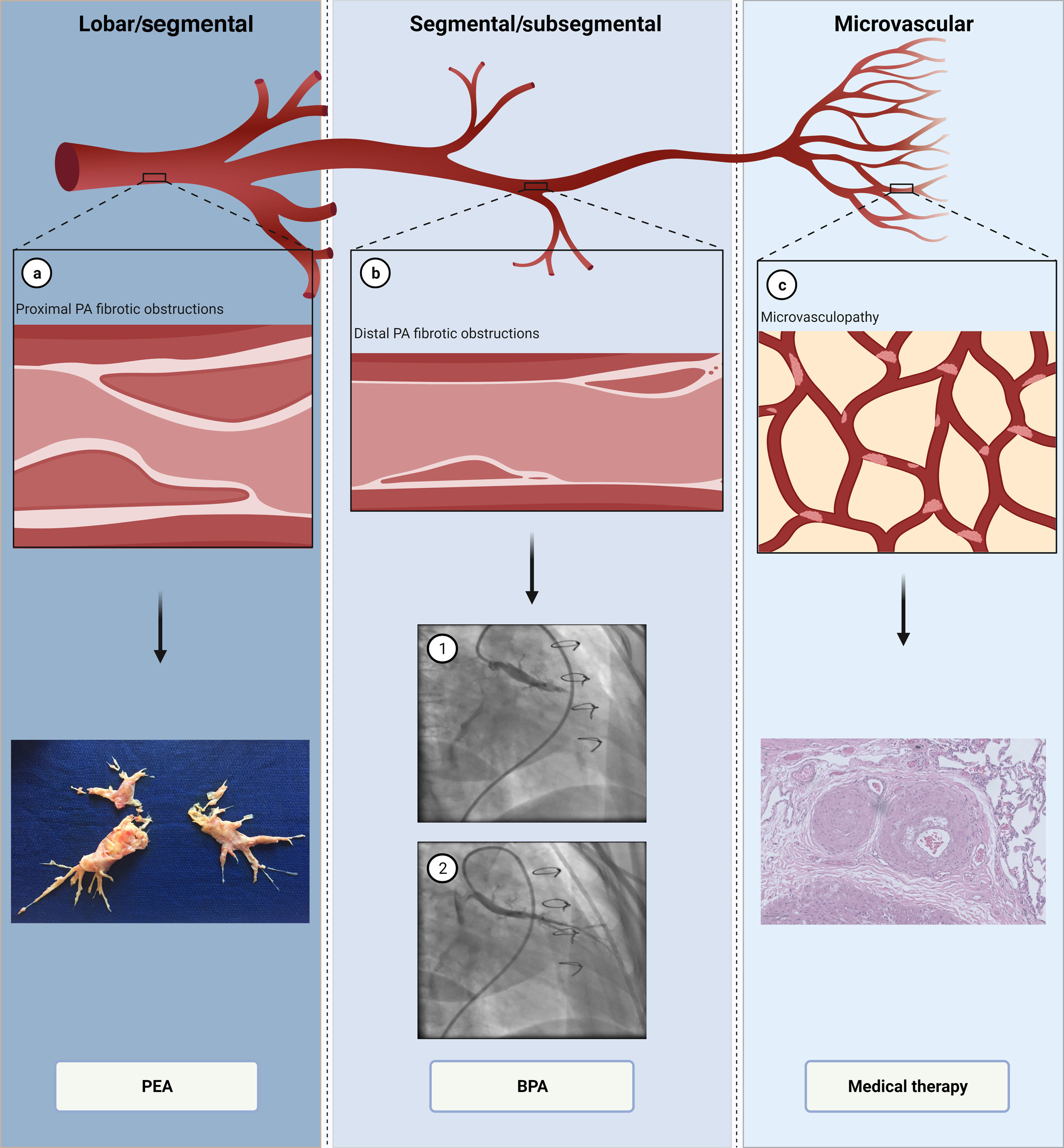
PathophysiologyThe pathophysiology of CTEPH is complex and not fully understood. While initially thought to be solely a consequence of persistent obstruction of the PA, involvement of small vessels similar to that observed in idiopathic PAH may be present in some patients.4,5 There are therefore two types of vascular lesions which contribute to increased pulmonary vascular resistance (PVR): persistent intravascular obstruction of the PA by organized fibrotic clots, and secondary microvasculopathy3,4 (Figure 2).
The pathophysiology and multimodality treatment of chronic thromboembolic pulmonary hypertension. (a) Proximal lesions in lobar and segmental arteries (top); fibrotic, collagen-rich lesions, treated by pulmonary endarterectomy (bottom); (b) distal lesions in segmental and subsegmental arteries (top); balloon pulmonary angioplasty in a segmental branch (bottom) before (1) and after (2); (c) microvasculopathy lesions (top); small arteries with intimal thickening and fibrosis, which are treated with medical therapy (bottom). BPA: balloon pulmonary angioplasty; PA: pulmonary artery; PEA: pulmonary endarterectomy.
Created with BioRender.com.
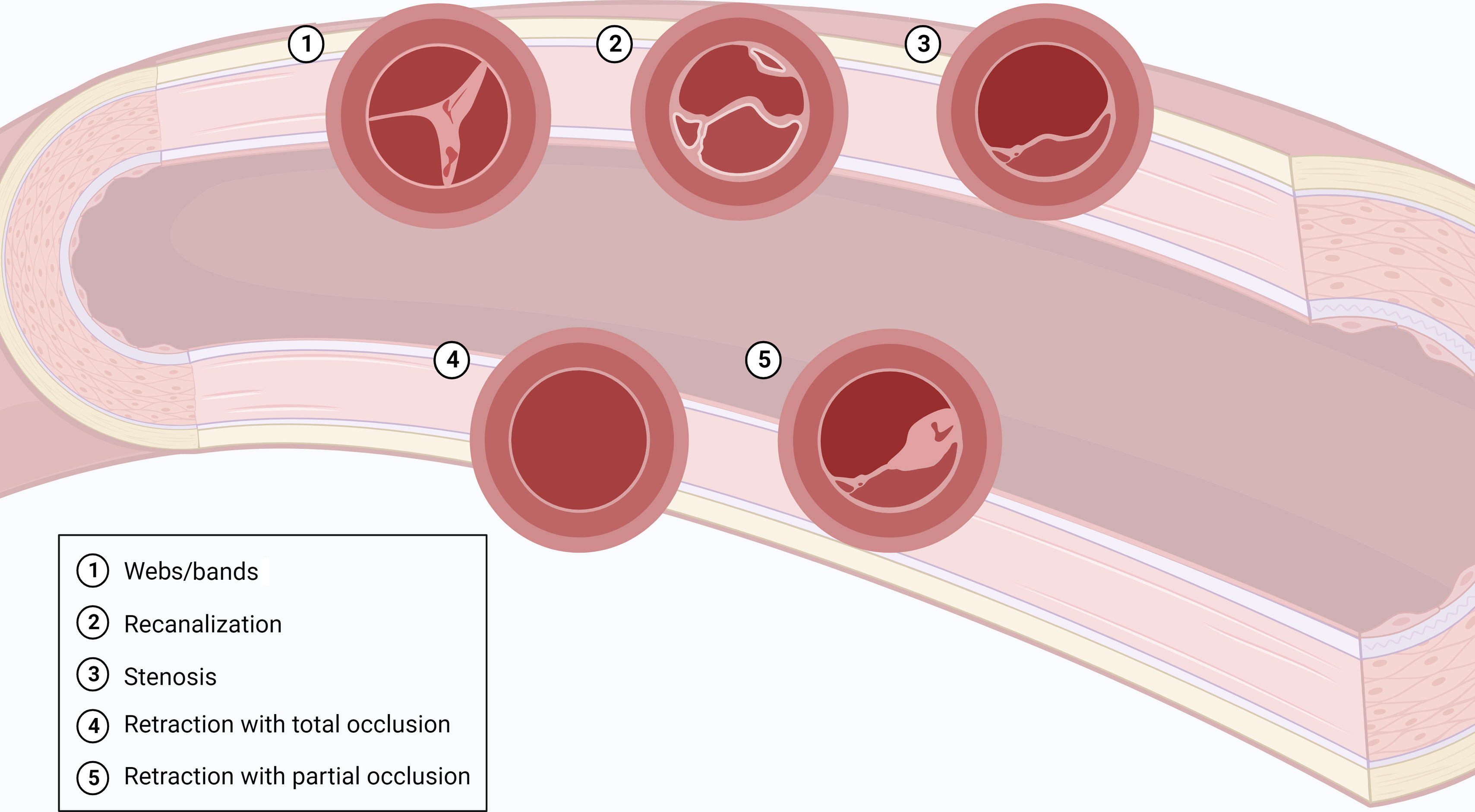
Intravascular obstruction can involve all segments of the PA, from the main arteries down to distal branches at the intra-acinar level.3,4 At the proximal level, PA obstructions are recanalized, fibrotic, organized clots, which remain adherent to the vessel wall and correspond to slits, webs, stenosis or pouching on vascular imaging3,4 (Figure 3). These collagen-rich clots cannot easily be detached from the vessel walls, in contrast to what is seen with fresh clots in acute PE4 (Figure 2a). Longstanding exposure to elevated pulmonary artery pressure (PAP) will also contribute to the formation of atheromatous lesions in the pulmonary vessels, with consequent increased wall stiffness.3,4,30 Distally, chronic obstruction of small pulmonary vessels is typically seen as partially recanalized lesions, with multiple lumina (colander-like lesions)3,4 (Figure 4).
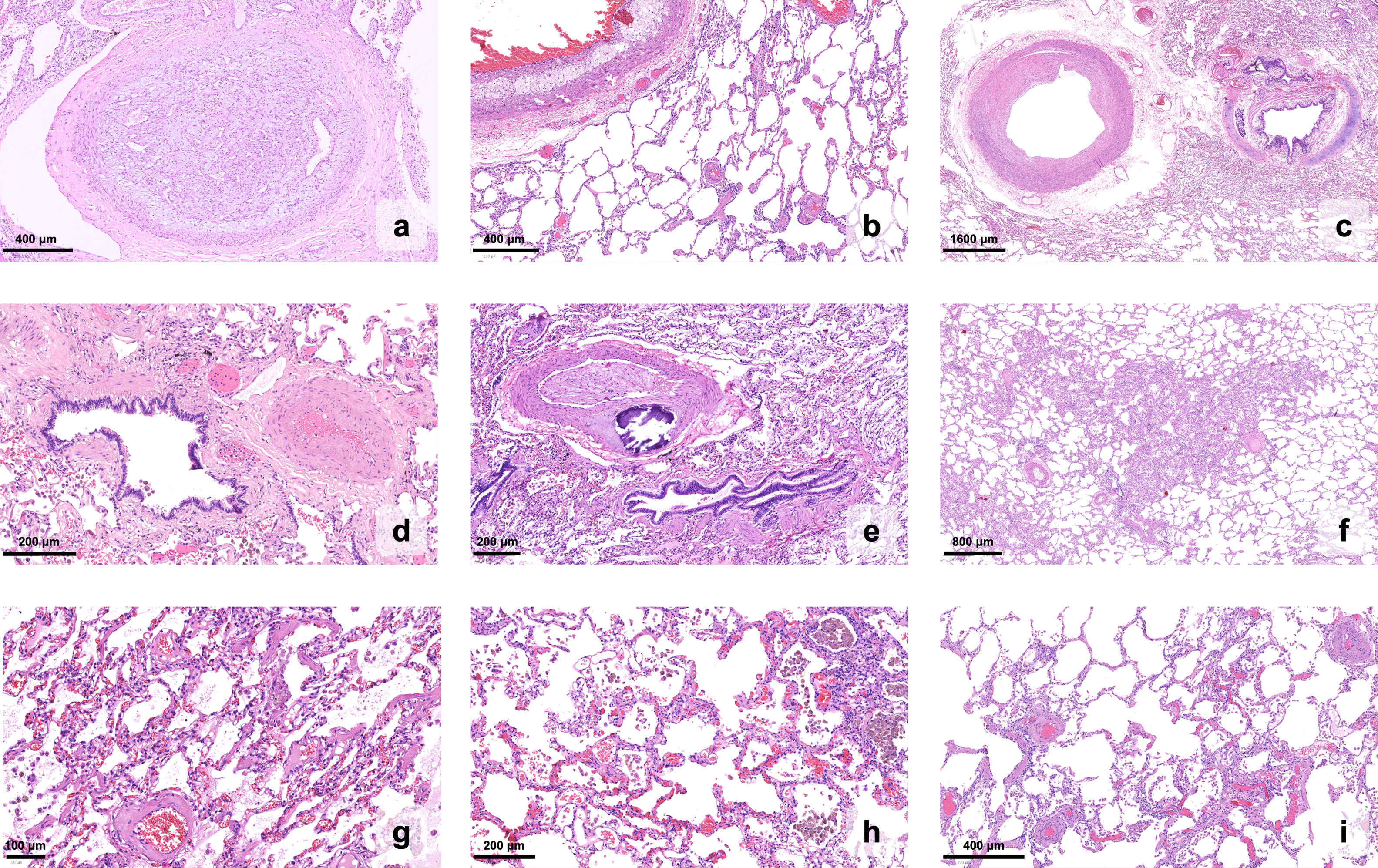
Histopathology of chronic thromboembolic pulmonary hypertension in explanted lungs (H&E stain). (a) A thrombus underwent organization and recanalization within the lumen of a proximal branch of the pulmonary artery; (b) aggregates of foamy histiocytes denote atheromatous lesions in a large-caliber vessel, pictured alongside small parenchymal venules with remodeling; (c–e) proximal and subsegmental branches of the pulmonary artery exhibit a range of fibromuscular proliferation and fibrosis (±calcification) of the wall; (f) an area of hemangiomatosis stands out from the surrounding parenchyma, due to broadened alveolar walls; (g and h) higher magnification reveals capillary proliferation, with congestion and extravasated red blood cells; hemosiderophages can be seen within the alveolar spaces; (i) small parenchymal venules, usually inconspicuous, become readily recognizable due to marked thickening of the wall.
Microvasculopathy lesions were described in CTEPH patients by Moser and Bloor, through histopathological examination of lung tissue.31 The authors depicted intimal remodeling, with eccentric fibrosis and fibromuscular proliferation, as well as plexiform lesions, all of which are also present in idiopathic PAH (Figures 2c and 4). These lesions seem to predominate in non-obstructed pulmonary vessels; it was accordingly proposed that pulmonary blood flow would shift from obstructed arteries to non-obstructed territories, leading to higher local pressures, increased wall stress and vascular remodeling.31,32 Interestingly, this mechanism appears to partially explain not only the discrepancy between perfusion defects and PVR, but also the development of CTEPH in PE patients who are adequately anticoagulated and show no signs of recurrence.1 However, the same mechanism does not explain the presence of microvasculopathy lesions in territories located distally to obstructed pulmonary vessels, as noted by Moser and Bloor.31 In these territories, remodeling of precapillary arterioles, as well as of capillaries and post-capillary venules (resembling hemangiomatosis and veno-occlusive disease, respectively), was later described32,33 (Figure 4). Bronchial artery and vasa vasorum anastomoses with pulmonary vessels distal to the obstructed artery may play a major role in the development of microvasculopathy: although they are necessary to prevent lung ischemia in the affected territories, pulmonary vessels become exposed to high systemic circulation pressures, increasing wall stress and vascular remodeling.33 Bronchopulmonary shunting may also be observed when anastomoses develop with the post-capillary territory.33 The presence of microvasculopathy in both obstructed and non-obstructed areas may explain the development of persistent PH after treatment of obstructive lesions.1
Endothelial dysfunction also appears to play a central role in the development of microvasculopathy. Similarly to PAH, some pathways may be dysregulated in CTEPH and are being targeted with PAH-specific drugs:
- •
Nitric oxide (NO) is secreted by endothelial cells through the action of nitric oxide synthases (NOS). It inhibits platelet aggregation and stimulates soluble guanylate cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP).34 This substance, through activation of a cGMP-dependent kinase, mediates vasodilatory and antiproliferative effects on vascular smooth muscle cells.34 In CTEPH patients, NO levels appear to be reduced.35 Additionally, levels of asymmetric dimethylarginine, an NOS inhibitor, are increased and correlate with severity of pulmonary vascular disease and its outcomes.36 The importance of NO-sGC-cGMP pathway dysregulation in the pathogenesis of CTEPH is further demonstrated by the results of the CHEST-1 and CHEST-2 trials, in which riociguat, an sGC stimulator, improved patients’ functional capacity and pulmonary vascular resistance.37,38
- •
Endothelial cells also produce prostacyclin (PGI2), which has vasodilatory, antiplatelet, antiproliferative and immunomodulatory properties. PGI2 levels are decreased in patients with PAH.39 Subcutaneous treprostinil, a synthetic prostacyclin analog, is approved in the European Union for inoperable or persistent/recurrent CTEPH, after demonstration of its safety and improvement in functional capacity.40
- •
Levels of endothelin (ET)-1, a vasoconstrictor and pro-proliferative substance produced by endothelial cells, are also elevated in patients with CTEPH and correlate with disease severity.41 ET-1 levels may be useful for preoperative identification of patients at risk for persistent PH after PEA.41 The endothelin receptor antagonist macitentan has shown some promising results regarding PVR improvement in a phase 2 trial.42 However, the phase 3 MACiTEPH study to evaluate the efficacy and safety of macitentan 75 mg in inoperable or persistent/recurrent CTEPH was interrupted due to futility (ClinicalTrials.gov identifier: NCT04271475).
Initially, increases in PVR lead to increased RV afterload and adaptive, concentric hypertrophy, which results in decreased wall stress and increased pumping effectiveness.5,43 Over time, continuous exposure to progressively higher PVR leads to maladaptive RV remodeling, with eccentric remodeling, RV dilatation, diastolic dysfunction, and fibrosis.5,43 Consequently, increased wall tension develops and leads to increased oxygen consumption, further worsening RV function. Decreased RV stroke volume leads to low systemic CO, with worsening of RV perfusion, and eventually worsening RV dysfunction culminates in RV failure.5,43
DiagnosisSince treatment of CTEPH is potentially curative, physicians should have a high level of suspicion in every patient who develops symptoms after a PE episode.2 Signs and symptoms are non-specific and may be present in other PH etiologies and cardiovascular or pulmonary conditions.2 Dyspnea on exertion, exercise intolerance, hemoptysis (possibly due to bronchial artery hypertrophy) and manifestations of RV failure such as lower limb edema, abdominal distention, lightheadedness or syncope may be present.2,17 In more advanced cases, symptoms may occur due to compression of adjacent structures secondary to pulmonary artery dilatation (thoracic compression syndrome).2 Examples include exertional chest pain or even acute coronary syndromes due to extrinsic compression of the left main coronary artery,44,45 Ortner's syndrome due to recurrent laryngeal nerve compression, and respiratory infections or atelectasis due to compression of the bronchi.46 In CTEPD without PH at rest, symptoms may be attributable to exercise-induced PH and/or increased dead space ventilation.2
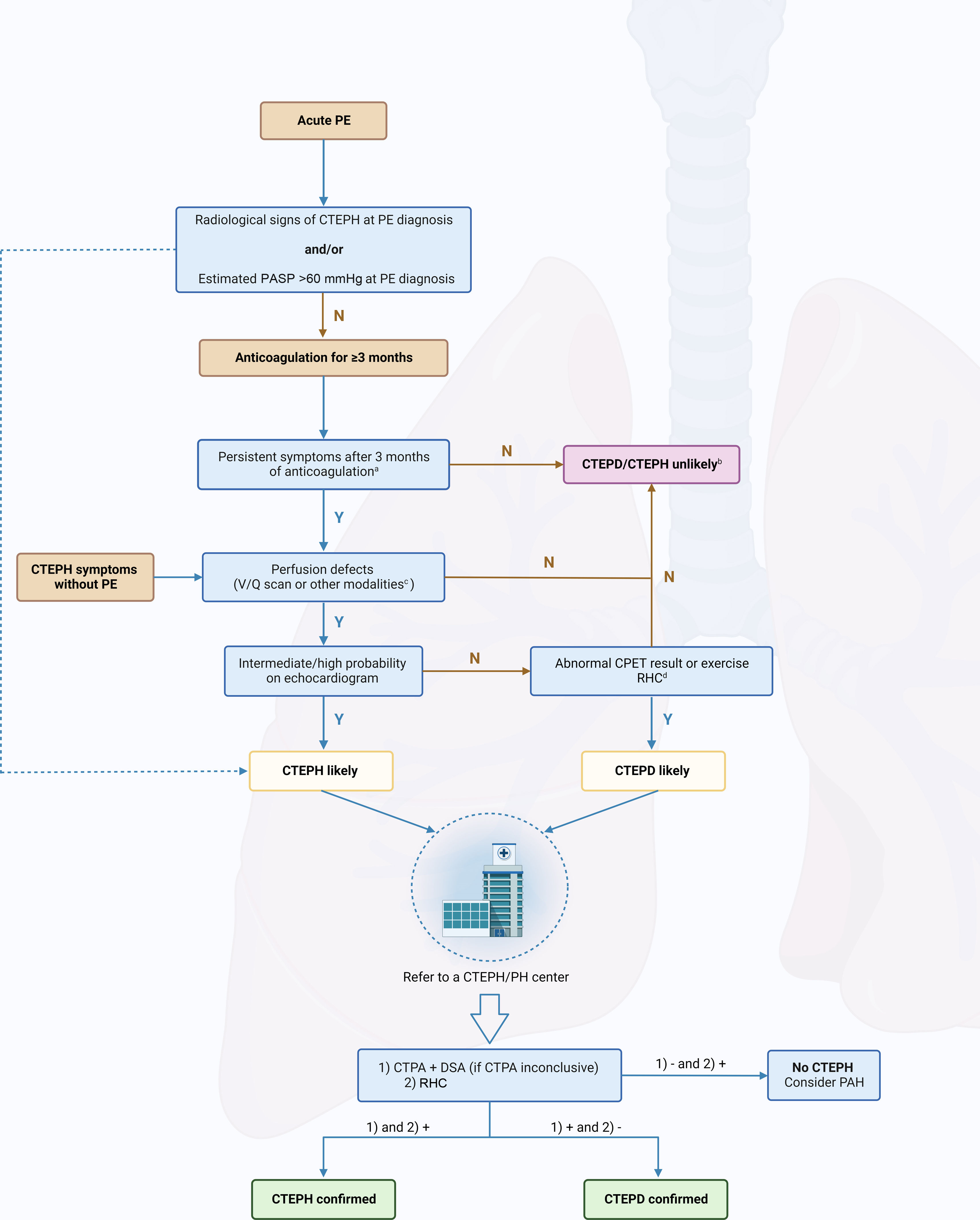
CTEPH should also be suspected at the time of acute PE when CTPA shows findings suggestive of chronic fibrothrombotic deposits – bands, webs, arterial narrowing or retraction, and/or dilated bronchial arteries (Figure 5) – or when the echocardiogram depicts an estimated PASP >60 mmHg and/or features of RV dysfunction and hypertrophy.14 In asymptomatic patients with risk factors (Figure 1) or a high CTEPH prediction score (Table 1),20 diagnostic assessment should also be considered.2 A proposed diagnostic algorithm is shown in Figure 6. Initial assessment of patients with suspected CTEPH should include a transthoracic echocardiogram to determine the probability of PH (Table 2) and a V/Q scan to look for mismatch perfusion defects.2 In general, especially considering that CTPA performed by chest radiologists is not widely accessible, a V/Q scan remains the gold standard exam for exclusion of CTEPD without PH at rest, due to its high sensitivity (96.2–97.4%) and negative predictive value of almost 100%47,48 (Figure 7). Other perfusion techniques, such as dual-energy CT scan or magnetic resonance perfusion, may be superior to a V/Q scan (Figure 8). However, these are more technically challenging and expensive, have limited availability, and lack multicenter validation.3
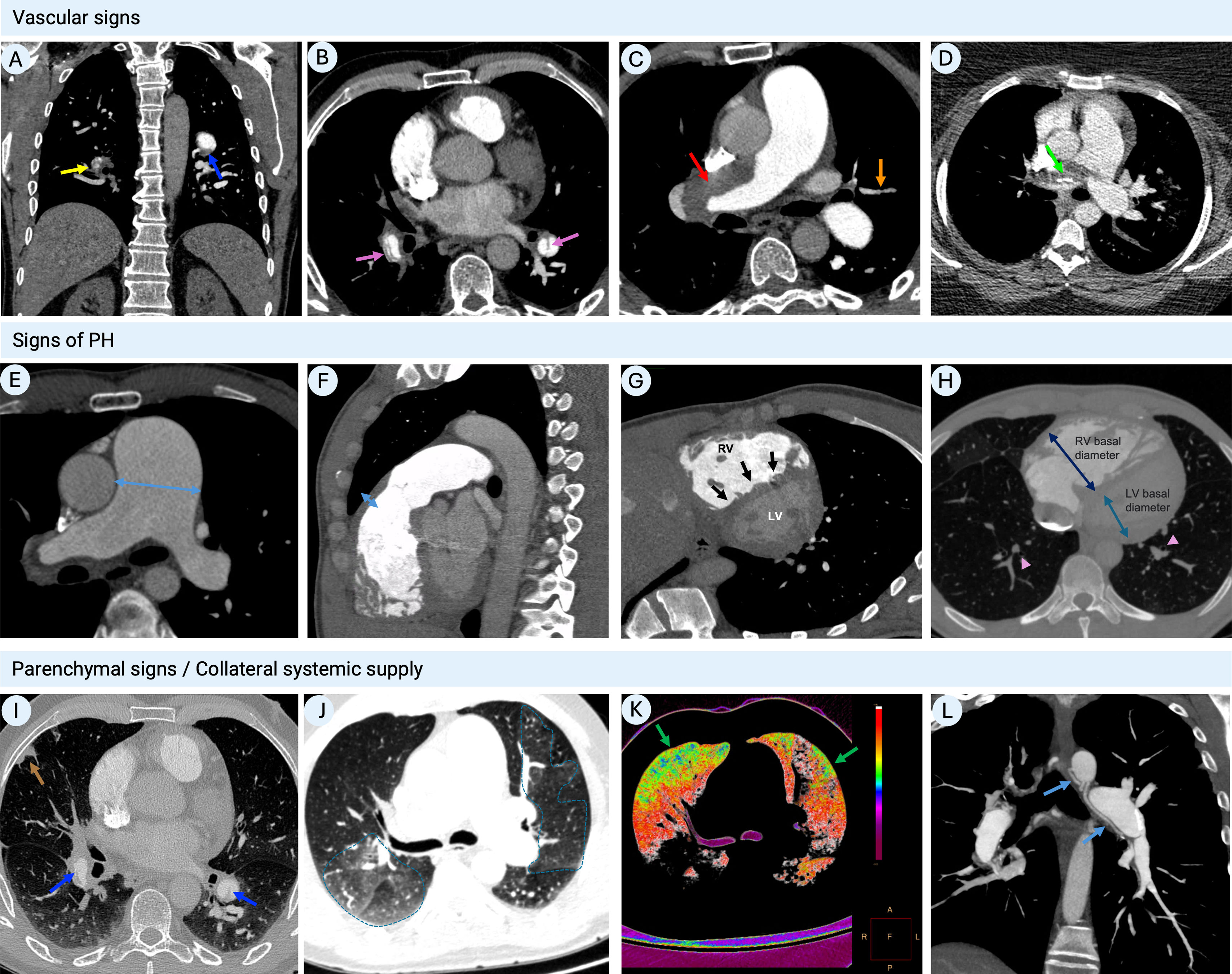
Computed tomography pulmonary angiography findings in chronic thromboembolic pulmonary hypertension. (A) Coronal image showing fibrothrombotic retraction with partial obstruction (yellow arrow) and eccentrically located thrombus with a broad base forming obtuse angles with the vessel wall, due to incomplete thrombus resolution (blue arrow); (B) axial image with intravascular webs (purple arrows); (C) axial image with total left PA occlusion (red arrow) and segmental arterial retraction (orange arrow); (D) calcifications within fibrothrombotic deposits in the right PA, illustrating the chronic component of the disease (green arrows); (E) axial image depicting main PA dilatation (double-headed arrow); (F) sagittal image showing RV outflow tract hypertrophy (double-headed arrow); (G) cardiac short-axis image with flattening of the interventricular septum (black arrows) and D-shaped left ventricle; (H) axial image showing segmental artery-to-bronchus diameter ratio of >1:1 (arrowheads), flattening of the interventricular septum, RV hypertrophy and RV/LV diameter ratio >1 (double-headed arrow); (I) peripheral nodular opacity (brown arrow) in the right upper lobe secondary to previous pulmonary infarction along with intra-arterial fibrothrombotic deposits (blue arrows); (J) axial image showing sharply demarcated segmental and subsegmental areas of hypo- and hyperattenuation (dotted lines); (K) (same patient as in panel J) color maps showing the distribution of perfusion: spectrum from green to red indicates increasing perfusion (hypoperfusion indicated by green areas); (L) sagittal image with bronchial artery hypertrophy (blue arrows). LV: left ventricular; PA: pulmonary artery; RV: right ventricular.
Chronic thromboembolic pulmonary hypertension prediction score.
| Points | |
|---|---|
| Unprovoked PE | +6 |
| Known hypothyroidism | +3 |
| Symptom onset >2 weeks before PE diagnosis | +3 |
| RV dysfunction on CT or echocardiography | +2 |
| Known diabetes mellitus | -3 |
| Thrombolytic therapy or embolectomy for PE | -3 |
CT: computed tomography; PE: pulmonary embolism; RV: right ventricle.
Low risk: −6 to 6 points; high risk: >6 points.
Diagnostic algorithm in patients with suspected chronic thromboembolic pulmonary disease/hypertension. CO: cardiac output; CTEPD: chronic thromboembolic pulmonary disease; CTEPH: chronic thromboembolic pulmonary hypertension; CPET: cardiopulmonary exercise test; CTPA: computed tomography pulmonary angiography; DECT: dual-energy computed tomography; DSA: digital subtraction angiography; mPAP: mean pulmonary artery pressure; MRI: magnetic resonance imaging; N: no; PAH: pulmonary arterial hypertension; PASP: pulmonary artery systolic pressure; PE: pulmonary embolism; PETCO2: end-tidal partial pressure of carbon dioxide; RHC: right heart catheterization; SPECT: single-photon emission computed tomography; V/Q: ventilation/perfusion; VE/VCO2: ventilatory equivalent for carbon dioxide; VO2/HR: oxygen pulse; VO2: oxygen uptake; Y: yes.
aCould also be considered in rapidly deteriorating patients.
bFollow-up echocardiogram could be considered if symptoms persist.
cSPECT V/Q, DECT or MRI perfusion.
dLow PETCO2, high VE/VCO2, low VO2/HR and low peak VO2 on CPET or mPAP/CO slope between rest and exercise >3 mmHg/l/min on RHC.
Created with BioRender.com.
Echocardiographic probability of pulmonary hypertension.
| Peak tricuspid regurgitation velocity (m/s) | Presence of other echocardiographic PH signsa | Probability |
|---|---|---|
| ≤2.8 | − | Low |
| 2.8–3.4 | + | Intermediate |
| − | ||
| >3.4 | + | High |
| − or + |
| Other echocardiographic signs of PH | ||
|---|---|---|
| Ventricles | PA | IVC and RA |
| RV/LV basal diameter ratio >1.0 | RVOT AT <105 ms and/or mid-systolic notching | IVC diameter 21 mm and decreased inspiratory collapse |
| LV eccentricity index >1.1 | Early diastolic pulmonary regurgitation velocity >2.2 m/s | |
| TAPSE/PASP ratio <0.55 mm/mmHg | PA diameter >25 mm or >AR diameter | RA area (end-systole) >18 cm2 |
AR: aortic root; IVC: inferior vena cava; LV: left ventricular; PA: pulmonary artery; PASP: pulmonary arterial systolic pressure; PH: pulmonary hypertension; RA: right atrial; RV: right ventricular; RVOT AT: right ventricular outflow tract acceleration time; TAPSE: tricuspid annular plane systolic excursion.
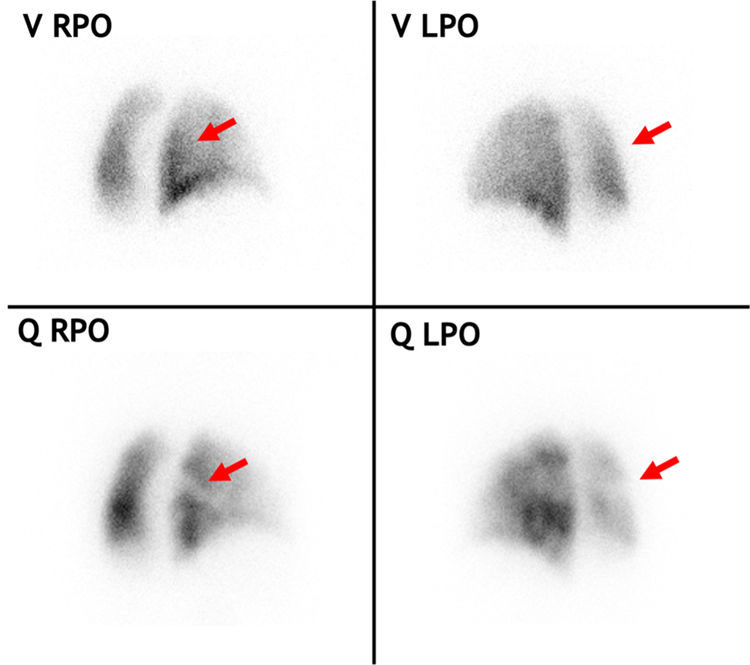
Planar V/Q scan in a chronic thromboembolic pulmonary hypertension patient with multiple perfusion defects. The red arrows highlight a ventilation/perfusion mismatch in the superior segment of the right lower lobe, seen from two different angles. LPO: left posterior oblique; Q: perfusion, RPO: right posterior oblique, V: ventilation.
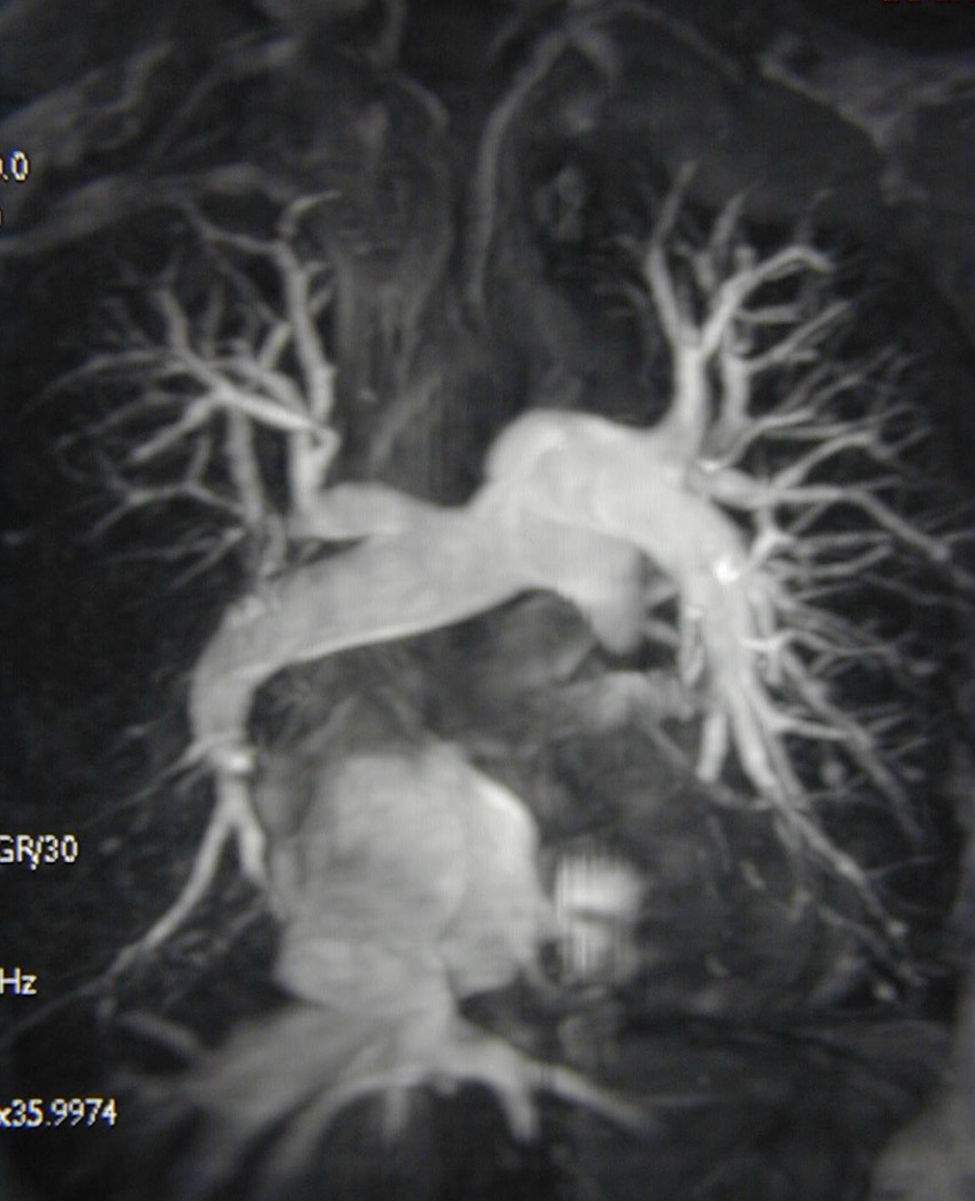
Magnetic resonance imaging angiogram demonstrating chronic thromboembolic pulmonary hypertension in an operable distribution, affecting the right middle and lower lobes as well as the left lower lobe. There is also evidence of tricuspid regurgitation with retrograde flow of contrast into the hepatic veins.
The presence of intermediate or high echocardiographic probability of PH and mismatch perfusion defects on imaging should prompt referral to a PH center for further assessment. In patients with perfusion defects but low echocardiographic probability of PH, additional exercise testing may be useful to detect patients with CTEPD without PH at rest, such as cardiopulmonary exercise testing (CPET) or exercise RHC,2 or a combination of the two to maximize the available information. Typical CPET findings include low end-tidal partial pressure of carbon dioxide (PETCO2), high ventilatory equivalent for carbon dioxide (VE/VCO2), low oxygen pulse (VO2/HR), and low peak oxygen uptake (VO2).2 Regarding exercise RHC, a mean PAP (mPAP)/CO slope >3 mmHg/l/min while the pulmonary artery wedge pressure/CO slope remains <2 mmHg/l/min reflects pulmonary vascular disease.2 Patients with such findings should also be assessed in a PH center.
CTPA is currently used to confirm the presence of CTEPD and to assess operability, providing detailed information on endovascular thrombi, vascular wall thickness, and the presence of bands/webs, stenosis and bronchial artery collateral circulation2,48 (Figure 5). CTPA also enables differential diagnosis of PH, as it can detect underlying parenchymal lung or mediastinal disease and other causes of vascular occlusion (PA sarcoma, arteritis, in-situ thrombosis or congenital anomalies).48 Technological advances have improved the diagnostic performance of this exam. A recent meta-analysis of 10 studies demonstrated high sensitivity (98%) and specificity (99%) for CTPA in the detection of CTEPD on a vessel-based analysis.49 However, these exams were assessed by expert chest radiologists, and CTPA still has lower sensitivity for detecting CTEPD in segmental and subsegmental vessels.48 Therefore, a negative CTPA does not exclude CTEPD, and digital subtraction angiography may be necessary to fully assess treatment options.2 Novel techniques such as cone-beam CT and area-detector CT are being increasingly used, as they provide better visualization of segmental and subsegmental disease and are useful for guidance of BPA therapy,50–52 but they still require validation in prospective trials.2
Diagnosis of CTEPH requires confirmation of precapillary PH through RHC, defined by mPAP >20 mmHg, pulmonary capillary wedge pressure (PCWP) ≤15 mmHg and PVR >2 Wood units.2 It is important to emphasize that PCWP may not always be reliable in CTEPH, due to occlusion of PA branches and consequent difficulty in wedging the Swan-Ganz catheter balloon tip. In such cases, measurement of left ventricular end-diastolic pressure is warranted.2 The pulmonary artery occlusion technique may also be used to partition PVR into larger arterial and small arterial plus venous components, but this has not been generally considered a part of hemodynamic assessment.1 Because there are no direct imaging methods to quantify microvasculopathy, it is nowadays subjectively assessed by correlation of perfusion defects with hemodynamic parameters, despite its lack of accuracy.1
When symptoms are clearly attributable to obstruction of intravascular pulmonary arteries but there is no PH at rest, such patients may be considered to have exercise-induced PH, which is defined by mPAP/CO slope between rest and exercise >3 mmHg/l/min, assessed by exercise RHC.2
TreatmentAnticoagulationLifelong anticoagulation is recommended in patients with CTEPH.2 Although there have been few randomized clinical trials, vitamin K antagonists (VKAs) have been the most used drug class in this setting, with a target international normalized ratio of 2–3.2 Direct oral anticoagulants (DOACs) are increasingly used; while some retrospective studies have indicated that DOACs may be more associated with the presence of acute or subacute thrombi at the time of PEA surgery or with a higher rate of venous thromboembolism recurrence, bleeding rates and survival appear to be similar to VKAs.53,54 Regarding CTEPD without PH at rest, the most recent guidelines recommend long-term anticoagulation based on individualized decision-making, as there is no evidence in favor or against.2
Mechanical treatmentIn view of the pathophysiology of CTEPH, two mechanical treatment options targeting fibrothrombotic vessel obstructions are available: PEA and BPA.1
Pulmonary endarterectomyPEA is the recommended treatment in patients with operable CTEPH. Its main objective is to remove all macroscopic obstructive material in order to reduce PVR and improve V/Q mismatch.1 PEA requires cardiopulmonary bypass and uses deep hypothermic circulatory arrest to reduce metabolic rate and protect vital organs during the absence of perfusion.1 Cooling to 20°C allows safe arrest periods of up to 20 min, which is usually enough for disease clearance on one side.1
Historically, PEA was associated with high inpatient mortality and morbidity, with the first reviews reporting mortality rates of 22%.55 Fragata and Telles56 published the initial experience with 19 PEA procedures performed at a single-center in Portugal, with a postoperative mortality rate reflecting the initial phase of the learning curve. However, this experience paved the way for future PEA practices in the country. As the surgical technique has been increasingly used, early (30-day) mortality has significantly improved, with high-volume centers reporting rates as low as 0.8%, according to a recent systematic review.57 Mortality is inversely related to the number of PEA procedures performed, and some centers report lower mortality rates over the years with increasing numbers of procedures.57–60 PEA also significantly improves right heart and pulmonary vascular hemodynamics, functional capacity and mid- to long-term survival, with reported five- and 10-year rates of 50–89.2% and 62–86.1%, respectively.57 One of the studies included in the aforementioned review also reported a 15-year survival of around 91% in patients with proximal disease, as compared with 29.6% in those with residual PH.61 Patients with operable disease who do not undergo surgery have worse outcomes, with five-year survival of 53% (vs. 83% in the PEA group), according to an observational study.62
Operability should be assessed in expert CTEPH centers by a multidisciplinary team, including an experienced PEA surgeon and a chest radiologist.2 Correlation between vascular disease on imaging and severity of PVR does not seem to be a reliable parameter to determine operability. Although a proportionate PVR may indicate absence of significant microvasculopathy, imaging tends to underestimate the extent of disease, since chronic thromboembolic material retracts the lumen of the artery and small caliber branches enlarge after PEA.63 Therefore, there is no upper limit of PVR beyond which PEA is contraindicated.63 In fact, even though patients with PVR values >12.5 Wood units present with higher mortality risk, they still benefit from PEA, as long as complete resection of thromboembolic material and significant postoperative PVR improvement are achieved.63,64
Technical operability depends on the surgeon's experience and the anatomical location of the disease.2,48 The intraoperative classification of CTEPH, revised in 2016, is based on the most central component of the disease – level 0 (no surgical evidence of disease), level I (one of the main PAs) and IC (complete obstruction of one of the main PAs), level II (lobar branches), level III (segmental branches) and level IV (subsegmental branches).65 Appropriately trained PEA surgeons should be able to operate on levels I, II and III (proximal); however, levels III (distal) and IV are more dependent on the surgeon's and center's experience.48 Criteria defining the level of expertise have been proposed: surgical mortality <5% (level I); as for level I plus number of PEA procedures/year ≥50 (level II); and as for level II plus ability to operate on distal disease plus ability to provide PEA, BPA and medical therapy (level III).66 Current recommendations also state that there should be one expert center performing PEA per 40–50 million population.66 The rationale for this is that high-volume centers report lower levels of mortality and have more experience in operating on distal disease; therefore, operability assessment may be different than in low-volume centers, which report higher percentages of inoperable patients, possibly due to lower levels of expertise.7,48 In these cases, a second opinion may be necessary.
Although all patients with technically operable disease should be offered PEA, some conditions or comorbidities may limit the benefit of surgery (such as in patients with malignancy and limited lifespan). Attempting to restore perfusion in patients with severe parenchymal lung disease and ventilation compromise may not improve symptoms and can increase the risk of respiratory failure.65 Likewise, patients with left ventricular systolic dysfunction may not be able to cope with increased RV cardiac output after PEA.65 Other conditions which increase operative risk, such as absence of history of PE or deep vein thrombosis, right heart failure, World Health Organization (WHO) functional class IV, inconsistency on imaging modalities, absence of lower lobe disease and higher diastolic PAP, should be considered by the multidisciplinary CTEPH team.48 It is also important to emphasize that older age is not a contraindication for PEA, as there are studies demonstrating benefit in this group of patients.67,68
Complications may arise in the postoperative period, such as persistent PH with difficulty in weaning from cardiopulmonary bypass, airway hemorrhage or reperfusion pulmonary edema.63 The latter usually develops within 72 h of PEA and its management is generally supportive (oxygen, positive pressure ventilation and diuretics), with improvement in a few days.1 In more severe cases, venovenous extracorporeal membrane oxygenation (ECMO) may be necessary as a bridge to recovery. In a single-center study, increasing ECMO utilization proved useful in patients with severe CTEPH (PVR >12.5 Wood units), with a significant decrease in mortality.59
Residual PH is frequent after PEA and is associated with worsened survival and quality of life.57 Although there are no specific criteria to define residual PH, previous meta-analyses report incidences of 20–25%.57,69 Cannon et al. demonstrated that mPAP ≥38 mmHg and PVR ≥5.3 Wood units correlated with worse long-term survival, and mPAP ≥30 mmHg was associated with initiation of vasodilator drugs, presumably because of clinical worsening.70 There are currently several treatment options for residual PH, including redo PEA, which may be considered when proximal disease remains substantial.71 These options should always be discussed in a multidisciplinary team, particularly when there is segmental and/or subsegmental disease.1 Mortality in patients with residual PH is substantially higher, with reported figures ranging from 7.7% to 40%.72
Patients with CTEPD without PH at rest may benefit from PEA after careful consideration of the risk/benefit ratio. Two studies have reported clinical and/or hemodynamic improvement after PEA.73,74
Balloon pulmonary angioplastyBPA is a catheter-based intervention in which a balloon is inflated to dilate branches of the PA and restore blood flow. There are several types of lesions which correlate with angioplasty success rates and complications: ring-like stenoses (type A), web lesions (type B), subtotal occlusions (type C), total occlusions (type D), and tortuous lesions (type E).75 BPA leads to rupture of these fibrotic lesions, with subsequent increase in antegrade flow and improvements in lung perfusion and RV afterload.1
The use of BPA was first reported in 18 patients in 2001; however, due to the development of reperfusion edema in 11 of them, with three requiring mechanical ventilation, the technique was abandoned.76 Over the following decade, Japanese interventionalists refined the procedure and presented promising results: a multicenter registry with a total of 308 patients revealed improvement of hemodynamic parameters, significant reduction of concomitant PH drug therapy and oxygen, and overall survival of 96.8% at one and two years and 94.5% at three years.77 BPA also improves RV function, symptoms, and functional capacity, as has been demonstrated in several other centers.78–83 In the most recent European guidelines, it is currently recommended for treatment of selected patients with inoperable CTEPH or persistent/recurrent PH after PEA.2 This selection should be carried out by multidisciplinary teams in expert centers.
Treatment goals are not universally agreed. According to a recent European consensus document on BPA, patients should ideally experience symptom relief at rest and during exercise, with treatment of all lesions if possible.84 Further goals should include improvement in quality of life, hemodynamics, and gas exchange. The minimum hemodynamic goal of BPA is a final mPAP <30 mmHg, due to its prognostic significance.
BPA is associated with other complications, such as vascular injury due to wire perforation and lung injury with hemoptysis and/or hypoxia.2 Growing operator experience has led to a lower level of complications and periprocedural mortality, which was recently reported as 0.2%.85 Nowadays, BPA is performed in multiple sessions in order to reduce the risk of reperfusion injury.1 Therefore, BPA should be performed in high-volume CTEPH centers.2
Besides severe contrast allergy, there are no absolute contraindications to BPA.1 However, tortuous lesions constitute a lesion-based relative contraindication, due to their complexity and higher risk of complications.1
Regarding CTEPD without PH, selected symptomatic patients may benefit from BPA, with clinical and hemodynamic improvement.2,86
Drugs used to treat pulmonary hypertensionThe theoretical target of PH drugs in CTEPH is precapillary microvasculopathy, similar to that observed in PAH. Several pulmonary vasodilators have been used in CTEPH, particularly in persistent/recurrent cases after PEA or in inoperable cases. The current European guidelines2 recommend riociguat in such cases (class I, level B recommendation) and consideration of high-dose subcutaneous treprostinil in patients who remain in WHO functional class III–IV (class IIb, level B recommendation).2 This is based on the results of the CHEST and CTREPH trials, respectively, which demonstrated improvement in 6-min walk test (6MWT) distance, hemodynamic parameters, functional class and N-terminal pro-brain natriuretic peptide levels.37,38,40 These results led to approval of the drugs for CTEPH in the European Union.
Other drugs such as bosentan and sildenafil are used off-label, as they lack proven efficacy.87,88 A recent small Japanese trial also demonstrated the benefit of selexipag in CTEPH patients regarding hemodynamic parameters.89 However, a more recent study with the same drug was terminated early due to lack of efficacy at interim analysis (ClinicalTrials.gov identifier: NCT03689244). Table 3 summarizes the completed trials of medical therapy in CTEPH.
Randomized controlled trials of medical therapy in chronic thromboembolic pulmonary hypertension.
| Study | Authors (year) | Drug | No. of patients | Follow-up (weeks) | Primary outcome | Relevant secondary outcomes |
|---|---|---|---|---|---|---|
| Sildenafil88 | Suntharalingam et al. (2008) | Sildenafil (vs. placebo) | 19 | 12 | 6MWD (+17.9 m, p=0.385) | Improved PVR and FC |
| BENEFiT87 | Jaïs et al. (2008) | Bosentan (vs. placebo) | 157 | 16 | 6MWD (+2.9 m, p=0.054)PVR (−146 dyn·s·cm−5, p<0.0001) | No difference in FC or TTCW |
| CHEST-137 | Ghofrani et al. (2013) | Riociguat (vs. placebo) | 261 | 16 | 6MWD (+39 m, p<0.001) | Improved PVR, FC, mPAP and NT-proBNP |
| MERIT-142 | Ghofrani et al. (2017) | Macitentan (vs. placebo) | 80 | 16 | PVR (−206 dyn·s·cm−5, p=0.041) | Improved 6MWD, CO, and NT-proBNP |
| CTREPH40 | Sadushi-Kolici et al. (2019) | SC treprostinil (30 ng/kg/min vs. 3 ng/kg/min) | 105 | 24 | 6MWD (+45 m, p=0.00028) | Improved PVR, FC, mPAP, cardiac output and NT-proBNP |
| Selexipag89 | Ogo et al. (2022) | Selexipag (vs. placebo) | 78 | 20 | PVR (−98.2 dyn·s·cm−5, p=0.006) | Improved CI and Borg dyspnea score. No difference in 6MWD or FC |
CI: cardiac index; CO: cardiac output; FC: functional class; mPAP: mean pulmonary artery pressure; NT-proBNP: N-terminal pro-brain natriuretic peptide; PVR: pulmonary vascular resistance; SC: subcutaneous; TTCW: time to clinical worsening; 6MWD: 6-min walk distance.
Given the advances in the various treatment options available, a multimodality strategy may be adopted to fully cover the whole spectrum of vascular lesions in CTEPH patients. Again, as there are no standardized guidelines for patient selection, this should be done case by case in expert centers by a multidisciplinary team consisting of a PEA surgeon, a BPA expert, a PH specialist and a thoracic radiologist.3 A proposed management algorithm with all three modalities is shown in Figure 9.
Multimodal management of patients with chronic thromboembolic pulmonary hypertension. BPA: balloon pulmonary angioplasty; CTEPD: chronic thromboembolic pulmonary disease; CTEPH: chronic thromboembolic pulmonary hypertension; PEA: pulmonary endarterectomy; PH: pulmonary hypertension. aSome patients with CTEPD without PH at rest might benefit from PEA and/or BPA.
Created with BioRender.com.
As patients can have both operable and inoperable lesions, combining PEA and BPA may be required. One study reported favorable outcomes in patients with accessible lesions in one lung and inoperable lesions in the other, with improvement in PVR and WHO functional class at mid-term follow-up.90 BPA could also be beneficial in residual PH after PEA, with reports of hemodynamic and functional class improvement.91,92
The role of medical therapy in persistent/recurrent PH following PEA has been detailed previously in this article. The use of vasodilators as bridging therapy in patients with preoperative PVR is more controversial. Most studies in the literature report hemodynamic improvement prior to PEA, but no additional benefit after it.3,63 In the International CTEPH registry, bridging therapy was associated with higher mortality, possibly due to delaying surgery.93 However, a more recent retrospective study demonstrated better one-year survival in patients previously treated with vasodilators.94 More trials are needed to further clarify this issue.
Concomitant use of BPA and medical therapy has been commonly applied, since the latter reduces pre-BPA mPAP. Feinstein et al. demonstrated a correlation between development of reperfusion injury and pre-BPA mPAP >35 mmHg.76 Additionally, patients who are started on vasodilators may benefit from symptom improvement between BPA sessions.63 The results of the recently published RACE trial comparing riociguat with BPA in newly diagnosed inoperable CTEPH highlight the potential benefits of multimodality treatment.95 In that study, patients were randomized to either BPA or riociguat and had the option of crossover at 26 weeks if predefined goals were not met. Although patients first treated by BPA had a greater reduction in PVR, they experienced a higher rate of treatment-related adverse events than patients who were first treated with riociguat. The current guidelines accordingly recommend PH therapy before BPA when PVR is >4 Wood units, as this has demonstrated additive benefits and improved safety.95
Lung transplantationGiven the various treatment options for CTEPH, lung transplantation is now rarely considered in this patient group.3 It could be considered in highly selected cases, as an alternative to failed PEA or inoperable disease not amenable to other medical or surgical treatment; however, data on the outcomes are scarce and some studies report high morbidity and mortality.96
RehabilitationThe role of rehabilitation in CTEPH and PAH is well established, although exercise physiology is slightly different for the two conditions.97 Most of the pivotal rehabilitation trials were based on in-hospital training in tertiary referral set-ups. Small non-randomized studies showed that home-based rehabilitation may also be an effective and safe option. However, exercise training in PH should be individually adjusted and closely supervised by both PH experts and physiatrists who are experienced in the rehabilitation of severely compromised patients. Patients should be on optimized treatment and in a stable clinical condition before entering a rehabilitation program.2
Another potential role for rehabilitation is in the recovery phase after PEA and BPA. To date, the potential role of rehabilitation after PEA has only been studied in a small non-randomized retrospective study,98 in which significant changes in 6MWD were observed during the three-month rehabilitation period. In a prospective randomized study,99 the authors studied the impact of rehabilitation directly after BPA, demonstrating that a combined approach of BPA and rehabilitation sessions was more successful than BPA alone. Based on these studies, a carefully monitored rehabilitation program after surgical or BPA treatment could be considered standard of care.
Larger-scale RCTs are needed to define the optimal training methodology and timing of exercise training in CTEPD.
Novel techniquesPulmonary denervation (PD) is a novel technique that has been explored in PH. Regarding CTEPH, one study comparing PD with riociguat in patients with residual PH after PEA showed substantial reduction of PVR and improvement of 6MWT distance.100
Follow-upCurrent European guidelines suggest regular follow-up of CTEPH patients, including invasive assessment with RHC, 3–6 months after intervention and yearly non-invasive follow-up with echocardiography and assessment of exercise capacity, in order to rule out recurrent PH.2 Although there is no consensus on the therapeutic target in CTEPH, experts aim for WHO functional class I-II, improvement in quality of life and normalization or near normalization of hemodynamics at rest (assessed by RHC at 3–6 months after intervention).2
ConclusionCTEPD/CTEPH is a complex disease that combines unresolved vascular occlusions and microvasculopathy. Recent advances in the field have led to better diagnostic performance, as well as development and improvement of treatment modalities that complement each other and improve patients’ survival and quality of life. These patients should be managed in expert centers in a multidisciplinary fashion. Future studies are still needed to further clarify the gaps in evidence.
Conflicts of interestThe authors have no conflicts of interest to declare.