Chest pain is a frequent symptom in patients with pulmonary arterial hypertension (PAH). Left main coronary artery (LMCA) extrinsic compression from a pulmonary artery (PA) is an increasingly recognized cause of angina or complications, such as acute myocardial infarction, left ventricular dysfunction, arrythmia, and sudden death.
We report the case of a 45-year-old patient with pre-capillary pulmonary hypertension (PH), a patent ductus arteriosus corrected surgically during adolescence, and chronic constrictive bronchiolitis. In 2016, the patient began to report oppressive chest pain and worsening fatigue. Computed tomography coronary angiography (CTCA) showed extrinsic LMCA compression by a dilated PA, which was confirmed by invasive coronary angiography and intravascular ultrasound. After stent implantation, the patient reported symptom resolution, and has been asymptomatic ever since.
Imaging studies, in particular CTCA, play an important role in the diagnosis of LMCA compression in patients with PAH. The reported case supports the efficacy and safety of stent implantation as a therapeutic option, as already demonstrated in the literature. It shows the complexity of decision making on the operability of systemic-to-pulmonary shunts and reinforces the importance of continuous diagnostic testing.
A dor torácica é um sintoma frequente em doentes com hipertensão arterial pulmonar (HAP), sendo a compressão extrínseca do tronco comum da coronária esquerda (TC-CE) pela artéria pulmonar (AP) uma causa cada vez mais frequentemente associada a angina ou a complicações como enfarte do miocárdio, disfunção ventricular, arritmias e morte súbita.
Apresenta-se o caso de uma doente de 45 anos com hipertensão pulmonar pré-capilar e antecedentes de encerramento de canal arterial e bronquiolite constritiva crónica. Em 2016 refere episódios de precordialgia opressiva e agravamento das queixas de cansaço. A angiotomografia computorizada (angio-TC) do tórax revelou compressão extrínseca do TC-CE por dilatação da AP, com confirmação por coronariografia invasiva e ecografia intracoronária. Após angioplastia com colocação de stent a doente refere resolução sintomática que se mantém até à atualidade.
O estudo imagiológico, com destaque para a angio-TC, desempenha um papel fulcral no diagnóstico de dor torácica por compressão do TC-CE em doentes com HAP. O caso apresentado corrobora a eficácia e segurança da angioplastia coronária com stent como opção terapêutica já demonstrada na literatura previamente publicada, ilustra a complexidade da tomada de decisão sobre a operabilidade dos shunts sistémico-pulmonares e reforça a importância da investigação diagnóstica contínua, inerente ao dinamismo dos processos fisiopatológicos, e a escolha apropriada das estratégias terapêuticas entre a multiplicidade de opções disponíveis.
Pulmonary hypertension (PH) is a hemodynamic condition defined by a mean pulmonary arterial pressure (PAP) ≥20 mmHg and is one of a number of clinical conditions, including pulmonary arterial hypertension (PAH).1 Despite the most recent advances in diagnosis, risk stratification and therapy, morbidity and mortality are still high in patients with PAH.2 Increased pulmonary vascular resistance (PVR), secondary to progressive remodeling of the distal small arteries, inevitably leads to right heart failure (HF) and mortality. However, early diagnosis is in most cases hampered by the non-specificity of symptoms, including dyspnea, fatigue, and chest pain.
Chest pain is a relatively frequent symptom in patients with PAH, present in about 7 to 29% of cases.3–6 Angina-type pain has classically been attributed to right ventricular ischemia, a consequence of the increased metabolic needs of a hypertrophied and overloaded right ventricle, or pulmonary artery (PA) distension,7,8 rather than atherosclerotic coronary artery disease. However, extrinsic compression of the left main coronary artery (LMCA) from a dilated PA in PAH has been increasingly associated with angina9 or other complications, such as myocardial infarction, left ventricular dysfunction,10 arrhythmias and even sudden death.7
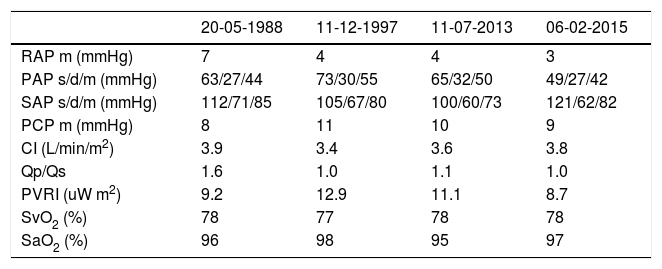
Clinical caseWe present the case of a 45-year-old female patient with a history of patent ductus arteriosus (PDA) closure in adolescence and chronic constrictive bronchiolitis, probably related to recurrent respiratory infections since childhood. Diagnostic catheterization, dated 1988 and performed following investigation of complaints of fatigue and exertional dyspnea, revealed the presence of PDA with left-right unidirectional shunt (Qp/Qs=1.6) and PH with pre-capillary hemodynamic phenotype (Table 1), with a PVR of 6.9 uW (9.2 uW m2); the systemic cardiac index was normal. The patient underwent PDA ligation that same year, which was uneventful, leading to symptom resolution at clinical follow-up. Approximately eight years after the procedure, the patient reported recurrence of progressive fatigue and dyspnea complaints (World Health Organization (WHO) functional class II). The echocardiogram revealed there was a high probability of PH. A year later, in 1997, hemodynamic and angiographic re-evaluation was performed, which documented persistence/recurrence of precapillary PH (Table 1), with normal cardiac output and a residual shunt of minor PDA (2 mm). This was not, however, hemodynamically significant. At this time, diltiazem 240 mg/day was introduced without clinical improvement. It was discontinued about one year after initiation. As the complaints of fatigue persisted, inhaled iloprost 50 μg 6x/day was started in December 1999. However, the patient discontinued the medication due to perceived therapeutic ineffectiveness and her inability to reconcile the time and frequency of administration with her social and professional activities.
Right catherizations performed.
| 20-05-1988 | 11-12-1997 | 11-07-2013 | 06-02-2015 | |
|---|---|---|---|---|
| RAP m (mmHg) | 7 | 4 | 4 | 3 |
| PAP s/d/m (mmHg) | 63/27/44 | 73/30/55 | 65/32/50 | 49/27/42 |
| SAP s/d/m (mmHg) | 112/71/85 | 105/67/80 | 100/60/73 | 121/62/82 |
| PCP m (mmHg) | 8 | 11 | 10 | 9 |
| CI (L/min/m2) | 3.9 | 3.4 | 3.6 | 3.8 |
| Qp/Qs | 1.6 | 1.0 | 1.1 | 1.0 |
| PVRI (uW m2) | 9.2 | 12.9 | 11.1 | 8.7 |
| SvO2 (%) | 78 | 77 | 78 | 78 |
| SaO2 (%) | 96 | 98 | 95 | 97 |
CI: cardiac index; PAP s/d/m: pulmonary artery pressure, systolic, diastolic, mean; PCP m: pulmonary capillary pressure mean; PVRI: pulmonary vascular resistance index; Qp/Qs: ratio between pulmonary output and systemic output; RAP: right atrial pressure; SaO2: arterial oxygen saturation; SAP: systemic arterial pressure; SvO2: mixed venous oxygen saturation; uW: Wood units.
In 2013, the patient began to be followed at the Portuguese national treatment center for PH (until then, she had been followed in Switzerland), reporting no clinical complications. In July 2013, hemodynamic re-evaluation was performed via right heart catheterization (Table 1), which showed globally similar results to those of 1997. Therapy with sildenafil 25 mg 3x/day and ambrisentan 10 mg 1x/day was initiated, with an adequate clinical and hemodynamic response (Table 1).
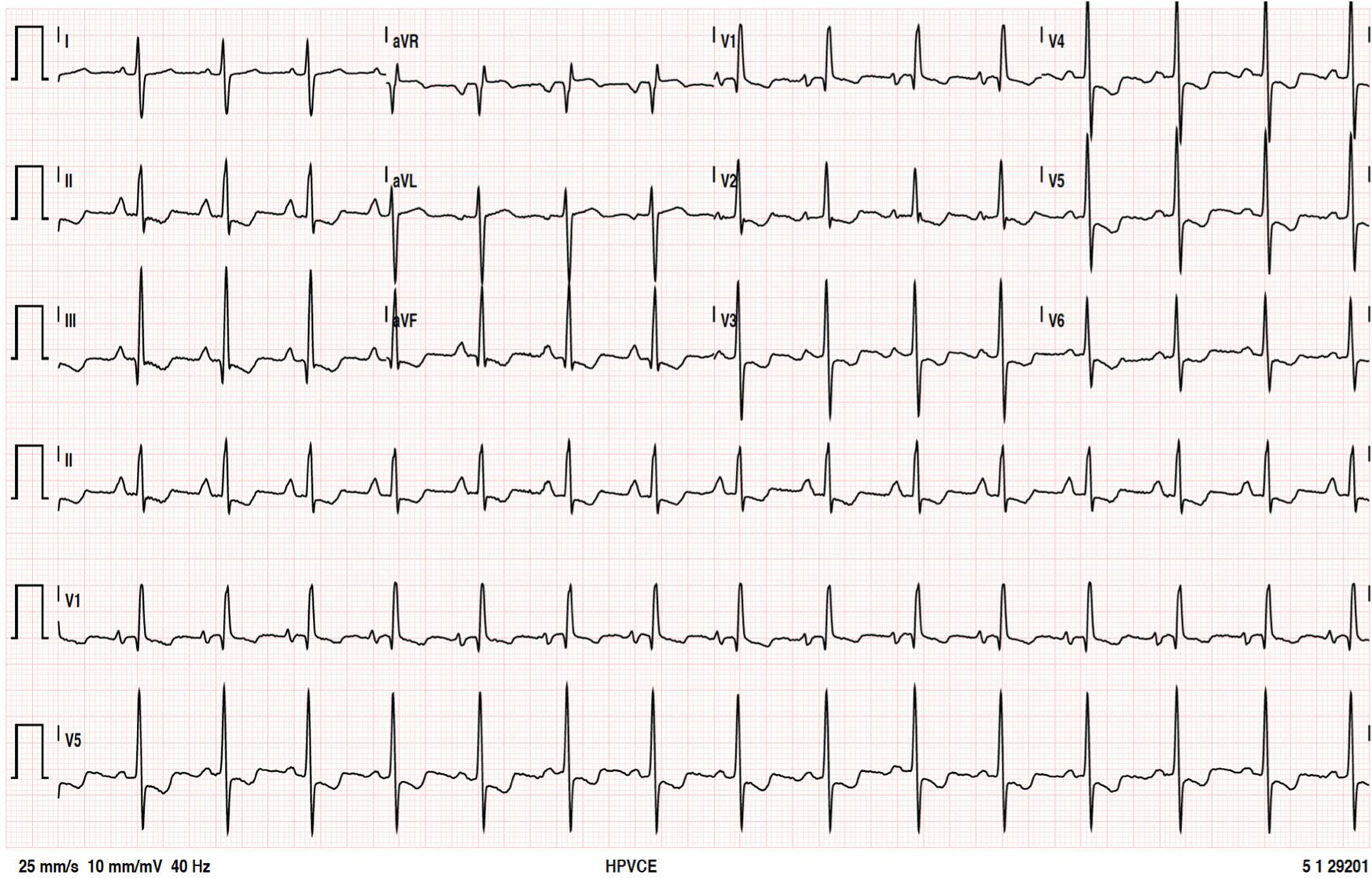
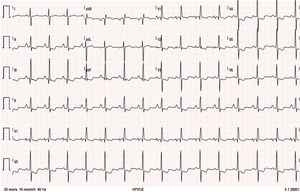
The patient remained clinically stable, in WHO functional class II, until June 2016, when she reported episodes of oppressive chest pain with sub-mandibular irradiation, precipitated by moderate exertion (e.g., climbing a flight of stairs) with concomitant worsening of fatigue, in the absence of other symptoms or signs of HF; she denied palpitations, presyncope, or syncope. The 12-lead electrocardiogram (Figure 1) at rest showed no changes compared to previous tests. The presence of sinus rhythm, pulmonary P wave, right deviation of the electrical axis of the QRS in the frontal plane and clear criteria for right ventricular overload are of note.
Twelve-lead electrocardiogram. Sinus rhythm, HR=90 bpm; P pulmoale wave; normal atrioventricular and intraventricular conduction; right electrical axis of the QRS in the frontal plane; anticlockwise deviation of the electrical axis of the QRS in the axial plane together with other criteria for right ventricular overload.
The chest teleradiography revealed a slight increase in the cardiothoracic index at the expense of the right heart chambers, increased pulmonary artery caliber, slight peripheral oligohemia, and absence of relevant pleuroparenchymal changes.
The transthoracic echocardiogram (overlapping with previous studies) showed a slight increase in the dimensions of the right chambers, marked ectasia of the main pulmonary artery, and normal biventricular systolic function, absence of valve disease or pericardial effusion. Systolic pressure in the pulmonary artery was estimated at 67 mmHg.
The hemogram and overall biochemical profile were normal. Of note was the absence of N-terminal pro-B-type natriuretic peptide elevation (87 ng/mL). Pulmonary function tests showed ventilatory mechanics with an obstructive pattern: forced vital capacity: 1.97 L/74.4%; forced expiratory volume: 1.18 L/52.4%; Tiffeneau index: 60.1%; total lung capacity: 3.4 L/84.1%; residual volume: 1.26 L/93.4% - with no response to a bronchodilator. The presence of reduced alveolar-capillary carbon monoxide transfer capacity should be noted: 51% (58% after correction for alveolar volume).
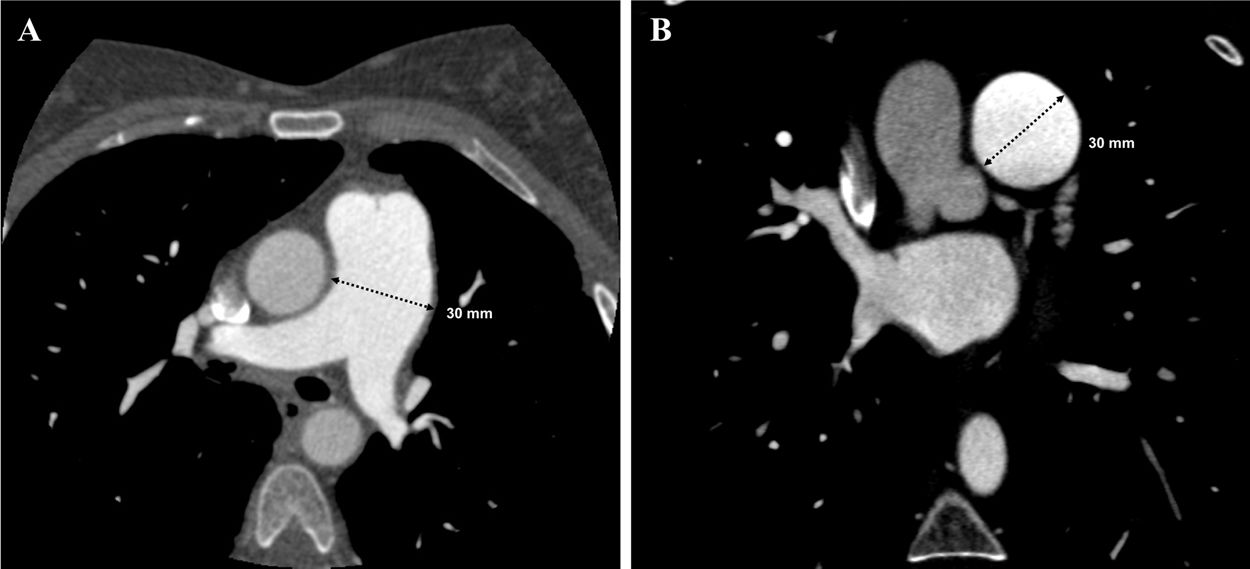
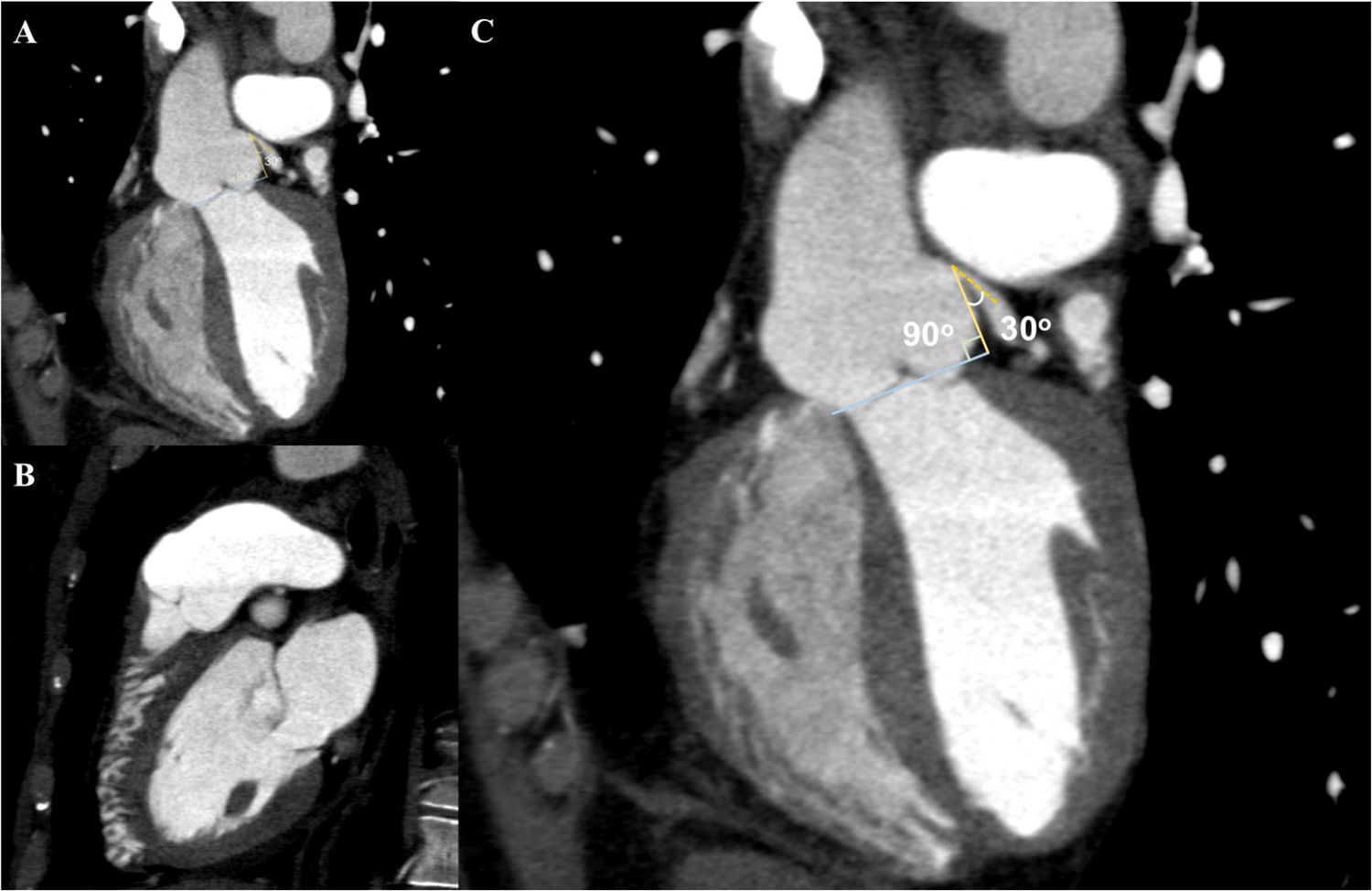
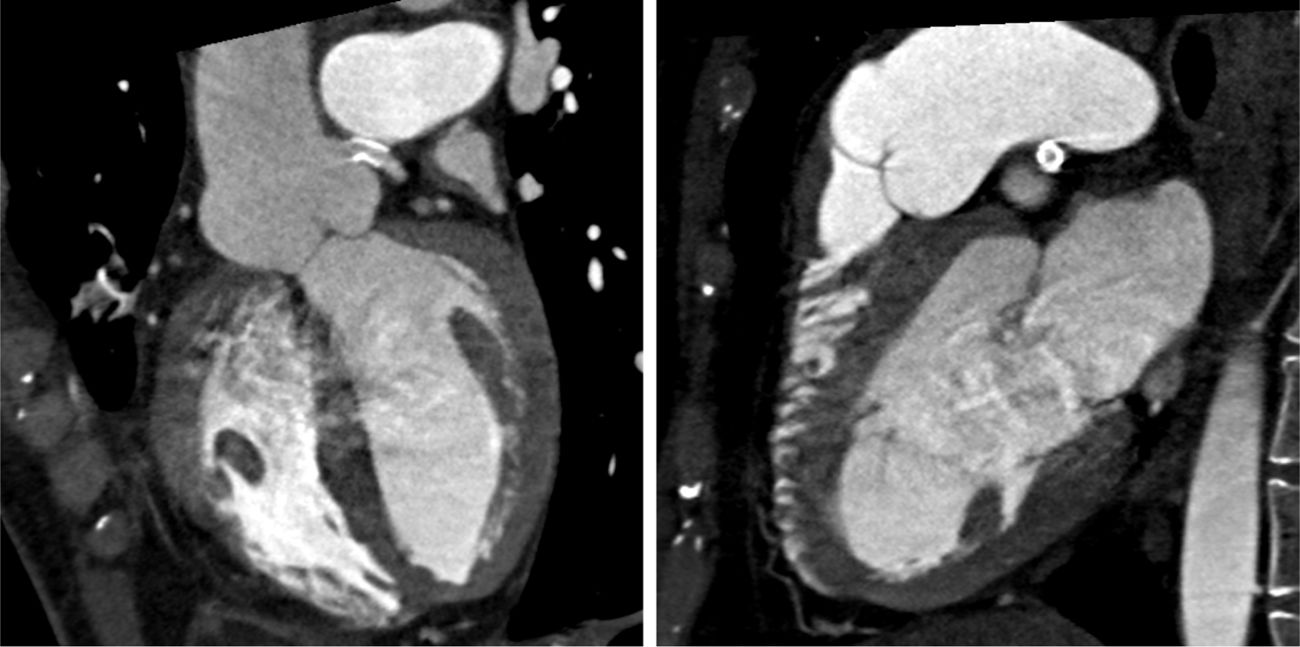
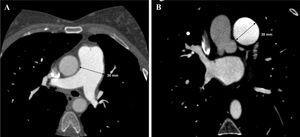
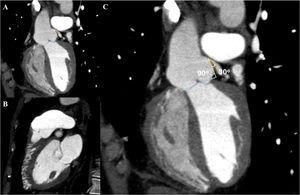
In view of the clinical context – angina in a young woman diagnosed with PAH – we decided to perform a computed tomography coronary angiogram (CTCA). It documented the presence of extrinsic compression of the proximal third of the LMCA due to dilation of the main PA (30 mm) (Figure 2), revealing a LMCA take-off angle in relation to the left aortic sinus of 30° (Figure 3). Left coronary dominance and the absence of other changes in coronary permeability, such as the absence of coronary artery atherosclerotic disease are of note. The main pulmonary arteries and segmental and subsegmental branches presented increased dimensions and preserved permeability. No passage of contrast was observed at the level of the ductus arteriosus. The CTCA also revealed the presence of malacia of the left main, left lower lobe and intermediate bronchi. In the forced expiratory flow test, extensive areas of air retention were observed (left upper lobe, apical segment of the left lower lobe, right lower lobe, middle lobe and subsegmental in the right upper lobe), which were not altered by a bronchodilator.
Computerized tomography coronary angiogram. (A/B) Orthogonal projection images, demonstrated compression of the proximal section of the LMCA from a dilated PA, with major stenosis. (C) A close-up of panel (A), which shows the reduction in the take-off angle (30°) of the LCMA in relation to the aortic sinus, secondary to compression by the PA.
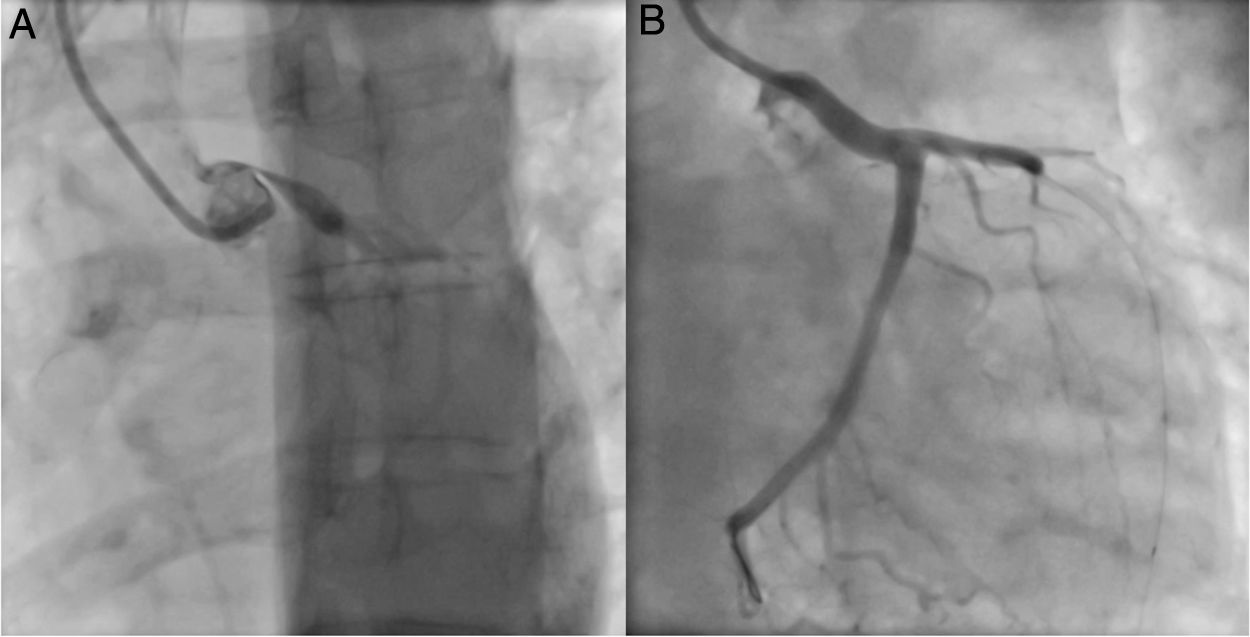
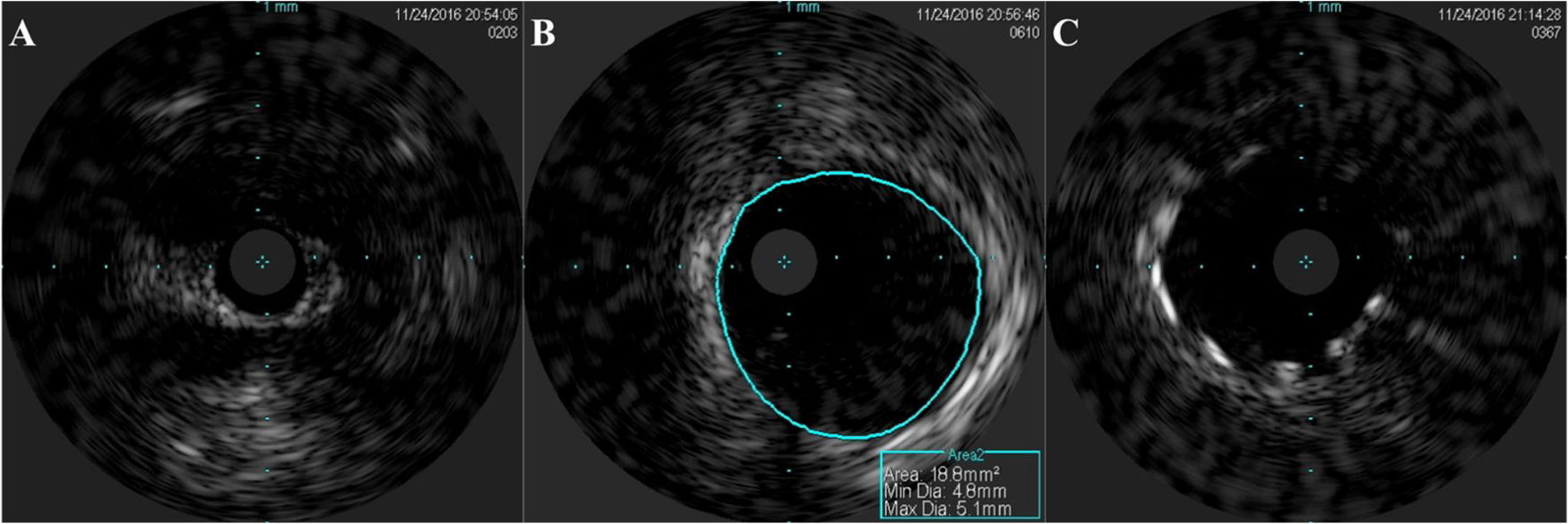
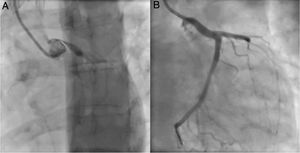
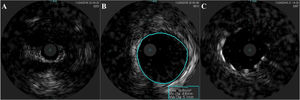
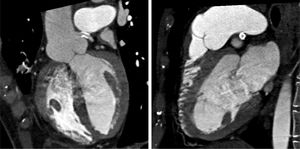
An invasive coronary angiography was performed, which confirmed the extrinsic compression of the LMCA due to dilation of the main PA (Figure 4a). An intravascular ultrasound (IVUS) was carried out (Figure 5b) to visualize the LMCA dimensions. Angioplasty was performed, placing a 4 mm×9 mm sirolimus-coated stent in the proximal and middle segments of the LMCA. The procedure was uneventful, with excellent angiographic results, documented by CTCA and IVUS (Figures 4b and 5c). The patient reported resolution of angina symptoms and relief from fatigue. Approximately one year after the procedure, despite the absence of symptoms, we decided to perform a CTCA of the coronary arteries, essentially to reassess the patency of the previously implanted stent. Normal positioning and normal conformability were observed (Figure 6), and additional compression-associated changes were ruled out. Approximately three years after coronary intervention, the patient remains clinically stable, with no recurrence of complaints of chest pain.
This clinical case illustrates the complexity of the approach required for a patient with PH, highlighting the essential role of continuous diagnostic tests, inherent to the dynamism of pathophysiological processes, and the appropriate choice of therapeutic strategies from among the multitude of available options. It leads us naturally to a discussion of the main topic – thoracic outlet syndrome – but also highlights other major topics, such as multifactorial PH and the “operability” of congenital heart defects in the context of pulmonary vascular disease.
Left main coronary artery compression due to PA dilatation was first reported in 1957.11 Since then, several clinical cases, predominantly single ones, have been published.
A recent prospective study9 of 765 patients with PAH aimed to assess the prevalence of LMCA compression from a dilated PA. It concluded that prevalence was at least 6% in this population, increasing to 40% in patients with angina or angina-like symptoms. Prevalence proved to be higher than expected based on previously published observational studies.
The mechanism of LMCA compression by the PA is strictly related to PA dilation, highlighting the primary role of multimodality imaging, in particular CTCA. Some papers9,12 have identified PA dilatation ≥40 mm as an independent predictor of significant LMCA compression, especially in patients with angina. However, with reference to present clinical case, in which the PA diameter was ≤40 mm, it should be noted that despite the importance of vessel caliber, other parameters should be considered. In the study by Galiè et al., systematic evaluation by CTCA9 was used in the diagnosis and evaluation of the efficacy and safety of coronary angioplasty with stenting in patients with PAH and LMCA compression from a dilated PA. The authors grouped patients with PAH and angina (or angina-like) symptoms into four groups based on the anatomical and spatial relationship of the LMCA and PA: (1) normal distance: minimal distance >1 mm; (2) contiguity: distance ≤1 mm without dislocation; (3) dislocation: deformity of the LMCA due to dilated PA with a take-off angle of the LMCA in relation to the left aortic sinus <60°, with or without significant lumen compression; (4) significant stenosis: stenosis ≥50% of the LMCA due to compression from PA. There was a high prevalence of stenosis ≥50% confirmed by CTCA in the last two patient groups – 30.6% and 91.4%, respectively.
It should be noted that the use of imaging methods for assessment of myocardial perfusion does not seem to be useful in the diagnosis of LMCA compression from a dilated PA. This is explained by the dynamic nature of the anatomical relationship between the structures involved and conditioned by variables such as, for example, physical exercise. Additionally, the expected scenario of global myocardial ischemia in the context of LMCA compression from a PA may prevent its detection by imaging methods such as perfusion scintigraphy. Coronary functional assessment of lesions by fractional flow reserve and instantaneous wave-free ratio is standard practice in percutaneous coronary intervention for atherosclerotic disease. Recently, the first case report13 was published on the use of these techniques in the functional evaluation of LCMA compression from a PA, and further studies are needed to validate them in this context.
It is interesting to note that the mean age of patients with LMCA stenosis due to a dilated PA in published cases tends to be lower than the mean age of patients in PH records.14,15 This difference may be explained by the predominance of patients with PAH associated with congenital heart disease.9,12,16 This population, although very heterogeneous, has a higher survival rate associated with high output and higher pulmonary pressure values, which favor PA dilation.
With regard to the therapeutic approach to LMCA compression from a PA, coronary angioplasty with stenting has been the strategy used in most published cases, taking into account the risks inherent to surgical intervention under general anesthesia and extracorporeal circulation in patients with PAH.9,17,18
All data regarding percutaneous therapy for LCMA compression from a PA are based on case reports or small series. The decision on the type of stent – medicated or non-medicated – should, as in atherosclerotic coronary artery disease, consider individual patient characteristics and data regarding coronary anatomy. In contrast to the 48.9% rate of medicated stent placement in the series by Galiè et al.,9 only unmedicated stents were used in the series by Akbal et al.18 Due to the larger LCMA diameter, the benefit of the pharmacological effect of the stent is doubtful, and the durability of the radial force against extrinsic compression is crucial.
From a surgical perspective, coronary revascularization procedures with bypass and/or pulmonary artery repair can be considered. The outcome in the follow-up of the patient in this clinical case reinforces the efficacy and safety of the percutaneous therapeutic strategy demonstrated by the largest series of patients published in this group of patients.9
If we consider the patient's clinical status from the perspective of pulmonary vascular disease, based on the hemodynamic results at the time of diagnostic catheterization in 1988, the hemodynamic phenotype of precapillary PH with left-right shunt was already present. This subgroup of patients includes large intra- or extra-cardiac defects, especially post-tricuspid, in whom the severity of the pulmonary vasculopathy is not yet sufficient to lead to shunt reversal and consequent Eisenmenger syndrome. In these cases, the severity of the pulmonary vascular remodeling changes, demonstrated by PVR, determines the indication for closure of the defect.19 The “operability” of a congenital defect should be based on the probability of a favorable outcome after closure of the defect, taking into account clinical, echocardiographic and hemodynamic variables. Unfortunately, the current state of the art does not yet allow the precise selection of patients who will evolve favorably in hemodynamic terms after correction of the defect. Current recommendations17,20,21 for closing a defect are relatively conservative when there is evidence of intrinsic pulmonary vascular disease, based on the uncertainty of long-term benefit. In the present clinical case and in the light of current knowledge, closure of the ductus arteriosus would not be recommended, given the presence of high PVR (>8 Wood units/m2) at the time of diagnosis. However, there was an important interfering factor, potentially contributing to the increase in PVR, the presence of chronic lung disease, functionally and anatomically compatible with constrictive bronchiolitis.22,23 Therefore, the multifactorial etiology of PH, which also fits into group three of the clinical classification, is admissible.17 The documented airway obstructions, with air retention and alveolar hypoxia in hypoventilated areas would condition localized vasoconstriction and consequent additional hyperflow in normally ventilated areas, with increased PAP and PVR. Therefore, as the mechanisms that led to the increase in PVR are somewhat distinct, if only the pathological repercussion of the hyperflow caused by the defect in the pulmonary vasculature had been considered as a decisive factor of “operability”, the potential hemodynamic and clinical benefit resulting from its closure would have remained in doubt. Indeed, the patient has remained clinically and hemodynamically stable in the long term.
ConclusionLeft main coronary artery compression from a dilated PA should always be considered in patients with PAH and angina or angina-like symptoms. There is a direct relationship between the mechanism of compression and a dilated PA, in addition to the anatomical and spatial positioning of the PA and the LCMA. Imaging methods, particularly CTCA, therefore play an essential role in diagnosis. In the case of dynamic coronary stenosis, conventional ischemia tests, including myocardial perfusion imaging, have low diagnostic sensitivity.
Coronary angioplasty with stenting has been the preferred strategy in most published cases, given the efficacy and safety demonstrated by the largest published patient series in this patient group and also corroborated by this clinical case.
With regard to the operability of systemic-to-pulmonary shunts, current recommendations are relatively conservative when vascular structural damage with elevated PVR (>8 uW/m2) is present. However, recommendations for the closure of systemic-to-pulmonary shunts do not address multifactorial PH, especially when it results from a combination of diffuse shunt hyperflow and anatomical and functional changes in the context of chronic lung disease. Ductus arteriosus closure in the context of multifactorial PAH in the present case resulted in clinical and hemodynamic benefits over 30 years of follow-up. This outcome points to the importance of considering other etiologic factors for PAH when making a decision to intervene in cardiovascular shunts.
The present case highlights the importance of continuous diagnostic testing, inherent to the dynamism of the pathophysiological processes, and the appropriate choice of therapeutic strategies from among the multitude of available options.
Conflicts of interestThe authors have no conflicts of interest to declare.