Cardiopulmonary exercise testing (CPET) provides a noninvasive and integrated assessment of the response of the respiratory, cardiovascular, and musculoskeletal systems to exercise. This information improves the diagnosis, risk stratification, and therapeutic management of several clinical conditions. Additionally, CPET is the gold standard test for cardiorespiratory fitness quantification and exercise prescription, both in patients with cardiopulmonary disease undergoing cardiac or pulmonary rehabilitation programs and in healthy individuals, such as high-level athletes. In this setting, the relevance of practical knowledge about this exam is useful and of interest to several medical specialties other than cardiology. However, despite its multiple established advantages, CPET remains underused. This article aims to increase awareness of the value of CPET in clinical practice and to inform clinicians about its main indications, applications, and basic interpretation.
A prova de esforço cardiorrespiratória (PECR) fornece uma avaliação não invasiva e integrada das respostas ao exercício dos sistemas respiratório, cardiovascular e músculo-esquelético. Essas informações melhoram o diagnóstico, a estratificação de risco e a abordagem terapêutica de diversas condições clínicas. Além disso, a PECR é o teste gold standard para a quantificação da aptidão cardiorrespiratória e a prescrição de exercício, tanto em doentes com doença cardiopulmonar em programas de reabilitação cardíaca ou pulmonar, como em indivíduos saudáveis, incluindo atletas de alto rendimento. Neste contexto, o conhecimento prático da relevância deste exame é útil e transversal a diversas especialidades médicas para além da cardiologia. No entanto, apesar das suas múltiplas vantagens reconhecidas, a PECR continua subutilizada. Este artigo tem como objetivo aumentar a consciencialização do valor da PECR para a prática clínica e informar os médicos sobre as suas principais indicações, aplicações e interpretação básica.
Standard exercise testing remains a clinical tool with many applications in clinical practice, providing important information for patients with a wide spectrum of conditions.1 Combining this test with ventilatory gas exchange measurements provides incremental information, leading to more accurate quantification of cardiorespiratory fitness (CRF) and to the identification of exercise-limiting pathophysiological mechanisms, both of which are highly useful in clinical practice for cardiology, as well as several other areas, including pneumology, internal medicine, oncology, surgery, neurology, sports medicine, and physical medicine and rehabilitation.1,2
Cardiopulmonary exercise testing (CPET) provides a noninvasive and dynamic integrative assessment of the exercise responses involving the respiratory, cardiovascular (CV), and musculoskeletal systems. It is considered the gold standard in the assessment of cardiorespiratory function and is extremely useful in the diagnostic investigation of unexplained exercise intolerance.3 However, its utility goes beyond diagnosis as it also helps with prognostic stratification and therapeutic evaluation in different clinical contexts, and in guiding exercise prescription, not only in patients undergoing cardiac or pulmonary rehabilitation, but also in healthy athletes who aim to enhance their performance.3,4
Despite being recommended by several scientific societies across a wide range of settings, CPET is still underused for multiple reasons such as its complexity and the lack of trained personnel to interpret it, lack of awareness of practicing clinicians of its utility, its availability, and costs.2–4
This article aims to address some of those barriers by reviewing the main indications, applications, and basic interpretation skills concerning CPET in contemporary clinical practice.
How to perform cardiopulmonary exercise testingGiven the wide range of physiological data and differential diagnosis, knowing the clinical context of the individual and the question of the referring physician is a critical step when performing a CPET.5–7 CPET should be performed by healthcare professionals qualified and trained in emergency situations. A physician must be present during the test and an emergency cart with a defibrillator must be quickly available.5,8 The laboratory where a CPET is carried out must have a controlled environment with a temperature between 16 and 24°C and humidity between 30 and 60%, while the equipment must be correctly calibrated.6
The test should be clearly explained, potential doubts clarified, and informed consent obtained.8 It is also important to agree on the type of gestural communication to adopt during the test and to emphasize the relevance of performing maximum effort. Before a CPET session or test, the gas analyzer must be calibrated: gas volume at the beginning of each session of tests and gas concentration before each test. Also, immediately before, a spirometry and/or a maximal voluntary ventilation (MVV) test should be performed, which are essential to determine the breathing reserve (BR) and identify possible ventilatory limitations at rest and during exercise.5 In addition to continuous gas exchange assessment, during CPET, the electrocardiogram (ECG), blood pressure (BP) and peripheral oxygen saturation (SpO2) are also monitored.
Ergometers and protocolsErgometers are mechanical or electrical types of equipment that allow the definition of the work (intensity of effort) that the user will perform during the test. The most used are the cycle and treadmill ergometers, but there are others available, such as arm and ergometers for athletes’ evaluation in specific sports such as swimming, rowing, cross-country skiing, or kayaking.6,8,9
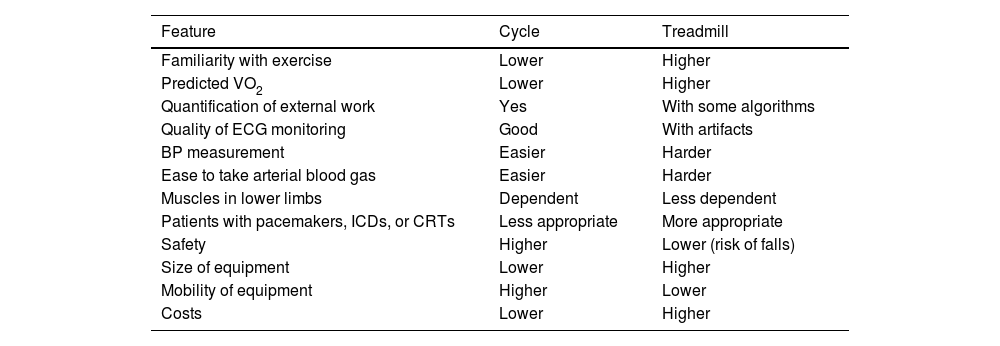
In a hospital environment the treadmill and cycle ergometers are preferred as they can replicate the most common physical activity types. The treadmill, which also involves upper limb muscles, enables users to attain 5–10% higher oxygen consumption (VO2) and represents an activity that most people do in their daily lives (walking or running).8 A comparison of the main advantages of these two types of ergometers is presented in Table 1.
Comparison between cycle and treadmill ergometers.
| Feature | Cycle | Treadmill |
|---|---|---|
| Familiarity with exercise | Lower | Higher |
| Predicted VO2 | Lower | Higher |
| Quantification of external work | Yes | With some algorithms |
| Quality of ECG monitoring | Good | With artifacts |
| BP measurement | Easier | Harder |
| Ease to take arterial blood gas | Easier | Harder |
| Muscles in lower limbs | Dependent | Less dependent |
| Patients with pacemakers, ICDs, or CRTs | Less appropriate | More appropriate |
| Safety | Higher | Lower (risk of falls) |
| Size of equipment | Lower | Higher |
| Mobility of equipment | Higher | Lower |
| Costs | Lower | Higher |
CRT: cardiac resynchronization therapy; ICD: implanted cardioverter-defibrillator.
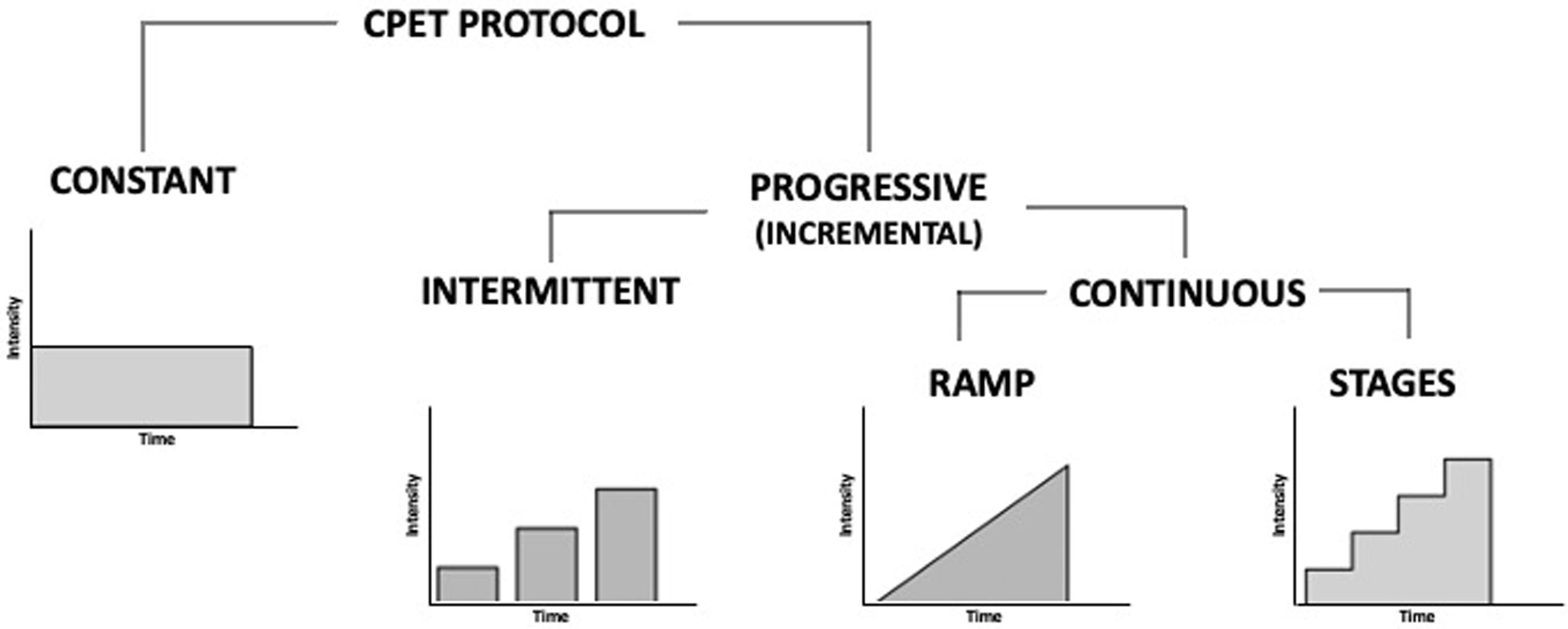
Exercise protocols need to be individualized considering the characteristics of the person who performs it and the indication for the exam.10,11 According to the load application, protocols can be classified as constant or progressive (incremental). Progressive or incremental load protocols can be intermittent (with pauses) or continuous, while the latter can be performed on a ramp or by stages (levels) (Figure 1).
Most laboratories perform incremental ramp or stagged tests. Ramp tests have the advantage of increasing the speed or resistance in a gradual and linear way, without jumps between stages, which allows a greater individualization of the protocol. With this methodology it is possible to obtain a linear increase in VO2, improving the precision to determine maximal VO2 (VO2max) and submaximal parameters, namely the ventilatory thresholds (VTs), which increases the reproducibility of the test.11
Constant load protocols can be used in specific situations, such as for the diagnosis of exercise-induced bronchospasm, evaluation of the contribution of carotid bodies in exercise hyperpnea, assessment of the lactate threshold (constant low-intensity work lasting 10 minutes), and determination of the VO2max.10,11
Key cardiopulmonary exercise testing variables to analyzeModern day gas analyzers perform breath-by-breath measurements of respiratory gases, which provide data with large variability and justifies performing data averaging: 20- or 30-second averaging are the most recommended modalities since they are a good balance between data variability and accuracy.
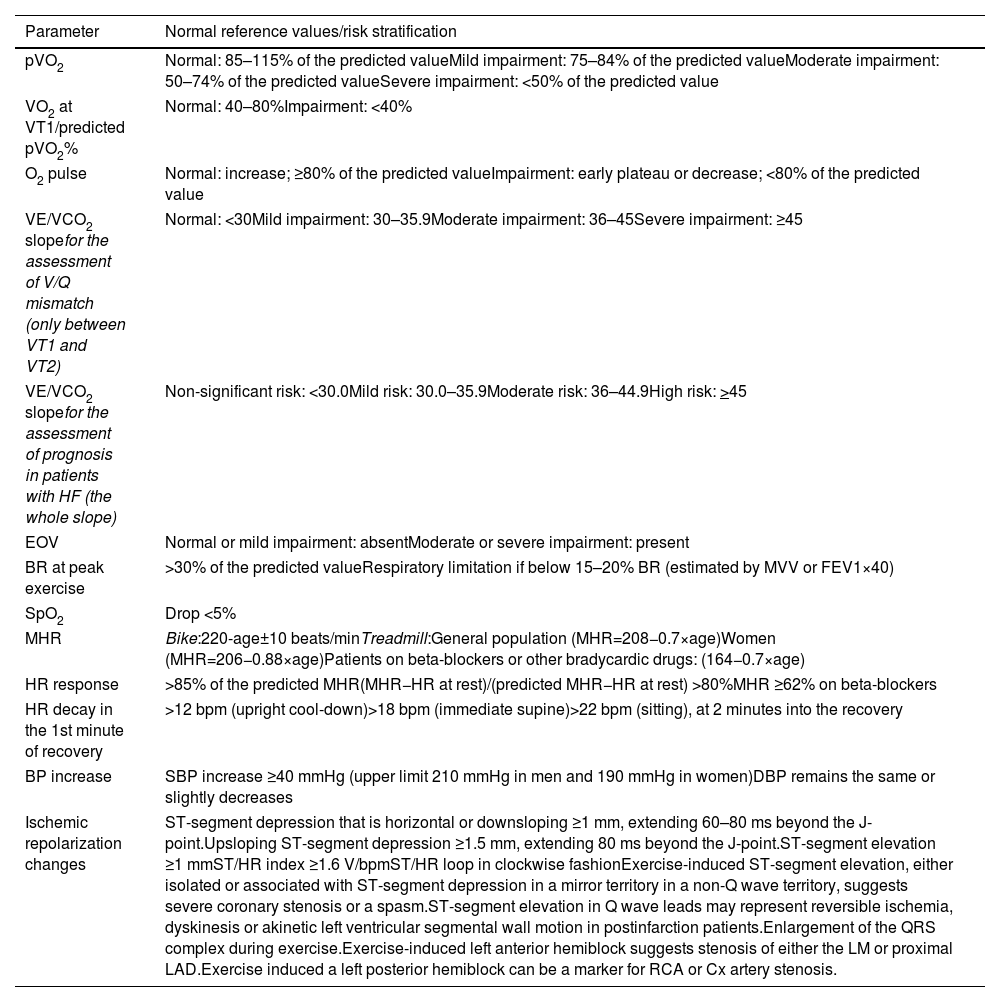
Cardiopulmonary exercise testing can generate a large number of variables, but there is a group of those that are more pertinent in current clinical practice. A general overview of normalized values, based on the most recent recommendations, is presented in Table 2, but the values described may vary, depending on the literature and the population under study.8,11–14 Some of these parameters are already evaluated in conventional exercise testing (i.e. without respiratory gas assessment, such as BP, heart rate (HR) and rhythm, and the ST-segment of the ECG), but others are associated with gas exchange and only available with a CPET. The most important parameters in clinical practice will be discussed later in this document.
Reference values for selected cardiopulmonary exercise testing parameters.
| Parameter | Normal reference values/risk stratification |
|---|---|
| pVO2 | Normal: 85–115% of the predicted valueMild impairment: 75–84% of the predicted valueModerate impairment: 50–74% of the predicted valueSevere impairment: <50% of the predicted value |
| VO2 at VT1/predicted pVO2% | Normal: 40–80%Impairment: <40% |
| O2 pulse | Normal: increase; ≥80% of the predicted valueImpairment: early plateau or decrease; <80% of the predicted value |
| VE/VCO2 slopefor the assessment of V/Q mismatch (only between VT1 and VT2) | Normal: <30Mild impairment: 30–35.9Moderate impairment: 36–45Severe impairment: ≥45 |
| VE/VCO2 slopefor the assessment of prognosis in patients with HF (the whole slope) | Non-significant risk: <30.0Mild risk: 30.0–35.9Moderate risk: 36–44.9High risk: >45 |
| EOV | Normal or mild impairment: absentModerate or severe impairment: present |
| BR at peak exercise | >30% of the predicted valueRespiratory limitation if below 15–20% BR (estimated by MVV or FEV1×40) |
| SpO2 | Drop <5% |
| MHR | Bike:220-age±10 beats/minTreadmill:General population (MHR=208−0.7×age)Women (MHR=206−0.88×age)Patients on beta-blockers or other bradycardic drugs: (164−0.7×age) |
| HR response | >85% of the predicted MHR(MHR−HR at rest)/(predicted MHR−HR at rest) >80%MHR ≥62% on beta-blockers |
| HR decay in the 1st minute of recovery | >12 bpm (upright cool-down)>18 bpm (immediate supine)>22 bpm (sitting), at 2 minutes into the recovery |
| BP increase | SBP increase ≥40 mmHg (upper limit 210 mmHg in men and 190 mmHg in women)DBP remains the same or slightly decreases |
| Ischemic repolarization changes | ST-segment depression that is horizontal or downsloping ≥1 mm, extending 60–80 ms beyond the J-point.Upsloping ST-segment depression ≥1.5 mm, extending 80 ms beyond the J-point.ST-segment elevation ≥1 mmST/HR index ≥1.6 V/bpmST/HR loop in clockwise fashionExercise-induced ST-segment elevation, either isolated or associated with ST-segment depression in a mirror territory in a non-Q wave territory, suggests severe coronary stenosis or a spasm.ST-segment elevation in Q wave leads may represent reversible ischemia, dyskinesis or akinetic left ventricular segmental wall motion in postinfarction patients.Enlargement of the QRS complex during exercise.Exercise-induced left anterior hemiblock suggests stenosis of either the LM or proximal LAD.Exercise induced a left posterior hemiblock can be a marker for RCA or Cx artery stenosis. |
Oxygen consumption is a key parameter providing a refined measure of CRF which is of major value in different settings.15 Optimal oxygen (O2) delivery is central to exercise performance, being influenced by several factors ranging from CV and respiratory function to hemoglobin plasma concentration, autonomic inputs, mitochondrial efficiency, and thermoregulation.15 Furthermore, age, gender, genetic background, and training can also affect peak VO2 (pVO2).16,17
Oxygen consumption can be expressed as an absolute value or adjusted to body weight and should also be reported in relation to age, gender, weight, height, and ergometer predicted values, through specific formulas. Many of these equations provided by the gas analyzer software are inaccurate and outdated.1,5 Today, the FRIEND trial equation is accepted as the best one to calculate the predicted value of VO2max.
Importantly, pVO2 is the highest VO2 obtained during exercise, while VO2max corresponds to a state of a VO2 plateau, despite increases in workload. Notably, while pVO2 provides a comprehensive overview of CRF, it should be acknowledged that exercise economy, encompassing cardiorespiratory efficiency, but also factors such as biomechanics, neuromuscular efficiency, and training, should be considered, as two subjects with a similar pVO2 may have different performances.18 Likewise, individuals with better exercise economy could require a different VO2 for the same workloads.
O2 pulse reflects the amount of O2 extracted at each heartbeat, providing information on both stroke volume (SV) and the arteriovenous oxygen difference.19 In the absence of factors such as anemia, hypoxia, and mitochondrial disorders, the O2 pulse trajectory parallels the one of SV. During exercise, the curve is expected to increase linearly almost till the end of the exercise period where a plateau is normally expected. An early flattening or decrease of its trajectory are abnormal responses. Indeed, a plateau or decrease in the O2 pulse trajectory during incremental exercise may reflect a reduction in SV in the setting of myocardial ischemia or left ventricle outflow obstruction.20
Respiratory exchange ratio (RER) is the ratio between carbon dioxide production (VCO2) and VO2, providing information on the type of energy substrate being metabolized. When calculated at peak effort it offers an objective insight on whether effort was maximal. Though different cut-offs may be considered, a value ≥1.10 has been considered a criterion for maximal effort attainment.5,21 A low peak RER suggests submaximal CV effort.
The ratio of minute ventilation (VE) to VO2 is called the ventilatory equivalent for O2 (EqO2), and the ratio of VE to VCO2 is called the ventilatory equivalent for CO2 (EqCO2), providing information about ventilatory efficiency. During CPET, the normal pattern of change in ventilatory equivalent for oxygen (VE/VO2) is a drop early in exercise to its nadir at the first VT (VT1), followed by an increase as the maximal exercise capacity approaches. This behavior is due to a steeper rise in ventilation in response to increased CO2 production in proportion to VO2 increase. Ventilatory equivalent for carbon dioxide (VE/VCO2) correspondingly decreases hyperbolically as the work rate increases. This balance may be disturbed in several clinical conditions, including chronic obstructive pulmonary disease (COPD), pulmonary hypertension (PH) and heart failure (HF). In these conditions, VE/VO2 and VE/VCO2 are increased due to an augmented dead space and/or alveolar hyperventilation. A steep VE/VCO2 slope (a high VD/VT) is associated with several cardiorespiratory diseases and is an independent marker of poor prognosis.
Ventilatory thresholds (VT) provide pivotal data on the metabolic response to exercise, and are paramount in exercise prescription. The first VT (VT1) represents a transition to a mixed aerobic and anaerobic metabolism, being characterized by increases in lactate and decreases in pH.5 This is accompanied by lactate buffering, with ensuing increases in VCO2 and ventilation, to maintain acid–base homeostasis. The second VT (VT2) represents a point where lactate increases rapidly and more substantially (as buffering becomes insufficient), with ensuing hyperventilation.22
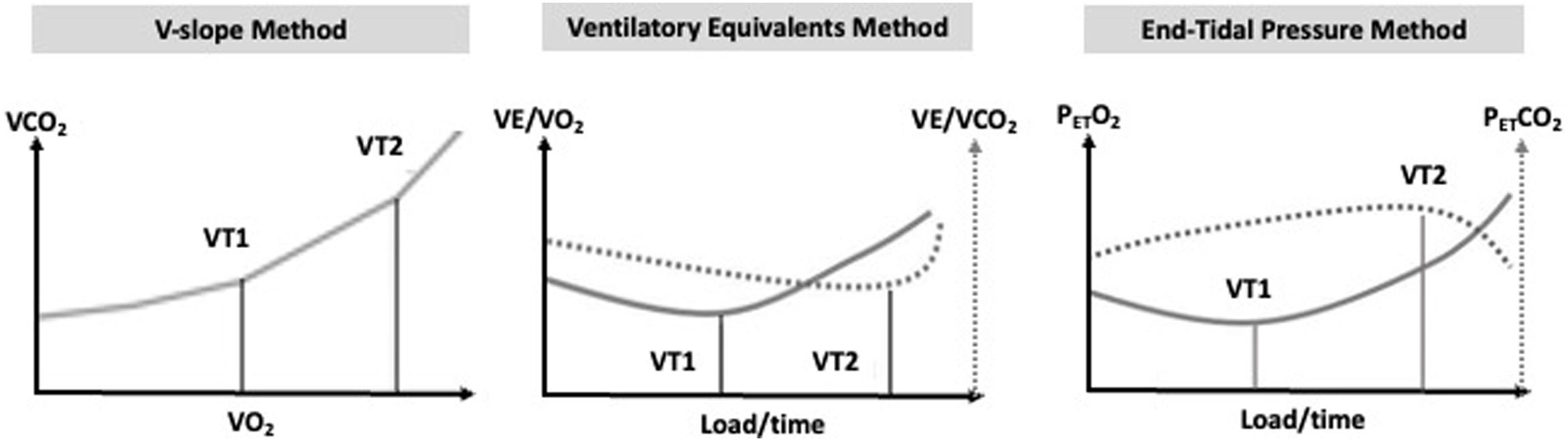
While different terms are sometimes used, such as anaerobic threshold (for VT1) and respiratory compensation point (for VT2), respectively, the terminology “VT” was adopted in the current literature. These metabolic transition points can be determined invasively (by blood analysis) or non-invasively. VT1 is commonly determined by the ventilatory equivalent method as the lowest point before an ensuing increase in the curve, or by the V-slope method (by an increase in the slope between VCO2 and VO2, which previously had a linear relationship, representing the increase in VCO2 due to lactate buffering).23,24 VT2 can be assessed by the ventilatory equivalent method, as the lowest point before a continuous increase, by a marked increase in ventilation (in relation to VCO2) and by the end-tidal carbon dioxide pressure (PETCO2), where a deflection occurs reflecting the marked ventilation increase.11Figure 2 illustrates the methods recommended for determining VTs. Importantly, an integrative approach employing different methods should be considered.
Partial pressure of end-tidal oxygen (PETCO2) reflects the gas exhaled precisely at the end of expiration, originating from the deep lung. The reported concentrations of end-tidal gas represent a mixture of gases from all alveoli, with some being well-perfused and others under-perfused.11 During the initial stages of moderate exercise, levels of end-tidal O2 (PETO2) typically decrease and start to rise during later stages due to increased CO2 production, resulting in acidemia and subsequently increased ventilation. PETCO2 levels increase initially, reflecting the rising CO2 production at the beginning of exercise, followed by a drop when acidemia stimulates ventilation beyond what is necessary to eliminate CO2.
Minute ventilation is a measure of the total volume of air breathed in one minute. During exercise, VE increases initially due to an increase in tidal volume, which can increase three to fivefold, reaching approximately 60% of the vital capacity. In later stages of exercise, breathing frequency will at least double, while tidal volume remains relatively unchanged. Younger and fitter individuals may experience a considerably higher increase in respiratory rate, reaching around 30–40 breaths per minute. A frequency higher than 55 breaths per minute is generally considered abnormal.25 If the tidal volume does not increase significantly during a CPET, it suggests the presence of lung disease.
Breathing reserve (BR) can be defined as the difference between the MVV at rest and the maximum ventilation achieved during exercise. MVV, measured in liters per minute, can be obtained through direct measurement (by instructing the individual to breathe as deeply and quickly as possible for 12 or 15 seconds and then multiplying the value by five or by four, respectively) or by estimation (MVV=forced expiratory volume in the first second (FEV1)×35 or 40). During a CPET, maximal VE should not exceed 80–85% of the predicted value in a healthy individual. If maximal VE exceeds 80% of the predicted value, it indicates a low BR, meaning there is little capacity for further increase in ventilation. A reduced (<15–20%) or absent BR suggests that the limitation to exercise is likely due to respiratory disease. However, it is important to note that BR tends to decrease with age and lower fitness levels. In cases of CV disease or other factors limiting exercise performance, BR is typically higher.
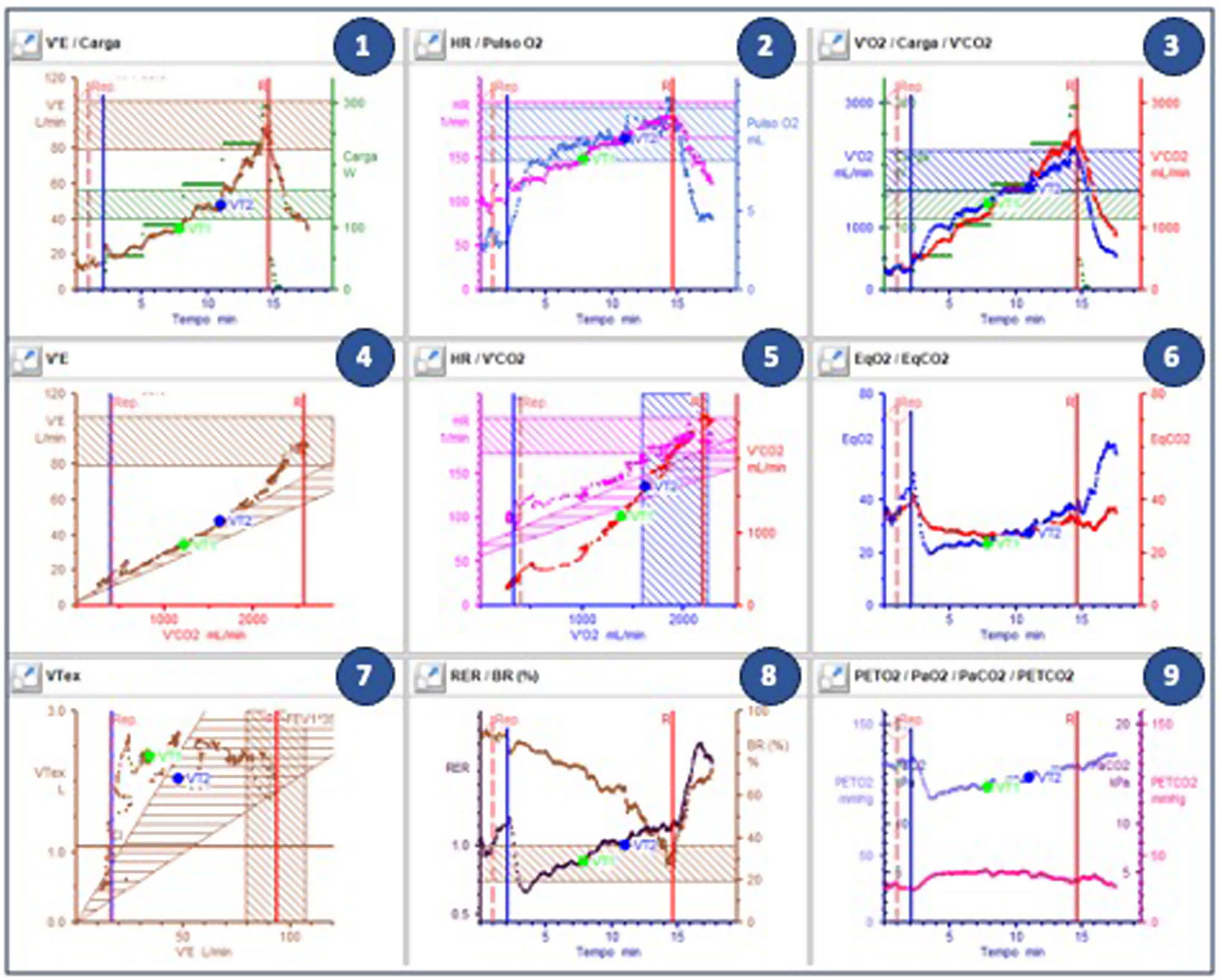
Interpretation of cardiopulmonary exercise testing resultsCardiopulmonary exercise testing has a large array of measured and calculated parameters that can be interpreted. To provide a simple yet comprehensive and visual information, Wasserman et al. arranged the CPET values into nine graphs, hence the name “9-panel plot” (Figure 3).11,25Figure 3 presents the classic and most used sequence, but other alignments may also be applied. Following a plot order and understanding the normal response and the most frequent abnormal patterns is essential for proper CPET interpretation.
Plot 3: The first question to be asked in a CPET is whether the test was maximal. The gold standard definition of a maximal CPET is a plateau or a VO2 curve drop, despite load increase. However, this finding may be difficult to attain in patients.
Plot 8: When a VO2 plateau is not identifiable, we look at this plot to check whether a RER (black dots) over 1.10 was attained at peak effort (the vertical red line). A RER of 1.10 may not be reached in cases of insufficient effort or causes of limitation other than circulatory limitation (e.g., respiratory, vascular PH, or musculoskeletal limitation).
Plot 3: We then inspect VO2 (blue dots) at peak exercise. While cut-off values differ, a value under 85% in the setting of a maximal CPET (RER >1.10) suggests a clear exercise limitation. In cycle-ergometer testing, it is possible to evaluate the VO2/work (W) ratio. Normal value is typically around 10 mL/W. However, in cases of heart disease, this relationship may decrease.
Plots 8 and 7: In the setting of dyspnea or the presence of exercise limitation, we then proceed to ascertain its etiology. A BR (panel 8, brown dots) <15–20% at peak exercise, defined after a good quality spirometry or MVV determination, suggests ventilatory limitation. It should be noted that in highly conditioned individuals with substantial tolerance to discomfort (e.g., athletes), a BR <20% can be reached without having true ventilatory limitation (usually in these cases a significant exercise time is attained, with a RER above 1.10). The pattern of the tidal volume (panel 7, brown dots) may inform whether the pattern of respiratory limitation is restrictive or obstructive. SpO2 is not always depicted in the 9-panel plot, but a decrease greater than 5% is abnormal and suggestive of limitations in gas exchange.
Plots 4, 6, and 9: Ventilation-perfusion (V/Q) mismatch due to low cardiac output (CO) or PH can also be a cause of exercise limitation. Ventilatory efficiency can be measured using two methods: (1) VE/VCO2 slope (plot 4) between VT1 and VT2; (2) nadir (VT2) of the ventilatory equivalents of CO2 (plot 6, red line). The results are usually similar. PETO2 and PETCO2 (plot 9) are also useful for assessing V/Q matching and detecting gas exchange abnormalities in the lungs. The more pronounced the ventilation, the lower the PETCO2 and the higher the PETO2.
Plot 1: VE increases proportionally with the load and CO2 concentration. In the case of a ramp protocol, it is expected to increase steadily from rest to VT1, have a steep increase from VT1 to VT2, and an even steeper increase after VT2. This pattern is difficult to observe when a staged protocol (e.g., Bruce) rather than a ramp protocol is used. If the patient has cyclic fluctuations with an oscillatory pattern in VE and expired gases, that persist ≥60% of the test with an amplitude ≥15% of the average resting value, exercise oscillatory ventilation (EOV) is noted. This is an important prognostic marker, especially in HF patients.
Plot 2: Peak HR (pink dots) can inform on the presence or absence of chronotropic incompetence. However, this information is difficult to interpret in the setting of beta-blocker therapy and may have little therapeutic impact. It can be useful in patients with pacemakers or cardiac resynchronization therapy (CRT), to identify insufficient rate response to exercise, which requires optimization in programming. More than the absolute and predicted value of peak O2 pulse (blue dots), the pattern of O2 pulse progression may be informative. It should increase and may have a plateau at maximal exercise. A marked and consistent decrease in O2 pulse during exercise, in non-athlete subjects, suggests a decrease in SV that may be caused by different phenomena such as myocardial ischemia, left ventricular outflow obstruction, or exercise-induced mitral regurgitation.
Plot 5: Like the VE curve in plot 1, the VO2/VCO2 relationship can be useful to identify VT1, and VT2, using the V-slope method. A low VT1 usually suggests circulatory limitation or severe muscular deconditioning.
Maximal versus submaximal testThe usual target of a CPET is to perform a maximal test. Submaximal tests should only be considered as an alternative for specific cases since their value for risk stratification is much less studied and reduced regarding a maximal test.8,26 It is widely accepted that a CPET may be considered maximal if a VO2 plateau or drop can be found at peak exercise despite by increasing workload. If a VO2 plateau or drop is not seen, but a RER >1.10, a BR <15%, a peak exercise HR over 90% of the predicted, or peak exercise lactate concentration ≥8 mmol/L (if measured) are reached, one may consider that an intense effort was achieved, and a near-maximal test was performed.5,10
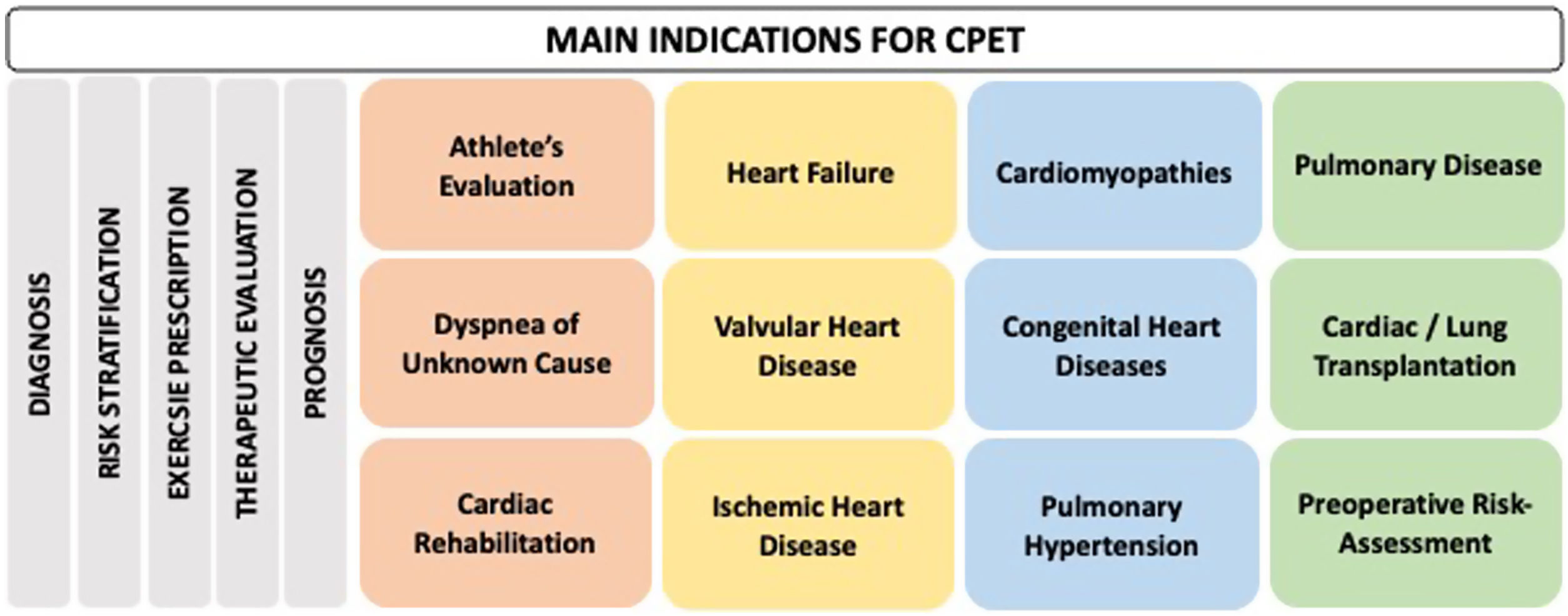
Indications for cardiopulmonary exercise testingCardiopulmonary exercise testing has multiple clinical indications, covering a broad spectrum of specialties and diseases. It is an important tool for diagnosis, risk stratification, exercise prescription, evaluation of the effect of several therapeutic interventions (pharmacological, percutaneous, and surgical) and prognosis assessment. Figure 4 shows some of the main indications for CPET.
Athlete evaluationIn asymptomatic athletes, CPET is important to detect subclinical cardiac disease, particularly in master athletes, in the assessment of baseline functional capacity, in revealing the sporting ability of young athletes, or when evaluating performance in different modalities, and training monitoring. CPET can assist in the diagnostic process and evaluation of non-specific symptoms such as exertional dyspnea, chest discomfort, or tiredness. Indeed, during their sporting careers, many athletes may experience these symptoms and the etiology may be cardiac, respiratory, muscular, or even psychological.6
In the context of sports performance, CPET allows the prescription of exercise through the documentation of VTs and the corresponding HR.27,28 In this way, this methodology helps to individualize the intensity of training, through the determination of different training zones:
- •
Zone 1: below the VT1 (light exercise)
- •
Zone 2: between VT1 and VT2 (moderate to high-intensity exercise)
- •
Zone 3: above the VT2 (very high-intensity exercise)
- •
Zone 4: corresponds to sprints and efforts above those previously mentioned.
Additionally, it plays an important role in diagnosing training overload and thus preventing overtraining syndrome.29
Cardiac rehabilitationCardiopulmonary exercise testing should be performed whenever available to stratify the risk for exercise, to prescribe exercise and to quantify the training benefits of cardiac rehabilitation.28,30 CPET is the gold standard to prescribe aerobic exercise, whether moderate continuous training (corresponding to the training zone between the two VTs), or interval training with low-intensity (below VT1) and high-intensity (above VT2) training intervals.31 Higher duration of the test and values of VO2 and HR at VTs and peak exercise, together with lower values of VE/VCO2 slope are some of the expected gains for a cardiac rehabilitation program.
Ischemic heart diseaseThe role of classical exercise testing in the diagnosis of coronary artery disease (CAD) has been progressively superseded by imaging modalities across several scenarios. CPET may add useful ancillary data, such as the O2 pulse trajectory, that can provide information concurring with possible ischemic contributions to exercise intolerance.32 Moreover, an abnormal relationship between pVO2 and work rate can also be of value in this setting.
Data derived from CPET can also provide prognostic information in CAD patients, namely with parameters such as pVO2 and the VE/VCO2 slope, giving inputs on the risk of further events and reinforcing its value in their comprehensive assessment.
CardiomyopathiesCardiopulmonary exercise testing is a safe and useful tool in patients with suspected/confirmed hypertrophic cardiomyopathy (HCM) to provide information on symptoms, severity, and prognosis, to aid planning management, and to monitor therapeutic efficacy.3,33,34 pVO2 can also help to distinguish left ventricular hypertrophy (LVH) associated with HCM from other forms of secondary LVH, such as hypertensive cardiomyopathy, “athlete's heart”, and athletes with HCM. It is suggested that in these cases, a pVO2 <84% of the age-gender predicted (AGP) is indicative of pathological LVH. A pVO2 >50 mL/kg/min or 120% of the AGP is proposed as a standard for differentiating an “athlete's heart” from HCM. Only a small percentage of athletes with HCM achieve >100% of the AGP pVO2.33 The functional information provided by the CPET should be integrated with data derived from other investigations for the appropriate differential diagnosis between “athlete's heart” and cardiomyopathies.
Although the application of CPET in arrhythmogenic cardiomyopathy is scarce, it has proven to be safe and potentially useful for risk stratification when considering advanced therapies (such as heart transplantation).35
Heart failureIn patients with HF with reduced ejection fraction (HFrEF), pVO2 has a prominent role in the prognostic stratification. However, submaximal exercise gas exchange variables have emerged that rival the prognostic utility of pVO2. Some of these encompass the VO2/W ratio (aerobic efficiency), VE/VCO2 slope (ventilatory efficiency), VO2 at VT1, oxygen uptake efficiency slope (OUES), and EOV. EOV represents a strong negative prognostic parameter in HF patients.36,37
The 2012 EACPR/AHA Scientific Statement3 proposed a multiparametric CPET data table developed by Arena et al., with an iteration of the figures by proposing color-coded interpretive tables applied to different diseases.38 A CPET score utilizing VE/VCO2 slope ≥34 (7 points), HR decay in the first minute of recovery ≤6 bpm (5 points), OUES ≤1.4 (3 points), resting PETCO2 <33 mmHg (3 points), and a pVO2 ≤14 mL/kg/min (2 points) has been validated to predict transplant/mechanical circulatory support-free survival in HF patients better than pVO2 alone, with a summed score >15 indicating the poorest prognosis.39,40 The use of this CPET score is helpful in risk stratifying HF patients in Weber class B (with pVO2 16–20 mL/kg/min) into low-risk and higher-risk subgroups.41 The latest criteria proposed two different pVO2 cut-offs for heart transplantation depending on whether the patient is (pVO2 ≤14 mL/kg/min) or not (pVO2 ≤12 mL/kg/min) on β-blocker treatment (Cl I, LOE B); in outpatients aged <50 years, a pVO2 <50% of the expected value (Cl IIa, LOE B).
The International Society for Heart and Lung Transplantation (ISHLT) guidelines indicate the use of a VE/VCO2 slope >35 as a determinant in listing for heart transplantation in the presence of a submaximal CPET (Cl IIb, LOE: C). The presence of a CRT does not alter the current pVO2 cut-off recommendations (Cl I, LOE: B).42,43 HF with preserved ejection fraction (HFpEF) represents worldwide most patients with HF. These patients may be functionally very limited, a limitation that can be objectively quantified by CPET. However, because CPET findings in HFpEF are nonspecific regarding HFrEF patients, the clinical utility of CPET in a patient with HFpEF suspicion is low. CPET can help to understand the nature and magnitude of symptoms, the pathophysiological mechanism, and the impact of noncardiac comorbidities that frequently limit elderly HFpEF patients. Lastly, CPET is also mandatory to correctly prescribe exercise to HFpEF patients integrated in cardiac rehabilitation programs.
Valvular heart diseaseIn valvular diseases, CPET can help unveil unreported symptoms, understand the mechanism's underlying symptoms, and better outline prognosis that helps to define treatment timings more appropriately. The ventilatory classification system may provide additional information in detecting elevated pulmonary pressures, with higher values indicating greater severity of the valvular heart disease and poorer prognosis.44 Combined stress echocardiography and CPET can be helpful in determining the mechanisms of exercise intolerance in patients with mitral stenosis. Those patients show the expected exercise-induced PH that may lead to hyperventilation and increased VE/VCO2 slope. Also, O2 pulse stops increasing due to lack of increase of ventricular filling during exercise because the valvular stenosis.
Current guidelines support the use of stress testing in asymptomatic severe aortic stenosis patients.45 A pVO2 ≤19 mL/kg/min for men and ≤15 mL/kg/min for women; O2 pulse ≤15 mL/beat for men and ≤11 mL/beat for women, were strong predictors of mortality in patients with moderate to severe aortic stenosis, irrespective of whether they undergo aortic valve replacement.46
Pulmonary hypertensionWhen evaluating a patient with an established or suspected PH diagnosis, CPET can be useful to elucidate the underlying pathophysiologic mechanism of exercise intolerance, to assess the severity of PH, to quantify the response to treatment, and to stratify mortality risk.
The pathophysiology of PH is characterized by reduced CO reserve due to increased right ventricle afterload and increased physiologic dead space due to marked inefficient ventilation. Variables such as pVO2, O2 pulse, and VO2/W ratio will be abnormally reduced due to the limited CO reserve. Likewise, the significant changes in VE, VE/VCO2 and PETCO2 during exercise, reflect the impaired ventilatory efficiency so distinctive of PAH.47
Congenital heart diseasesIt is safe to perform a CPET in the spectrum of congenital heart disease (CHD), not only for risk stratification, but also in assisting in the decision of timing of surgical or percutaneous interventions, as well as exercise counseling and training. The most reported CPET findings in CHD are reduced pVO2, early VT1, blunt HR increase, reduced tidal volume increase, and increased VE/VCO2 slope.48
As a general guideline, it is recommended to stop testing in the presence of severe desaturation (SpO2 ≤80%) when accompanied by symptoms and signs of severe hypoxemia. However, data concerning specific recommendations regarding cyanotic CHD are limited. A right-to-left shunt can manifest itself during the CPET by the onset or worsening of systemic arterial desaturation, augmentation of VE, usually associated with an abrupt decrease in PETCO2 and simultaneous increases in PETO2, RER, and ventilatory equivalents.49
Dyspnea of unknown causeDyspnea is a complex and multifactorial symptom characterized by the subjective feeling of breathing discomfort. It is a commonly reported symptom, and the underlying causes can be diverse and may include respiratory, CV, metabolic, or psychological factors.50 In fact, dyspnea experienced during exercise and daily activities may be an early symptom of various cardiopulmonary and neuromuscular diseases, leading to progressively less intense activities, resulting in muscle deconditioning and a decline in quality of life. Dyspnea is a predictor of quality of life, exercise tolerance, and mortality in several pathologies, being a better predictor than FEV1 in COPD or angina in ischemic heart disease.51
During CPET, dyspnea can be assessed using scales which are helpful to monitor its intensity throughout the test and to compare the severity of breathing discomfort with the level of exercise. The most used scale is the modified Borg scale, which ranges from 0 to 10. This scale has been widely validated and correlates well with aerobic stress and blood lactate levels during exercise.
Due to its subjective nature and multiple potential underlying causes, dyspnea requires a comprehensive evaluation to identify the factors contributing to the symptom. CPET plays a crucial role to clarify the underlying mechanisms of dyspnea during exertion. Interpretative algorithms enable identifying patterns of findings that are typical for different clinical conditions and allow clinicians to differentiate patterns of various conditions, such as COPD, asthma, HF, obesity, PH, and interstitial lung diseases.52 In some cases, modified protocols can be employed during CPET to detect specific conditions, which are suspected based on clinical data (e.g., identification of exercise-induced bronchoconstriction, and exercise-induced laryngeal obstruction).
Pulmonary diseaseCardiopulmonary exercise testing is extremely useful in the evaluation of patients with lung disease for quantifying exercise capacity and level of disability, providing diagnostic information, evaluating hypoxemia during exercise and underlying mechanisms, defining therapeutic strategies (such as pulmonary rehabilitation), assessing the preoperative risk of complications in lung surgery, and providing prognostic information.52,53 If BR is significantly reduced, it suggests that the respiratory system may be a limiting factor for exercise performance. It is possible to measure the flow-volume curve during exercise to detect ventilatory constraints.
In healthy individuals, as exercise intensity increases, the volume of air remaining in the lungs at the end of expiration declines while the inspiratory capacity increases, leading to improved ventilatory efficiency. However, patients with obstructive lung disease may have difficulty in emptying their lungs during incremental exercise compared to rest due to expiratory flow limitation (EFL) and increased respiratory rate, resulting in reduced expiratory time. Consequently, there is an increase in end-expiratory volume, in contrast to the decrease observed in individuals without lung disease, leading to a reduction in inspiratory capacity of at least 250 mL.54 Additionally, other measurements, including EFL >25% at peak effort, a lung volume ratio at the end of inspiration greater >90% of total lung capacity, and a tidal volume >70% of inspiratory capacity can be obtained.53 Assessing these parameters during exercise helps to identify the presence of dynamic hyperinflation, which can be responsible for dyspnea and a limiting factor for exercise.
The decision to perform arterial blood gas measurements during CPET depends on the specific goals of the test. In general, measuring partial pressure of O2 (PaO2) allows the calculation of gas exchange indices, such as the alveolar-arterial gradient. Measuring the partial pressure of CO2 (PaCO2) allows the calculation of the dead space over tidal volume (VD/VT) ratio, which is a measure of the efficiency of carbon dioxide exchange. Inefficient CO2 exchange is manifested by the high VD/VT ratio, often signaled by the high VE/VCO2 ratio with exercise.
Contraindications for cardiopulmonary exercise testingBeyond knowing the potential indications for CPET, it is also fundamental to know the main contraindications for this exam, especially corresponding to severe or uncontrolled CV conditions.1,13 In general, absolute contraindications for CPET encompass:
- •
Acute myocardial infarction (3–5 days)
- •
Unstable angina
- •
Uncontrolled arrhythmia causing symptoms or hemodynamic instability
- •
Active endocarditis
- •
Acute myocarditis or pericarditis
- •
Symptomatic severe aortic stenosis
- •
Decompensated HF
- •
Acute aortic dissection
- •
Uncontrolled asthma
- •
Acute pulmonary embolism
- •
Arterial desaturation at rest on room air <85%
- •
Physical disability that precludes safe and adequate testing
Other conditions represent relative contraindications for CPET, reinforcing the need of direct supervision by a physician. Among the relative contraindications, the following conditions are included:
- •
Untreated left main coronary stenosis or its equivalent
- •
Asymptomatic severe aortic stenosis
- •
Severe untreated arterial hypertension at rest (SBP >200 mmHg; SBP >110 mmHg)
- •
Significant tachyarrhythmias
- •
High-degree atrioventricular block or other significant bradyarrhythmia
- •
Thrombosis of the lower limb until treated
- •
Severe abdominal aortic aneurysm
- •
Recent stroke or transient ischemic attack
- •
Advanced or complicated pregnancy
- •
Psychiatric or mental impairment (inability to cooperate)
- •
Uncorrected medical conditions, such as significant anemia, important electrolyte imbalance, and hyperthyroidism.
Cardiopulmonary exercise testing is a comprehensive exam aimed at clarifying patient symptoms, differentiating underlying pathophysiological mechanisms, and estimating CRF, disease severity and prognosis. Standardization of CPET-derived data can optimize its accessibility and improve the individualized management of patients across a wide range of clinical contexts. Knowledge of the main indications, applications, and basic interpretation of CPET results is essential to harness its remarkable potential and apply its principal advantages in clinical practice.
Conflicts of interestThe authors have no conflicts of interest to declare.