The current study evaluates the effect of chelidonic acid on doxorubicin-induced cardiac toxicity. Chelidonic acid (CA) is a natural pyran-skeleton heterocyclic compound found in rhizomes of the perennial plant, celandine (Chelidonium majus).
MethodsWistar rats were given an intraperitoneal injection of doxorubicin (1.25 mg/kg, cumulative dose of 20 mg/kg) four times per week for a duration of four weeks to induce cardiotoxicity. CA treatment (10, 20, and 40 mg/kg orally for four weeks) was started together with doxorubicin.
ResultsCA treatment reduced myocardial damage and improved cardiac dysfunction in doxorubicin-treated rats. It improved blood pressure, restored ST wave height and normalized the QTc interval compared to the rats treated only with doxorubicin. Administration of CA for four weeks reduced left ventricular end-diastolic pressure. Moreover, CA treatment decreased the level of cardiac markers such as creatine kinase-myocardial band (CK-MB), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and cardiac troponin-T. Masson's trichrome, hematoxylin, and eosin staining of heart tissue revealed that CA attenuated the deleterious effects of doxorubicin and prevented further damage and fibrosis in rats.
ConclusionThe study findings confirm that CA treatment can protect the myocardium against doxorubicin-induced cardiotoxicity.
O presente estudo avalia o efeito do ácido quelidónico na toxicidade cardíaca induzida pela doxorrubicina. O ácido quelidónico é um composto heterocíclico natural do esqueleto pirano encontrado nos rizomas da planta perene, celidónia (Chelidonium majus).
MétodosRatos Wistar receberam injeção intraperitoneal de doxorrubicina (1,25 mg/kg, dose cumulativa de 20 mg/kg) quatro vezes por semana durante quatro semanas para induzir cardiotoxicidade. O tratamento com ácido quelidónico (10, 20 e 40 mg/kg por via oral durante quatro semanas) foi iniciado juntamente com a doxorrubicina.
ResultadosO tratamento com ácido quelidónico reduziu o dano miocárdico e melhorou a disfunção cardíaca em ratos tratados com doxorrubicina. O tratamento com ácido quelidónico melhorou a pressão arterial, bem como restaurou a altura ST e normalizou o intervalo QTc em comparação com os únicos ratos tratados com doxorrubicina. A administração de ácido quelidónico por quatro semanas reduziu a pressão diastólica final do ventrículo esquerdo. Além disso, o tratamento com ácido quelidónico diminuiu o nível de marcadores cardíacos como creatina quinase-banda miocárdica (CK-MB), lactato desidrogenase (LDH), aspartato aminotransferase (AST) e troponina T cardíaca. A coloração com tricrómico, hematoxilina e eosina de Masson do tecido cardíaco revelou que o ácido quelidónico atenuou os efeitos deletérios da doxorrubicina e preveniu maiores danos e fibrose em ratos.
ConclusãoOs achados do presente estudo confirmam que o tratamento com ácido quelidónico pode proteger o miocárdio contra a cardiotoxicidade induzida pela doxorrubicina.
In recent years, cancer has become more prevalent, and it is estimated that 13.1 million people will die of cancer by 2030.1,2 Treating cancer has become a highly complex process.3 Anthracyclines are a class of chemotherapeutic drugs used to treat various malignancies in both adult and pediatric patients. The most popular anthracycline, doxorubicin (also called adriamycin) (DOX), is used to treat a variety of malignant tumors, such as acute leukemia, lymphomas, ovarian, testicular, lung, thyroid, breast, among others.4,5 It is one of the most efficient anthracycline antibiotics and a frontline chemotherapeutic agent for the treatment of cancer.6,7 While doxorubicin has broad anticancer efficacy, due to its negative consequences, its therapeutic application is restricted, particularly in life-threatening cardiac toxicity.8 Clinically, DOX-induced cardiotoxicity is defined by an increase in ventricular wall thickening that can result in mortality, aberrant arrhythmias, congestive heart failure (HF), and a reduction in left ventricular ejection fraction.9 It shows dose-dependent cardiotoxicity, which is linked to a decrease in left ventricular function or HF in 7–26% of patients treated with doxorubicin (550 mg/m2).10,11
Arrhythmias, ischemia, systolic dysfunction, and HF are all manifestations of doxorubicin-induced cardiotoxicity, and the major causes are cardiac cell death and necrosis.12 Cardiomyocytes are more prone to doxorubicin toxicity than other tissues because they have lower antioxidant levels, a higher reliance on oxidative substrate metabolism, and a higher mitochondria volume than tumor cells.13,14 Chelidonic acid (CA) is a secondary metabolite found in several plants, such as Chelidonium majus L. It possesses anti-inflammatory, anti-allergic, anti-ulcerative colitis, and neurological sedative properties.15–18 CA also has many known therapeutic effects, such as being a mild analgesic, antimicrobial, and oncostatic; in the central nervous system, it acts as a sedative.19 It also inhibits TNF-α production in ulcerative colitis in rats.15 CA inhibits nuclear factor kappa B (Nf-kB) and activates mast cell caspase-1, a component of the AMP-activated protein kinase signaling pathway, to decrease the generation of IL-6. Our previous study reported the beneficial effects of CA in cisplatin-induced nephrotoxicity.20 CA can be useful to relieve the toxic effects of doxorubicin in myocardial tissue without interfering with its anticancer activity. This study, therefore, aimed to investigate any possible defense that CA might have against the cardiotoxicity that DOX causes in rats.
ObjectiveThis study was designed to investigate the possible cardioprotective effect of CA in DOX-induced cardiac toxicity in rats.
MethodsChemicalsChelidonic acid, 5-5-dithio bis-(2-nitrobenzoic acid), and thiobarbituric acid were procured from Sigma Aldrich (St. Louis, MO, USA). Doxorubicin hydrochloride USP (Batch# 062103092) was received as a gift sample from Khandelwal Laboratories, India. Kits of creatine kinase-MB and lactate dehydrogenase were purchased from Transasia Biomedicals Ltd., India.
Experimental animalsMale Wistar rats (170–200 g) were purchased from the National Institute of Biosciences in Pune, Maharashtra, India, and kept in an environment with a 12-hour light/dark cycle. Purified water and multi-nutritional pellet food (Nutrimix Laboratory Animal Feed, India) were available at all times.
Doxorubicin-induced cardiac toxicity in ratsDoxorubicin was solubilized in the normal saline solution and injected intraperitoneally at 1.25 mg/kg, four times per week for four weeks, the total cumulative dose was 20 mg/kg.21–23
Experimental designA total of 42 male Wistar rats were used in this study. They were split up into six groups at random, each containing seven rats. The details of each group are as follows:
0.5% carboxymethyl cellulose and saline solution were used as vehicles for CA and doxorubicin, respectively.24,25
Group 1. Normal: Rats were administered 0.5% carboxymethyl cellulose (1 ml/kg/day, p.o.) using oral gavage for a period of four weeks and saline solution (vehicle of DOX) (1 ml/kg, i.p.) four times per week for four weeks.
Group 2. DOX: Rats were administered 0.5% carboxymethyl cellulose (1 ml/kg/day, p.o.) using oral gavage for four weeks and DOX (1.25 mg/kg, cumulative dose 20 mg/kg, i.p.) four times per week for four weeks.
Group 3. DOX+DEX: Rats were administered DOX (1.25 mg/kg, cumulative dose 20 mg/kg, i.p.) and DEX (50 mg/kg, i.p.) 30 minutes before DOX administration.
Group 4. DOX+CA (10 mg/kg): Rats were administered DOX (1.25 mg/kg, cumulative dose 20 mg/kg, i.p.) and CA 10 mg/kg/day p.o. for four weeks.
Group 5. DOX+CA (20 mg/kg): Rats were administered DOX (1.25 mg/kg, cumulative dose 20 mg/kg, i.p.) and CA 20 mg/kg/day p.o. for four weeks.
Group 6. DOX+CA (40 mg/kg): Rats were administered DOX (1.25 mg/kg, cumulative dose 20 mg/kg, i.p.) and CA 40 mg/kg/day p.o. for four weeks.
At the end of the study, rats were anesthetized with urethane (1.25 gm/kg, i.p.). Each animal was positioned supine on an ECG board, and ECG was continuously recorded using normal three-lead skin electrodes, +ve, −ve, and neutral (the needle electrodes 29-gauge, 12 mm) in lead II position using a data acquisition system (AD Instruments, Australia).
Blood pressureTo perform a tracheotomy, the neck region of the rat was opened with a ventral midline incision, and then the right carotid artery was cannulated with a polyethylene tube attached to a three-way cannula and the mean arterial pressure, systolic pressure, and diastolic pressure were measured. Left ventricular end-diastolic pressure (LVEDP), and maximum and minimum rate of ventricular contraction (+dp/dt, −dp/dt) were recorded using a data acquisition system, Power Lab (AD Instruments, Australia).
Heart weight to body weight ratio (HW/BW ratio)Heart weight and body weight ratio were calculated after the measurement of blood pressure.
Estimation of cardiac injury markersBlood was withdrawn from animals from the retro-orbital plexus. A blood sample was collected from each animal in two separate Eppendorf tubes. One Eppendorf contains an anticoagulant for obtaining plasma and another without an anticoagulant for serum separation. Serum and plasma samples were separated and stored at −20°C, and the same were used for the determination of cardiac injury markers. Levels of CK-MB, LDH, and AST in serum were determined using diagnostic kits (Transasia Bio-Medicals Ltd., India). cTn-I level in plasma was determined using an ELISA kit provided by Abbkine, USA. All the parameters were estimated by using a biochemical analyzer (Erba Chem 7, Germany).
Preparation of tissue homogenateAfter the recording of hemodynamic parameters, animals were sacrificed. Heart tissue homogenate was prepared in ice-cold 50 mM phosphate buffer saline (pH 7.4) using a homogenizer (Polytron, Kinematica, Switzerland). The homogenate was centrifuged at 2000 g for 20 min at 4°C and the aliquots of the supernatant were collected and stored at −20°C for further evaluation. Total protein in heart tissue homogenate was measured using the Bradford Protein Assay as per Bradford26 method.
Estimation of oxidative stressLipid peroxidation (LPO) was measured as a malondialdehyde (MDA) concentration, and MDA level in heart tissue was measured by thiobarbituric acid reactive substances (TBARS) assay described by Ohkawa (1979)27. In the TBARS assay, total homogenate was used to determine thiobarbituric acid reactive substances. Reduced glutathione levels in heart tissue were estimated using the method given by Ellman.28 Post-nuclear fraction of homogenate which was prepared by centrifugation at 2500 g for 10 min was used to estimate catalase activity in heart tissue as per the method described by Luck.29 Superoxide dismutase activity was measured in post-mitochondrial fraction of homogenate, which was prepared by centrifugation at 10000 g for 20 minutes as per the method described by Paoletti and Mocali.30
Estimation of nitric oxideThe tissue homogenate was mixed with an equal quantity of Griess reagent, and absorbance was determined at 540 nm using a microplate spectrophotometer (BioTek-Epoch 2). NO concentration was expressed as μM/L of NO.31
Estimation of Nrf2 and release of pro-inflammatory cytokinesNrf2 and the release of TNF-α and IL-6 were measured by using sandwich ELISA methods according to the manufacturer's protocol (Abbkine, USA). Levels of C-reactive protein (CRP) were determined using diagnostic kits (Transasia Bio-Medicals Ltd., India).
Histopathological examinationHeart tissue was stored in a 10% neutral buffer formalin solution. The organ specimens were treated with xylene for dehydration and alcohol for 2 h. The infiltration and impregnation were carried out by treatment with paraffin wax twice. Specimens were cut into sections of 3–5 μm thickness and embedded in paraffin. Serial sections (3 μm) were cut using a microtome. The sections were stained with hematoxylin and eosin and Masson's trichrome. Sections were examined under the light microscope for myocardial injury and progression of fibrosis (Motic, Canada).
Statistical analysisThe data were expressed as mean±SEM for each group. Statistical analysis was performed using the one-way analysis of variance followed by Dunnett's post-hoc test. The data was analyzed using Graph Pad Prism 8 software (California, USA).
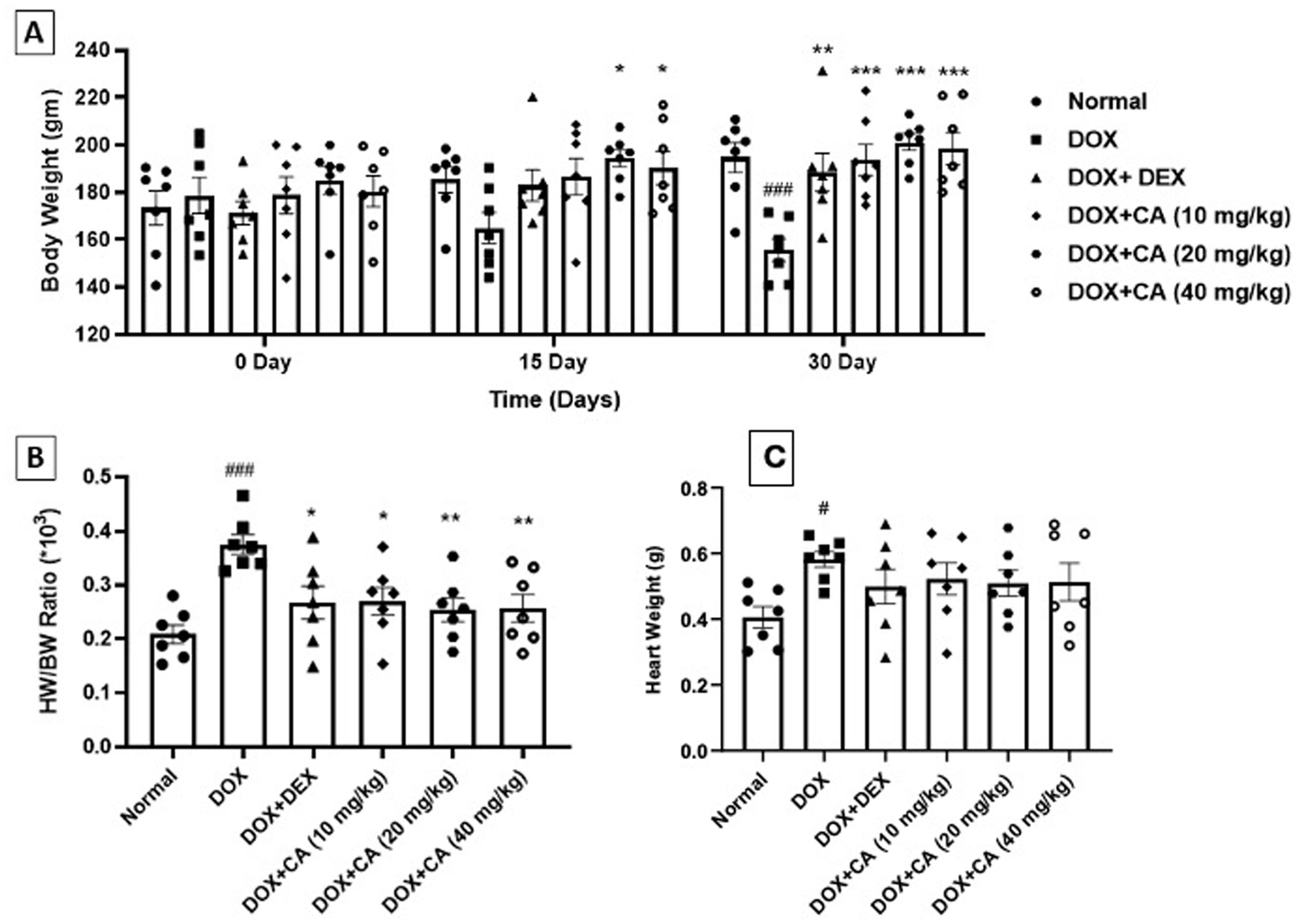
ResultsEffect of chelidonic acid on body weight and heart weight/body weight ratio (HW/BW ratio)DOX – treated group showed a significant decrease in body weight when compared with the normal group (p<0.001). CA treatment at a dose of 10, 20, and 40 mg/kg significantly elevated the body weight of animals as compared to the DOX-treated group (p<0.001). Standard dexrazoxane-treated rats showed a significant increase in body weight when compared with DOX-treated rats (p<0.01) (Figure 1A).
Effect of chelidonic acid on body weight and heart to body weight ratio. (A) Body weight, (B) heart weight to body weight ratio, and (C) heart weight. All values are expressed as mean±SEM (n=7), ***p<0.001, **p<0.01, and *p<0.05 when compared with the DOX – treated group. #p<0.05 and ###p<0.001 when compared with the normal group.
DOX-treated rats showed significant elevation in HW/BW ratio when compared with the normal group (p<0.001). CA treatment at doses of 10, 20, and 40 mg/kg significantly reduced the HW/BW ratio as compared with the DOX – treated rats. Dexrazoxane treated animals showed a significant decrease in HW/BW ratio as compared with only DOX-treated animals (p<0.05) (Figure 1B and C).
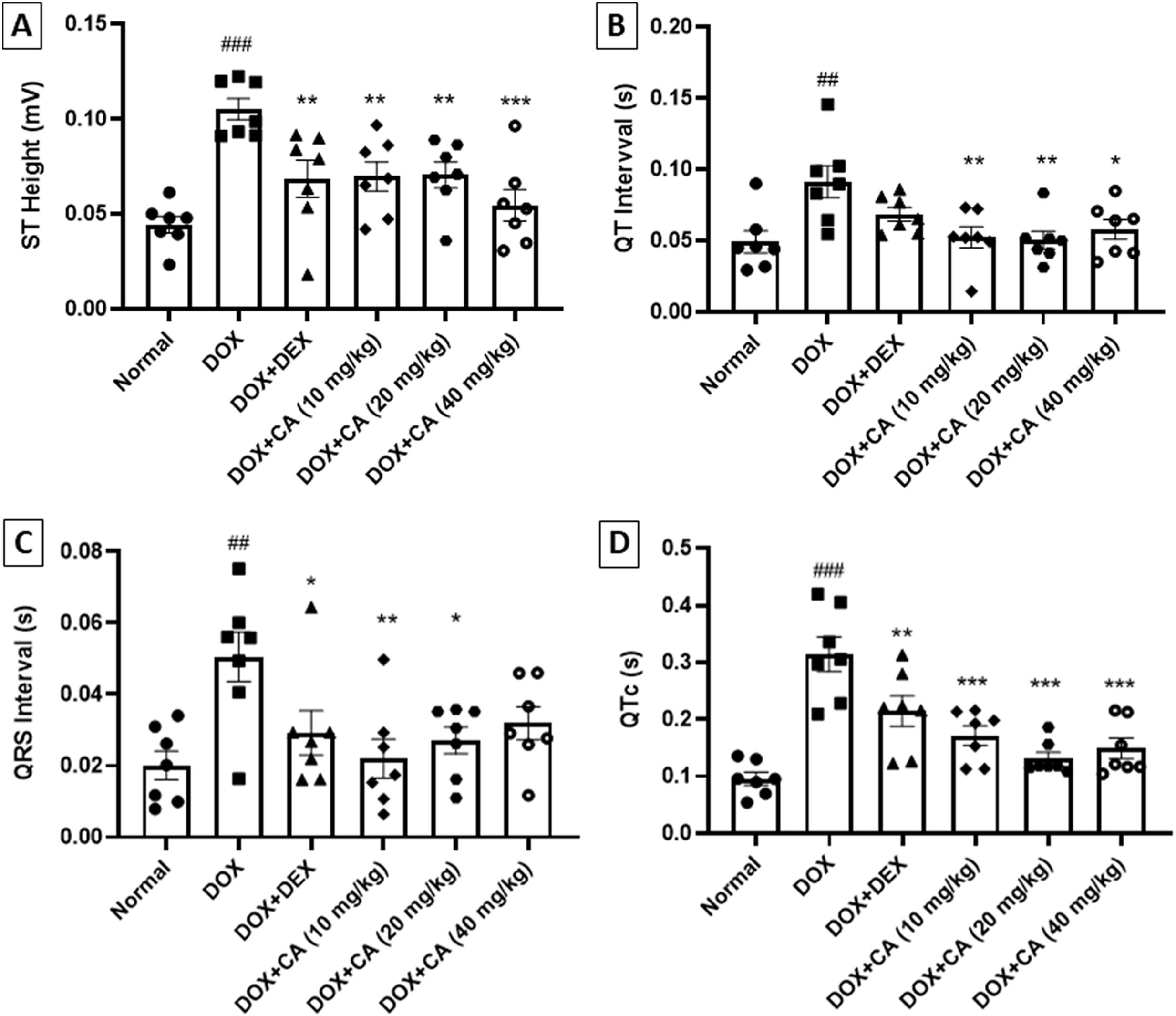
Effect of chelidonic acid on electrocardiogramChelidonic acid treatment normalized the electrocardiogram pattern in rats. The DOX - treated group showed significant elevation in ST height when compared with the normal group (p<0.001). CA treatment at doses of 10, 20, and 40 mg/kg significantly reduced the ST height when compared with DOX – treated rats (p<0.001 and p<0.01). Dexrazoxane-treated animals showed a significant decline in the ST height when compared with the DOX – treated group (p<0.01) (Figures 2 and 3A).
Effect of chelidonic acid on electrocardiography parameters. (A) ST height, (B) QT interval, (C) QRS interval and (D) QTc. All values are expressed as mean±SEM (n=7), ***p<0.001, **p<0.01, and *p<0.05 when compared with the DOX – treated group. ###p<0.001 and ##p<0.01 when compared with the normal group.
DOX - treated rats showed had increased QT interval when compared with the normal animals (p<0.01). CA at dose range significantly decreased the QT interval when compared with DOX – treated rats (p<0.01 and p<0.05) (Figure 3B).
QRS interval and QTc were significantly increased in DOX – treated animals when compared with the normal group (p<0.01 and p<0.001). CA treatment at doses of 10 and 20 mg/kg significantly reduced the QRS interval when compared with the DOX – treated group (p<0.01 and p<0.05). Dexrazoxane treated animals showed a significant decrease in the QRS interval. CA treatment at doses of 10, 20, and 40 mg/kg significantly decreased the QTc. Standard dexrazoxane-treated animals showed a significant decrease in the QTc (p<0.01) (Figure 3C and D).
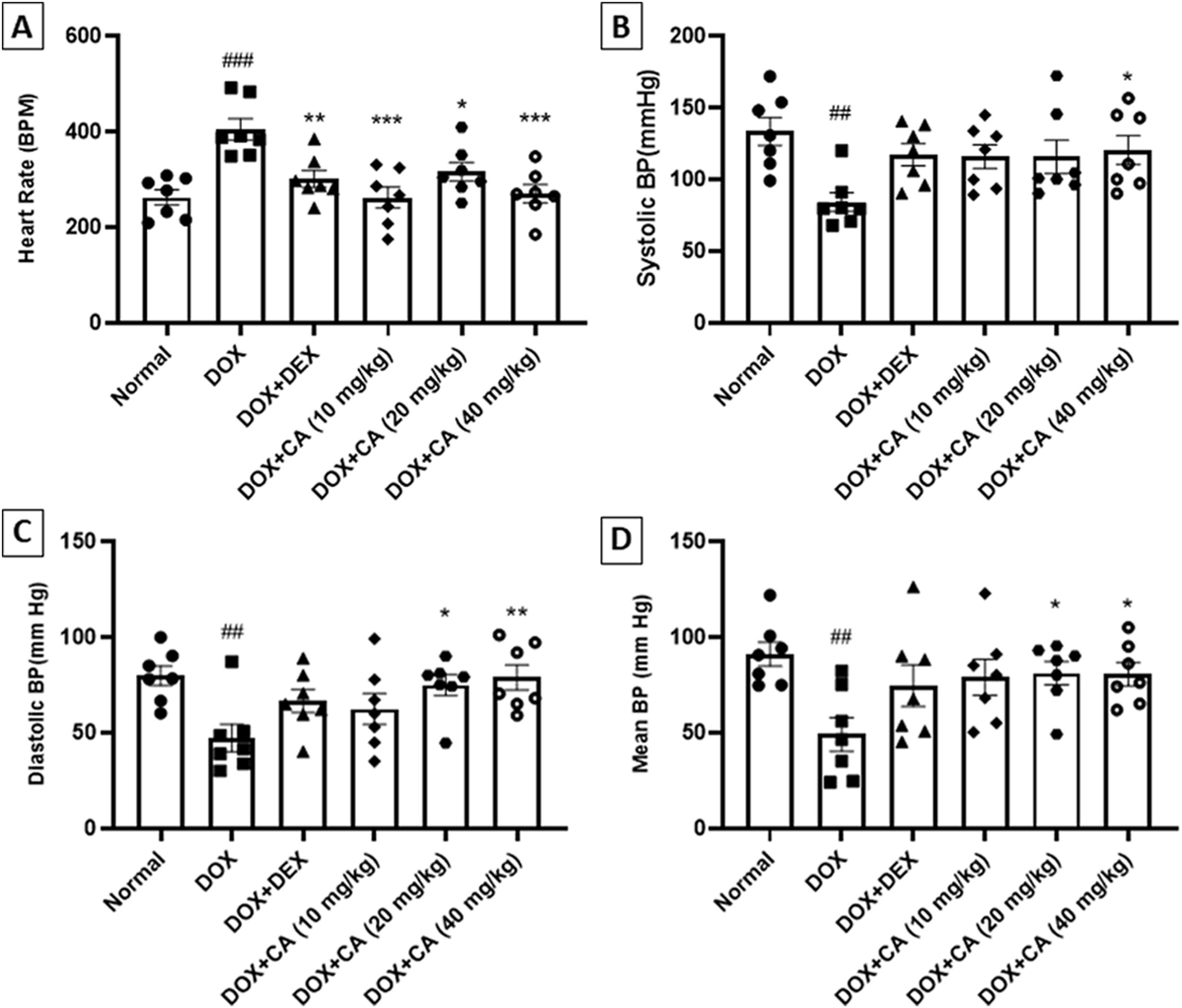
Effect of chelidonic acid on hemodynamic parametersDOX – treated group of animals exhibited an increase in heart rate when compared with the normal group (p<0.001). CA treatment at doses of 10, 20, and 40 mg/kg significantly declined the heart rate when compared with the DOX – treated group (p<0.001 and p<0.05). Dexrazoxane treated rats showed a significant decrease in heart rate when compared with the DOX - treated animals (p<0.01) (Figure 4A).
Effect of chelidonic acid on hemodynamic parameters. (A) Heart rate, (B) systolic blood pressure, (C) diastolic blood pressure, and (D) mean blood pressure. All values are expressed as mean±SEM (n=7), ***p<0.001, **p<0.01, and *p<0.05 when compared with the DOX – treated group. ###p<0.001 and ##p<0.01 when compared with the normal group.
Hemodynamic parameters in DOX-treated animals were significantly altered. DOX – treated animals showed a decrease in systolic blood pressure when compared with the normal group (p<0.01). CA at a dose of 40 mg/kg significantly increased the systolic blood pressure when compared with only DOX-treated group (p<0.05) (Figure 4B).
The DOX-treated animals showed a significant reduction in diastolic blood pressure when compared with the normal group (p<0.01). CA treatment at the entire dose range significantly increased the diastolic blood pressure when compared with the DOX - treated group (p<0.05 and p<0.01). Mean blood pressure was significantly reduced in DOX – treated animals when compared with the normal group (p<0.01). CA treatment at doses of 20 and 40 mg/kg significantly increased the mean arterial blood pressure when compared with the DOX – treated group (p<0.05). These changes in hemodynamic parameters were prevented by the CA treatments (Figure 4C and D).
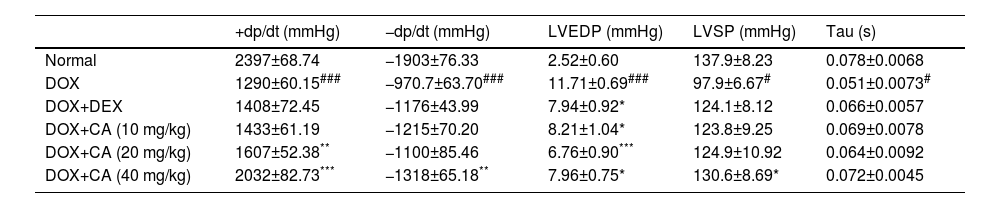
Effect of chelidonic acid on ventricular activityThe DOX-treated group showed significant elevation in LVEDP when compared to the normal group (p<0.001). CA treatment at doses of 10, 20, and 40 mg/kg significantly decreased it when compared with the DOX – treated group (p<0.05, p<0.001, and p<0.05). Dexrazoxane treated rats showed a significant reduction in LVEDP when compared with the DOX - treated group (p<0.05). DOX – treated group showed a significant decrease in LVSP when compared to the normal group (p<0.05) and isovolumic relaxation constant (tau) was significantly decreased in the DOX – treated group when compared with the normal group (p<0.05).
There was a significant decrease in +dp/dt and −dp/dt in the DOX – treated rats compared with the normal group (p<0.001). CA treatment at doses of 20 and 40 mg/kg led to a significant elevation in +dp/dt when compared with the DOX – treated group (p<0.01 and p<0.001). Also, CA treatment (40 mg/kg) significantly increased −dp/dt when compared with the DOX - treated group (p<0.01) (Table 1).
Effect of chelidonic acid on the ventricular activity.
| +dp/dt (mmHg) | −dp/dt (mmHg) | LVEDP (mmHg) | LVSP (mmHg) | Tau (s) | |
|---|---|---|---|---|---|
| Normal | 2397±68.74 | −1903±76.33 | 2.52±0.60 | 137.9±8.23 | 0.078±0.0068 |
| DOX | 1290±60.15### | −970.7±63.70### | 11.71±0.69### | 97.9±6.67# | 0.051±0.0073# |
| DOX+DEX | 1408±72.45 | −1176±43.99 | 7.94±0.92* | 124.1±8.12 | 0.066±0.0057 |
| DOX+CA (10 mg/kg) | 1433±61.19 | −1215±70.20 | 8.21±1.04* | 123.8±9.25 | 0.069±0.0078 |
| DOX+CA (20 mg/kg) | 1607±52.38** | −1100±85.46 | 6.76±0.90*** | 124.9±10.92 | 0.064±0.0092 |
| DOX+CA (40 mg/kg) | 2032±82.73*** | −1318±65.18** | 7.96±0.75* | 130.6±8.69* | 0.072±0.0045 |
All values are expressed as mean±SEM (n=7), ***p<0.001, **p<0.01, and *p<0.05 when compared with the DOX – treated group. ###p<0.001 when compared with the normal group.
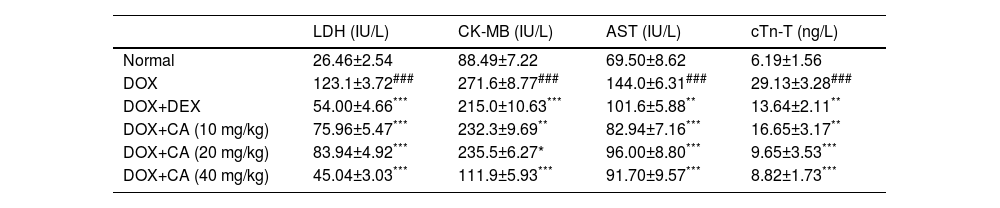
The DOX – treated rats exhibited significant elevation in cardiac markers such as LDH and AST. CA at doses of 10, 20, and 40 mg/kg significantly reduced LDH and AST levels when compared with the DOX – treated group. Dexrazoxane treated animals showed a significant decrease in the LDH when compared with the DOX – treated group (p<0.001). CK-MB concentration was significantly increased in the DOX - treated group and treatment with CA (10, 20, and 40 mg/kg) revealed a significant decrease in CK-MB when compared with the DOX – treated group (p<0.01, p<0.05, and p<0.001). The dexrazoxane-treated group showed a significant reduction in the CK-MB. In the DOX – treated group, there was a significant increase in cTn-T level when compared with the normal group (p<0.001). CA treatment at doses of 10, 20, and 40 mg/kg significantly decreased cTn-T level when compared with the DOX - treated group (p<0.01, p<0.001, and p<0.001). Dexrazoxane treated animals showed a significant decrease in the cTn-T when compared with the DOX – treated group (p<0.01) (Table 2).
Effect of chelidonic acid on cardiac marker enzymes.
| LDH (IU/L) | CK-MB (IU/L) | AST (IU/L) | cTn-T (ng/L) | |
|---|---|---|---|---|
| Normal | 26.46±2.54 | 88.49±7.22 | 69.50±8.62 | 6.19±1.56 |
| DOX | 123.1±3.72### | 271.6±8.77### | 144.0±6.31### | 29.13±3.28### |
| DOX+DEX | 54.00±4.66*** | 215.0±10.63*** | 101.6±5.88** | 13.64±2.11** |
| DOX+CA (10 mg/kg) | 75.96±5.47*** | 232.3±9.69** | 82.94±7.16*** | 16.65±3.17** |
| DOX+CA (20 mg/kg) | 83.94±4.92*** | 235.5±6.27* | 96.00±8.80*** | 9.65±3.53*** |
| DOX+CA (40 mg/kg) | 45.04±3.03*** | 111.9±5.93*** | 91.70±9.57*** | 8.82±1.73*** |
All values are expressed as mean±SEM (n=7), ***p<0.001, **p<0.01, and *p<0.05 when compared with the DOX – treated group. ###p<0.001 when compared with the normal group.
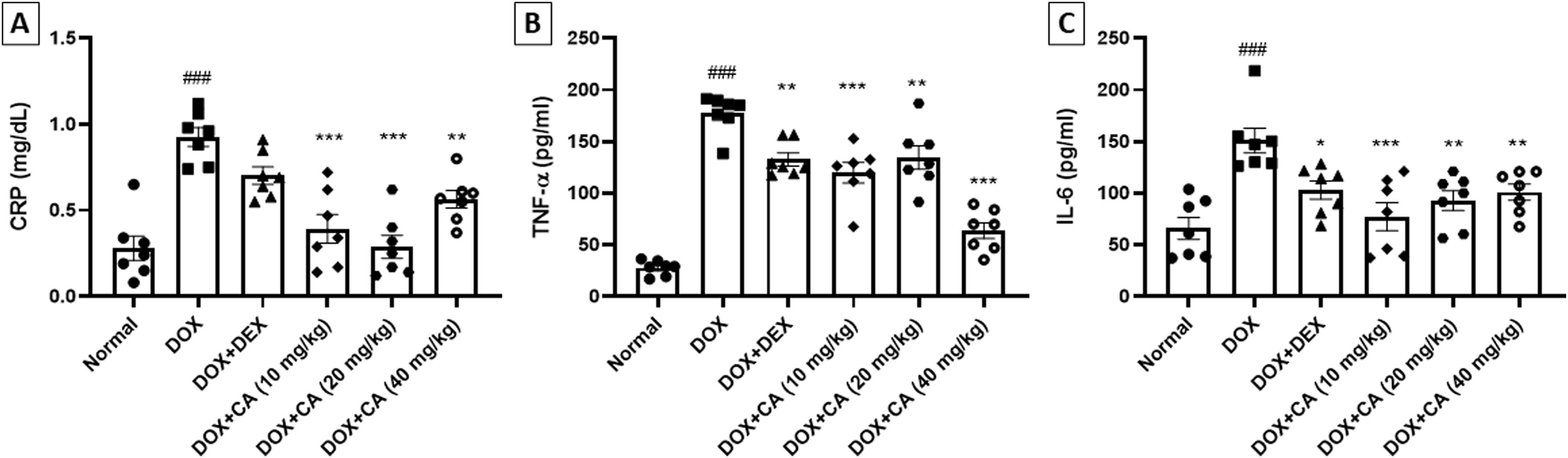
In the DOX – treated group, there was an increase in CRP, TNF-α and IL-6 concentration when compared with the normal group (p<0.001). CA treatment (10, 20, and 40 mg/kg) significantly decreased the CRP, TNF-α and IL-6 concentration when compared with the DOX – treated group (p<0.001 and p<0.01). In dexrazoxane-treated animals, a significant decrease in TNF-α and IL-6 concentrations was observed (Figure 5A–C).
Effect of chelidonic acid on inflammatory cytokines. (A) C-reactive protein (CRP), (B) tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). All values are expressed as mean±SEM (n=7), ***p<0.001, **p<0.01, and *p<0.05 when compared with the DOX – treated group. ###p<0.001 when compared with the normal group.
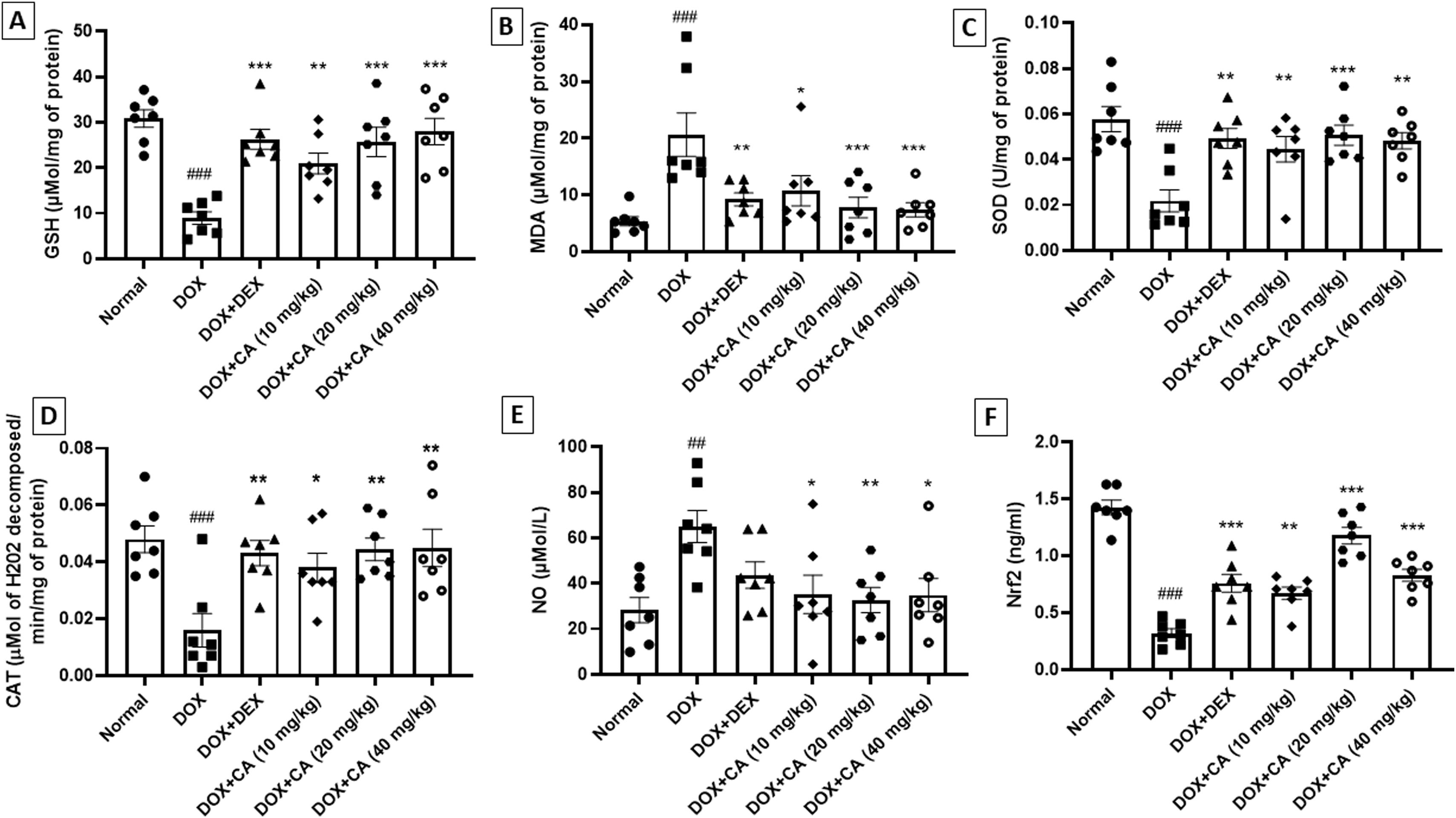
In the DOX – treated animals, there was a significant decrease in GSH and SOD levels and treatment with chelidonic acid (10, 20, and 40 mg/kg) significantly increased GSH and SOD levels when compared with the DOX – treated group (GSH: p<0.01, p<0.001, p<0.001 and SOD: p<0.01, p<0.001, p<0.01). Among dexrazoxane-treated rats, there was a significant increase in GSH and SOD levels. In the DOX – treated group, there was a noteworthy increase in the MDA level when compared with the normal group (p<0.001). CA treatment (10, 20, and 40 mg/kg) reduced the MDA level substantially. Among standard dexrazoxane-treated rats, there was a significant decrease in the MDA level when compared with DOX – treated group (p<0.01) (Figure 6A–C).
Effect of chelidonic acid on oxidative stress, NO, and Nrf2 concentration. (A) Glutathione (GSH), (B) malondialdehyde (MDA), (C) superoxide dismutases (SOD), (D) catalase (CAT), (E) nitric oxide (NO) and (F) nuclear factor erythroid 2-related factor 2 (Nrf2). All values are expressed as mean±SEM (n=7), ***p<0.001, **p<0.01, and *p<0.05 when compared with the DOX – treated group. ###p<0.001 and ##p<0.01 when compared with the normal group.
Catalase concentration was significantly decreased in DOX – treated animals when compared with the normal group (p<0.001). CA treatment (10, 20, and 40 mg/kg) significantly increased the catalase concentration. In the dexrazoxane-treated animals, there was a notable increase in catalase concentration when compared with the DOX – treated group (p<0.01). There was a noteworthy rise in NO concentration in the DOX – treated animals when compared with the normal group (p<0.01). CA treatment at doses of 10, 20, and 40 mg/kg reduced the NO concentration substantially (Figure 6D and E).
The Nrf2 concentration was significantly reduced in DOX – treated animals as compared with the normal group (p<0.001). CA treatment at doses of 10, 20, and 40 mg/kg significantly increased the Nrf2 concentration when compared with the DOX - treated animals (p<0.01, p<0.001, and p<0.001). There was a noteworthy rise in Nrf2 concentration in the standard dexrazoxane-treated group when compared with the DOX – treated group (p<0.001) (Figure 6F).
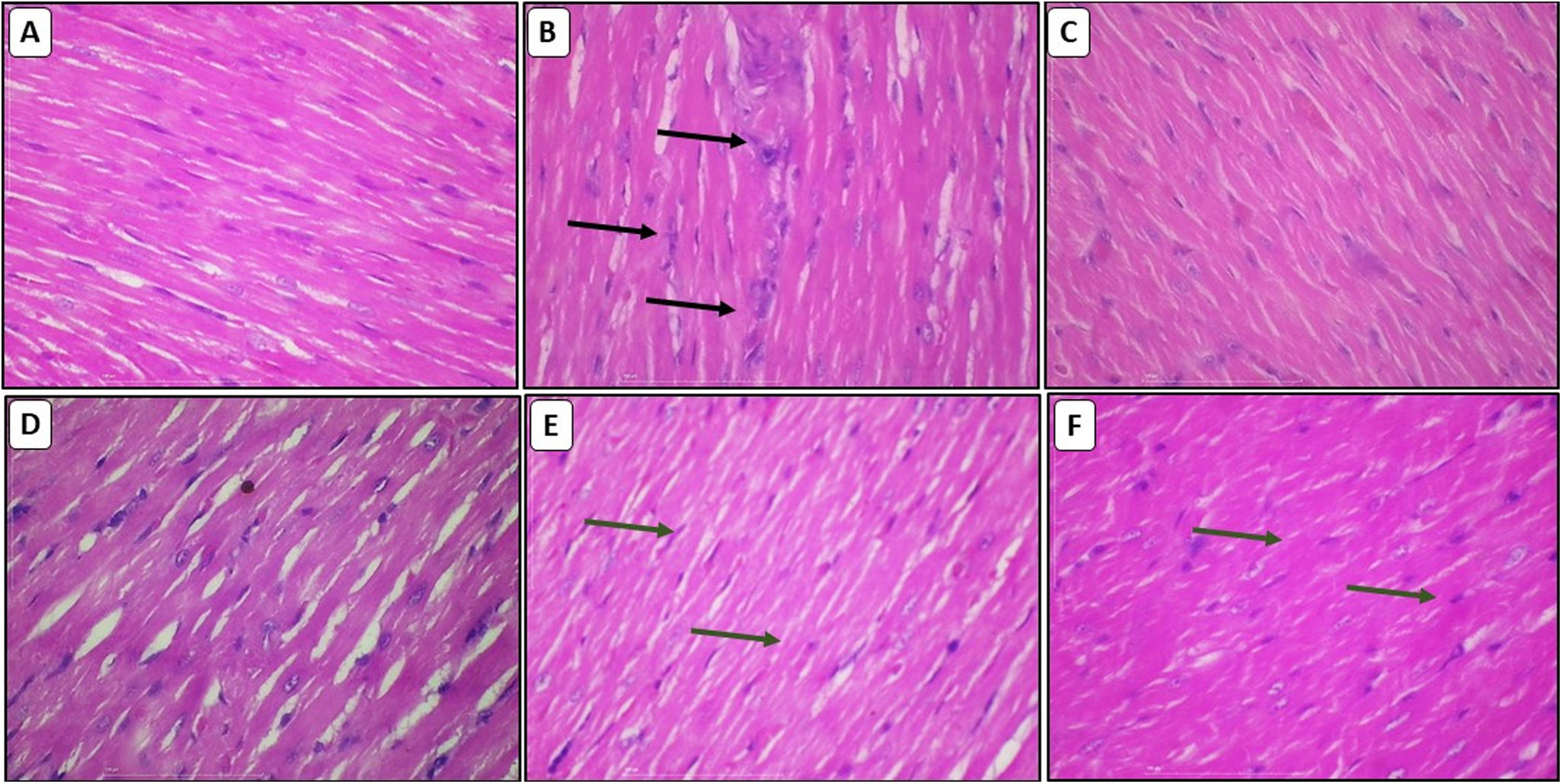
Effect of chelidonic acid on myocardial histologyH&E stainingH&E stain was used to investigate DOX-induced cardiomyopathy. The normal group displayed normal architecture in the cardiomyocytes. Cardiomyocytes in the DOX-treated group displayed intermuscular edema, myofibrillar loss, inflammatory cell infiltration, vacuolization, and cardiomyocyte degradation. CA treatment significantly reduced all these pathological alterations, and the cardiomyocyte architecture was essentially identical to that of the normal group (Figure 7A–F).
Effect of chelidonic acid on hematoxylin and eosin staining. (A) Normal, (B) DOX, (C) DOX+DEX, (D) DOX+chelidonic acid (10 mg/kg), (E) DOX+chelidonic acid (20 mg/kg), and (F) DOX+chelidonic acid (40 mg/kg) (n=7), black arrows indicated the damage and cell infiltration, while green arrows indicate the protection against myocardial damage.
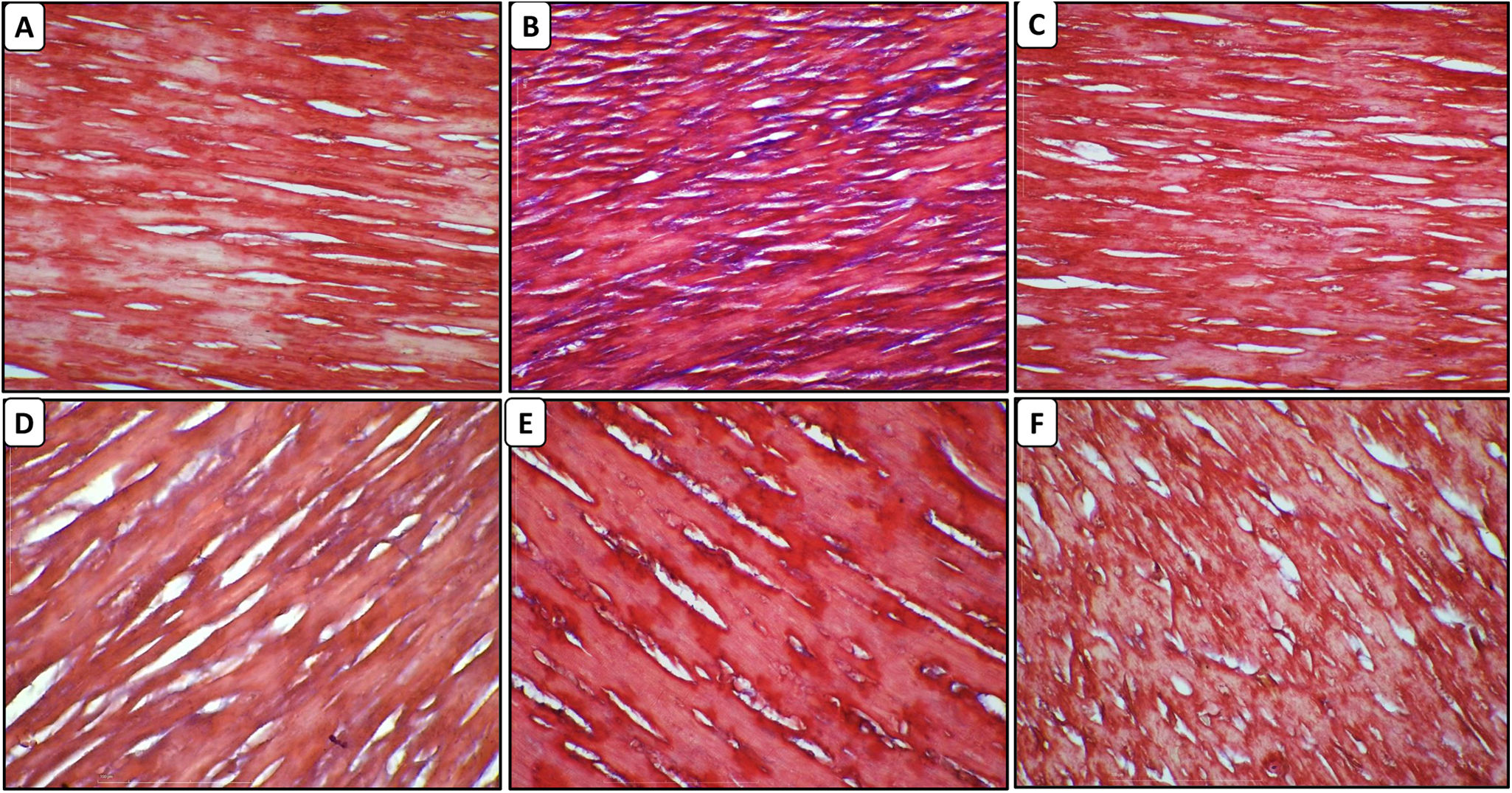
The cardiomyocytes of normal rats did not reveal any collagen deposition and fibrosis. In the DOX – treated animals, there was fibrosis and collagen deposition in the myocardium. Treatment with CA at a dose of 10, 20, and 40 mg/kg led to a reduction in collagen deposition and fibrosis. In the cardiac tissue of the dexrazoxane group, there was reduced collagen deposition (Figure 8A–F).
DiscussionThe most severe and detrimental side effect of doxorubicin is cardiotoxicity. Doxorubicin-induced cardiotoxicity is caused by a number of processes, including oxidative stress, autophagy, calcium dysregulation, mitochondrial dysfunction, apoptosis, necroptosis, and ferroptosis pathways.32
The widespread therapeutic use of doxorubicin in oncology still faces significant clinical challenges due to its associated cardiotoxicity.33 Therefore, it is necessary to ascertain new, reliable chemoprotective agents that could minimize or prevent the negative effects of doxorubicin. In the present study, probable benefits of CA were studied against DOX-induced cardiotoxicity. Study findings demonstrated that CA treatment reduced oxidative stress, inflammation, cardiac fibrosis, and necrosis caused by DOX in rats. Interestingly, CA also prevents DOX-induced cardiac dysfunction in rats.
Biologically active phytochemicals with health advantages are known as nutraceuticals. The use of nutraceuticals in treating a wide range of illnesses is currently gaining attention on a global scale. The field of nutraceuticals can be thought of as one of the pieces lacking an individual's overall health benefit.34
Rats given DOX displayed higher heart weight to body weight ratios and lower body weights. The enlarged, dilated, and hypertrophic atrium and ventricles may be responsible for a rise in heart weight. However, in the rats given CA treatment, all the aforementioned alterations were observed to be blocked.
Study findings on the heart's electrical potential investigations revealed that DOX challenge in rats caused cardiotoxicity, as seen by the changes in ECG indices.35 The effects of DOX on the ECG include an inverted p-wave, a longer PR interval, a prolonged QT, QRS, and QTc interval, as well as an elevation of the ST height. Many studies have reported that p-wave prolongation or increased PR interval in Wistar rats might be associated with high susceptibility to supraventricular arrhythmias following myocardial infarction.36 Supraventricular arrhythmias are associated with QRS complex narrowing. Large QRS complexes signify ventricular arrhythmias, and right and left bundle branch blockages, myocardial ischemia, and cardiac insufficiency all cause disturbances in intraventricular conduction.37
Chelidonic acid at a dose of 20 and 40 mg/kg considerably reduced the alterations in the p-wave, QRS complex, QT intervals, and ST segments. Heart rate was increased after prolonged doxorubicin treatment, which was in line with earlier studies that supported the theory that disturbances in calcium homeostasis may result from reactive oxygen species formation. The pacemaker cells in the SA node and other cells in the cardiac conducting system might increase or decrease excitability caused by the increase in intracellular calcium. Cardiovascular function, both systolic and diastolic, is compromised by doxorubicin. Additionally, doxorubicin eliminates the cardiac sarcoplasmic reticulum vesicle's ability to load calcium. Overall, doxorubicin alters calcium homeostasis and affects hemodynamic physiology.38 Hence, LVEDP, dp/dt max, and dp/dt min are the direct indicators of cardiac function. In the present study, treatment with CA significantly reversed the LVEDP, systolic, and diastolic blood pressure, which indicates that cardiac function increased significantly. Also, the changes in dp/dt max and dp/dt min were significantly attenuated by CA at 20 and 40 mg/kg.
Raised levels of AST, LDH, cTn-T, and CK-MB in serum are considered indicators of myocardial injury.39 DOX has significantly elevated AST, LDH, cTn-T, and CK-MB. This demonstrates that DOX caused cardiotoxicity in rats. These metabolic alterations caused by DOX were reversed by CA. The considerable rise in blood levels of AST, LDH, cTn-T, and CK-MB following DOX treatment is consistent with other studies that indicate DOX-induced oxidative stress can result in lipid peroxidation and the release of these enzymes into the serum.39,40
Inflammation of the cardiac tissue is a serious adverse event that has repeatedly been observed in animals given DOX. Additionally, there is strong evidence that DOX triggers a cascade of inflammatory reactions inside the myocardium by activating NF-κB and causing the release of several pro-inflammatory cytokines, including TNF-α, IL-6, and CRP levels.41,42 Recent research reports have shown that pro-inflammatory cytokines are rising inside cardiac tissue, which may be the pathophysiological cause of DOX-induced cardiomyopathy.43 NF-κB specifically regulates many chemokines, including TNF-α, IL-6, and many others, which are elevated in patients with inflammatory diseases. Consequently, it has been established that TNF-α and IL-6 are excellent targets for molecular treatments used to treat inflammatory illnesses. Shin et al., reported that, CA suppresses NF-κB translocation to the nucleus.16
According to the results of our research, cardiac TNF-α, IL-6, and CRP levels were significantly greater in the DOX group than in the normal group, supporting earlier studies that inflammation plays a significant role in the pathophysiology of DOX-induced cardiotoxicity. The results of the current study showed that CA therapy significantly decreased levels of TNF-α, IL-6, and CRP, indicating that CA has a reliable cytoprotective effect against the release of inflammatory mediators by DOX. Also, it was previously reported that CA reduces IL-6 production by blocking nuclear factor kappa B (Nf-kB) and activating caspase-1 in mast cells, which is a part of the AMPK signaling pathway.20
Doxorubicin administration resulted in a significant rise in lipid peroxidation in rats, which was preceded by marked elevations in MDA, a notable reduction in glutathione (GSH), and exhaustion of cardiac antioxidant enzymes such as catalase (CAT) and superoxide dismutase (SOD) due to excessive consumption by DOX-generated free radicals.44,45 The elevated levels of GSH, SOD, and catalase and decreased levels of MDA in our study are due to CA's antioxidant properties, which suppressed the oxidative process in the heart. Nuclear factor erythroid 2-related factor (Nrf2) is expressed less when ROS levels are elevated, which makes cells more susceptible to oxidative stress and death.46 DOX significantly raised the concentration of NO in the myocardium. NOS is successfully converted by DOX from a source of nitric oxide (NO) to one that produces superoxide.47
According to the reported studies, DOX reduces Nrf2 concentration which results in oxidative stress-related damage.48 Nrf2 activation can reduce the oxidative stress brought on by DOX and inhibit autophagy.49 These findings suggest that CA is involved in the regulation of Nrf2 concentration and that it has an inhibitory effect on oxidative stress, as seen by a drop in MDA and a rise in GSH levels, which points to its expected cardioprotective mechanism.
DOX treatment led to significant myocardial edema, cytoplasmic vacuolization, perinuclear vacuolization, disarray of myocardial fibers, and myofibrillar loss, which were all histological abnormalities. These cellular and structural alterations are comparable with various earlier studies on rats in DOX-induced cardiotoxicity.50,51
The histopathological results of our study showed that CA can lessen cardiac tissue lesions caused by DOX, as shown by the group receiving DOX experiencing a considerable improvement in severity. Masson's trichrome stained histology of a left ventricular segment from the heart of a rat treated with CA revealed sparse collagen deposition and fibrosis. These findings support earlier reports that CA has an anti-inflammatory response in addition to its direct impact on oxidative stress.14,18
ConclusionThe findings of this study demonstrated that CA restores blood pressure with ECG, inhibits oxidative stress, raises Nrf2 concentration, and reduces fibrosis to protect the heart from DOX-induced cardiotoxicity. Further studies are needed to prove the mechanism of CA in response to DOX-induced cardiotoxicity.
Ethical approvalAll animal experiments were approved by the Ethics Committee and followed the Guide for the Care and Use of Laboratory Animals. The Institutional Animal Ethics Committee (IAEC) of Shri Vile Parle Kelavani Mandal accepted and approved the protocol (protocol approval number – CPCSEA/IAEC/P-42/2021).
FundingThis research did not receive any specific grant from any funding agency.
Authors’ contributionsY.K., K.S. and S.K. designed the experiments. S.K. performed the animal experiments. S.K., Y.K. and K.S. interpreted the results and wrote the manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
Data availabilityThe data underlying this article will be shared on reasonable request to the corresponding author.


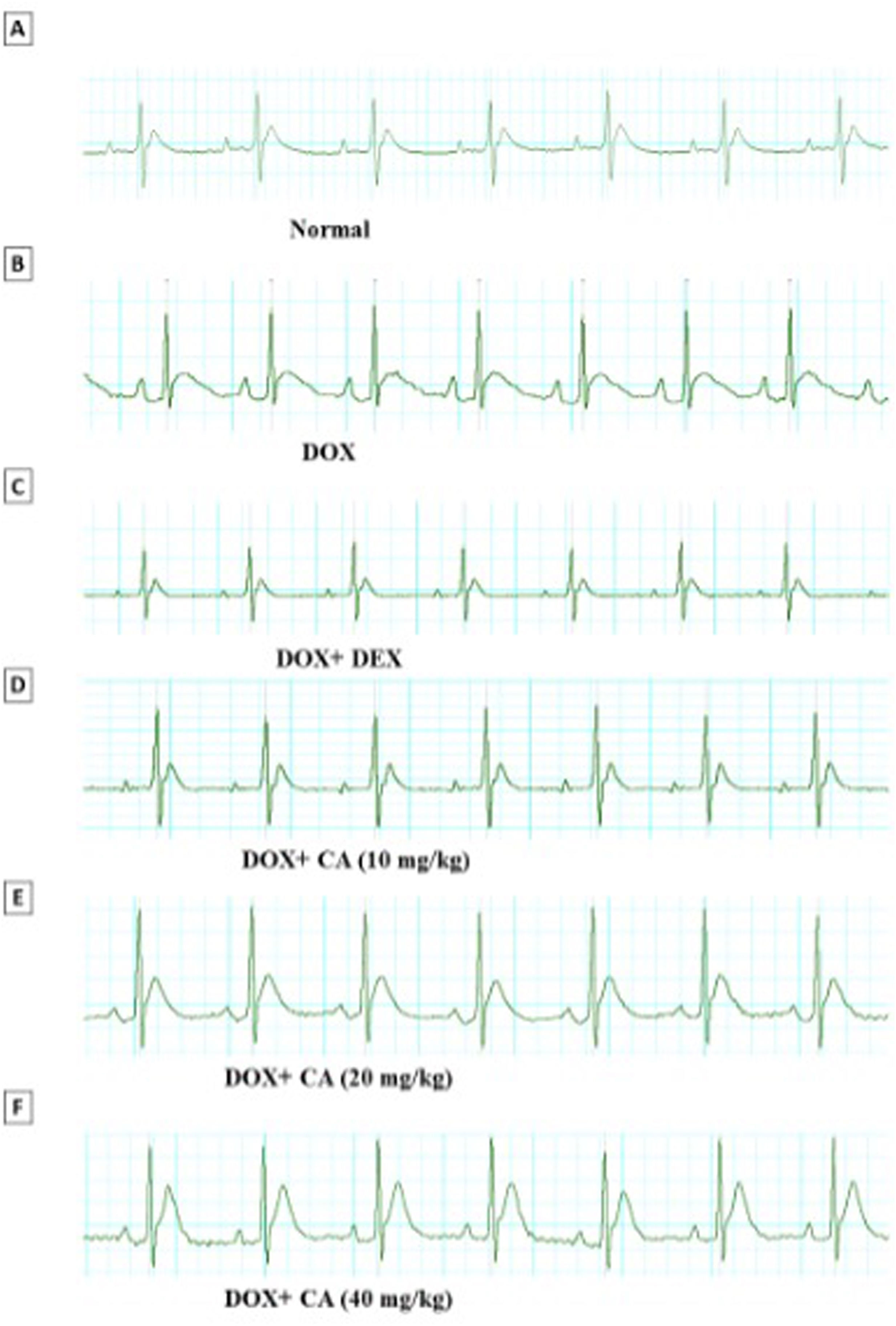

![Representative electrocardiogram images of various groups. (A) Normal, (B) DOX, (C) DOX+DEX, (D) DOX+chelidonic acid (10 mg/kg), (E) DOX+chelidonic acid (20 mg/kg), and (F) DOX+chelidonic acid (40 mg/kg)] (n=7). Representative electrocardiogram images of various groups. (A) Normal, (B) DOX, (C) DOX+DEX, (D) DOX+chelidonic acid (10 mg/kg), (E) DOX+chelidonic acid (20 mg/kg), and (F) DOX+chelidonic acid (40 mg/kg)] (n=7).](https://static.elsevier.es/multimedia/08702551/0000004400000003/v2_202509180520/S0870255124002646/v2_202509180520/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)








