Cardioneuroablation (CNA), a technique based on radiofrequency ablation of cardiac vagal ganglia, was developed to treat recurrent vasovagal syncope (VVS) with a predominant cardioinhibitory component, as an alternative to pacemaker implantation. The aim of our study was to evaluate the safety and success rate of CNA guided by extracardiac vagal stimulation in patients with highly symptomatic cardioinhibitory VVS.
MethodsProspective study of patients who underwent anatomically guided CNA at two cardiology centers. All patients had a history of recurrent syncope with a predominant cardioinhibitory component and refractory to conventional measures. Acute success was determined by the absence or significant reduction of cardiac parasympathetic response to extracardiac vagal stimulation. The primary endpoint was the recurrence of syncope during follow-up.
ResultsIn total, 19 patients (13 males; mean age 37.8±12.9 years) were included. Ablation was acutely successful in all patients. One patient had a convulsive episode after the procedure, which was deemed unrelated to the ablation, requiring admission to intensive care but without sequelae. No other complications occurred. At a mean follow-up of 21.0±13.2 months (range 3–42 months), 17 patients remained free of syncope. The remaining two patients had recurrence of syncope and, despite undergoing a new ablation procedure, required pacemaker implantation during follow-up.
ConclusionCardioneuroablation, confirmed by extracardiac vagal stimulation, appears to be an effective and safe treatment option for highly symptomatic patients with refractory VVS with a predominant cardioinhibitory component, providing a new potential approach as an alternative to pacemaker implantation.
A cardioneuroablação, técnica baseada na ablação por radiofrequência dos plexos ganglionares parassimpáticos cardíacos, foi desenvolvida para tratar a síncope vasovagal com componente predominantemente cardioinibitório como alternativa à implantação de pacemaker. O objetivo do nosso estudo foi avaliar a segurança e o sucesso da cardioneuroablação guiada pela estimulação vagal extracardíaca em doentes com síncope vasovagal cardioinibitória recorrente.
MétodosEstudo prospetivo de doentes submetidos a cardioneuroablação anatómica e guiada pela estimulação vagal extracardíaca. Todos os doentes apresentavam história de síncope recorrente predominantemente cardioinibitória e refratária às terapêuticas convencionais. O sucesso do procedimento foi definido pela ausência ou redução significativa da resposta parassimpática à estimulação vagal extracardíaca. O endpoint primário foi a recorrência de síncope durante o follow-up.
ResultadosNo total, 19 doentes (13 homens; idade média 37,8±12,9 anos) foram incluídos. O procedimento foi realizado com sucesso em todos os doentes. Um doente apresentou um episódio convulsivo não relacionado com a ablação após o procedimento, necessitando de admissão em unidade de cuidados intensivos, mas sem sequelas neurológicas. Não foram observadas outras complicações. No tempo de seguimento médio de 21,0±13,2 meses (entre 3 e 42 meses), 17 doentes mantiveram-se livres de síncope. Os outros dois doentes apresentaram recorrência de síncope e, apesar de submetidos a um novo procedimento de cardioneuroablação, necessitaram de implantação de pacemaker transvenoso durante o follow-up.
ConclusãoA cardioneuroablação, confirmada pela estimulação vagal extracardíaca, apresenta-se como uma opção terapêutica eficaz e segura no tratamento da síncope vasovagal cardioinibitória refratária e oferece uma nova abordagem potencial como alternativa à implantação de pacemaker.
Vasovagal syncope (VVS) is the most common etiology of sudden and transient loss of consciousness. It occurs due to cerebral hypoperfusion caused by inappropriate reflex peripheral vasodilation and/or bradycardia in response to a trigger.1,2 It mainly affects young patients without cardiac or neurological structural disease. Despite its benign prognosis, frequent episodes are associated with poor quality of life and increased risk of physical injury.3
Conventional therapies such as education, fluid and salt intake, physical maneuvers, orthostatic training, and pharmacological treatments have limited efficacy in the prevention of recurrent syncope.4,5 According to current recommendations, cardiac pacing is indicated in patients >40 years with severe, recurrent syncope and documented cardioinhibitory reflex.6 However, in younger patients no recommendation is given.6 Pacemaker therapy in these patients is of limited efficacy,7–12 and long-term complications after pacemaker implantation are not negligible. Thus, the decision to implant a pacemaker in young patients with recurrent vasovagal syncope (VVS) is a difficult one.
Vasovagal syncope can be classified as cardioinhibitory when bradycardia or asystole predominate, vasodepressor when a loss of vasoconstrictor tone is predominant or mixed when there is a combination of these two mechanisms.1,2,6 Cardiac innervation consists of parasympathetic, sympathetic, and sensory systems. While the neural body of the post-ganglionic sympathetic and sensory neurons are located in paravertebral ganglia chain and nodose ganglia, the parasympathetic ganglionated plexus (GP) are located in the heart, mainly in the atrial wall and sub-epicardially, surrounding the regions of the sinus and atrioventricular nodes.13–15 Enhanced vagal tone in these patients, together with the accessible location of parasympathetic GP, explains the rationale behind the cardioneuroablation (CNA) technique, developed by Pachon in the 1990s.16 This procedure consists of vagal denervation by using radiofrequency endocardial ablation of the neuromyocardial interface. Since its inception, reports have been published on its use,17–19 however, the most recent European Society of Cardiology guidelines on the management of syncope make only a small reference to this technique.6 European cardiac pacing guidelines make no reference to CNA as a possible alternative to pacing in reflex syncope.20
ObjectivesThe aim of our study was to assess the safety and success rate of CNA guided by extracardiac vagal stimulation in eliminating recurrent VVS in a real-world cohort of patients.
MethodsStudy designProspective non-randomized study of patients with recurrent VVS of a predominantly cardioinhibitory nature who underwent CNA in two Portuguese hospitals from January 2019 to April 2022. Patient data at the time of the procedure and at follow-up were collected to assess acute and follow-up outcomes.
All patients received detailed information on the methodology, benefits, and risks of the procedure, and signed an informed consent in accordance with the local ethics committee. The study was conducted according to the Declaration of Helsinki.
Inclusion criteria and sample characterizationInclusion criteria were: (i) a history of recurrent syncope with a predominant or exclusive cardioinhibitory component as determined by the occurrence of cardioinhibition during electrocardiogram (ECG) monitoring, implantable loop recorder or a head-up tilt test with symptom reproduction; (ii) refractoriness to conventional measures including optimal fluid intake, dietary advice, physical counterpressure training and pharmacological therapies whenever a vasodepressor component was associated; (iii) no other causes of loss of consciousness apparent based on the clinical history and initial study, including a 12-lead ECG, echocardiogram and 24-hour Holter.
Exclusion criteria included a previous history of heart surgery or pacemaker implantation, significant structural cardiomyopathy, or an additional indication for pacemaker implantation such as sinus node dysfunction or advanced AV conduction abnormalities. Vagally-mediated bradycardia was distinguished from conduction system disease based on the clinical history (age, context where syncope occurred, prodrome and triggers) and results of treadmill stress test, and 24-hour Holter.
Cardioneuroablation protocolAll procedures were performed under general anesthesia with intravenous propofol, fentanyl and occasionally rocuronium. Vital signs were monitored during the procedure. Brain function was monitored by BIS Aspect A-1000. A decapolar catheter was positioned in the coronary sinus and further advanced into the right internal jugular vein up to the jugular foramen for extracardiac non-contact vagal stimulation. Another quadripolar catheter was placed in the atria to perform atrial pacing. Intravenous heparin was administered after a single transseptal puncture guided by fluoroscopy to maintain an activated clotting time above 300 seconds. Intracardiac echocardiography was used at the discretion of the operator.
The three-dimensional (3D) geometry of the left atrium and pulmonary veins was constructed using a multipolar mapping catheter (PENTARAY®) with a 3D navigation system (CARTO-3, Biosense Webster Inc., Diamond Bar, CA). After electroanatomic map construction, extracardiac vagal stimulation was performed using the decapolar catheter placed in the internal jugular vein and a stimulator that releases square wave pulses of 50 microseconds width, frequency of 50 Hz and amplitude from 10 to 70 V, adjusted per patient weight (1 V/kg up to 70 V).21 Baseline response to vagal stimulation with or without atrial pacing was recorded. A positive vagal response was defined as transient asystole, severe sinus bradycardia and/or atrioventricular block during stimulation. A mixed sinus and AV nodal response was defined as the occurrence of asystole or severe sinus bradycardia, plus AV block (i.e., non-conducted p waves) with atrial pacing.
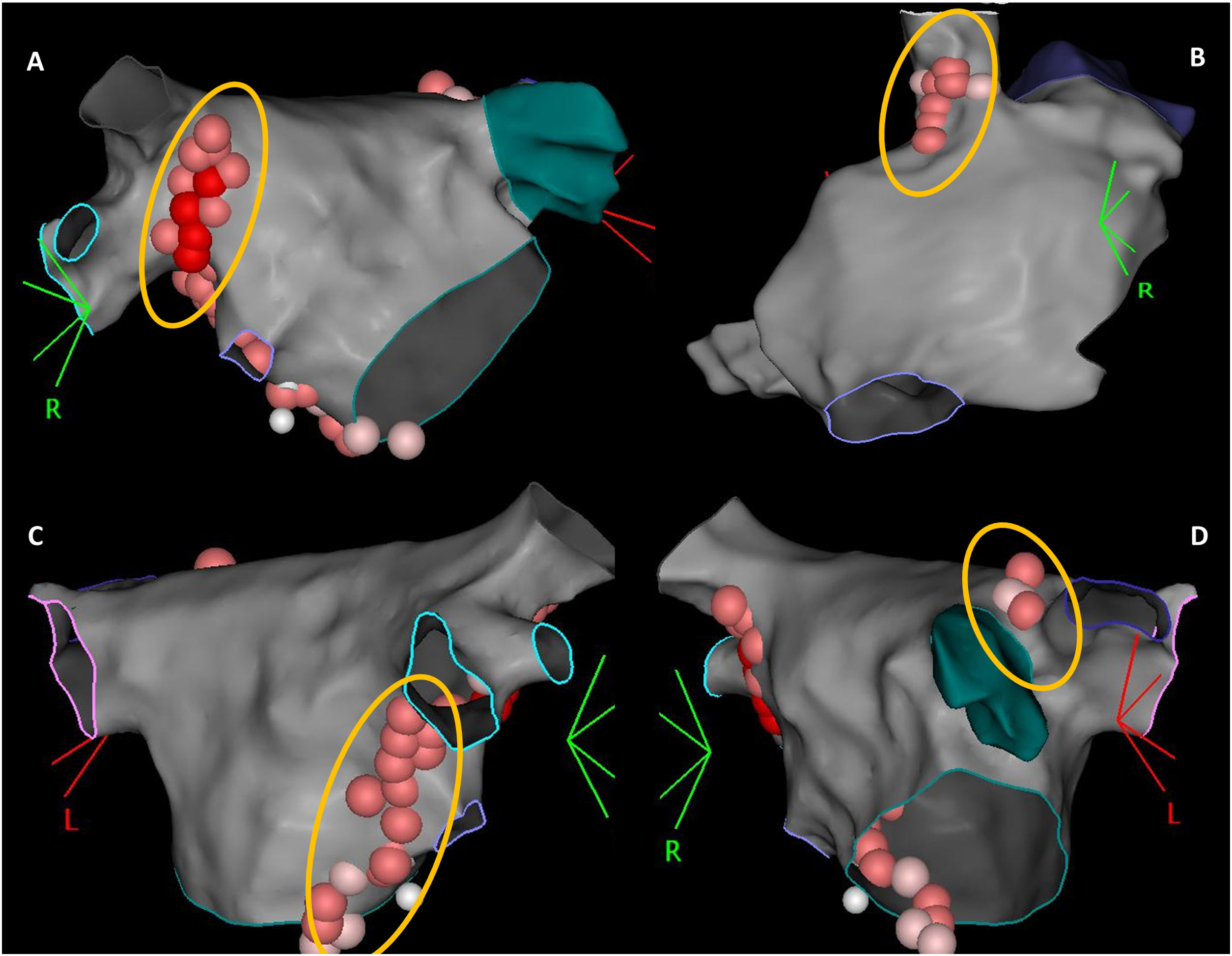
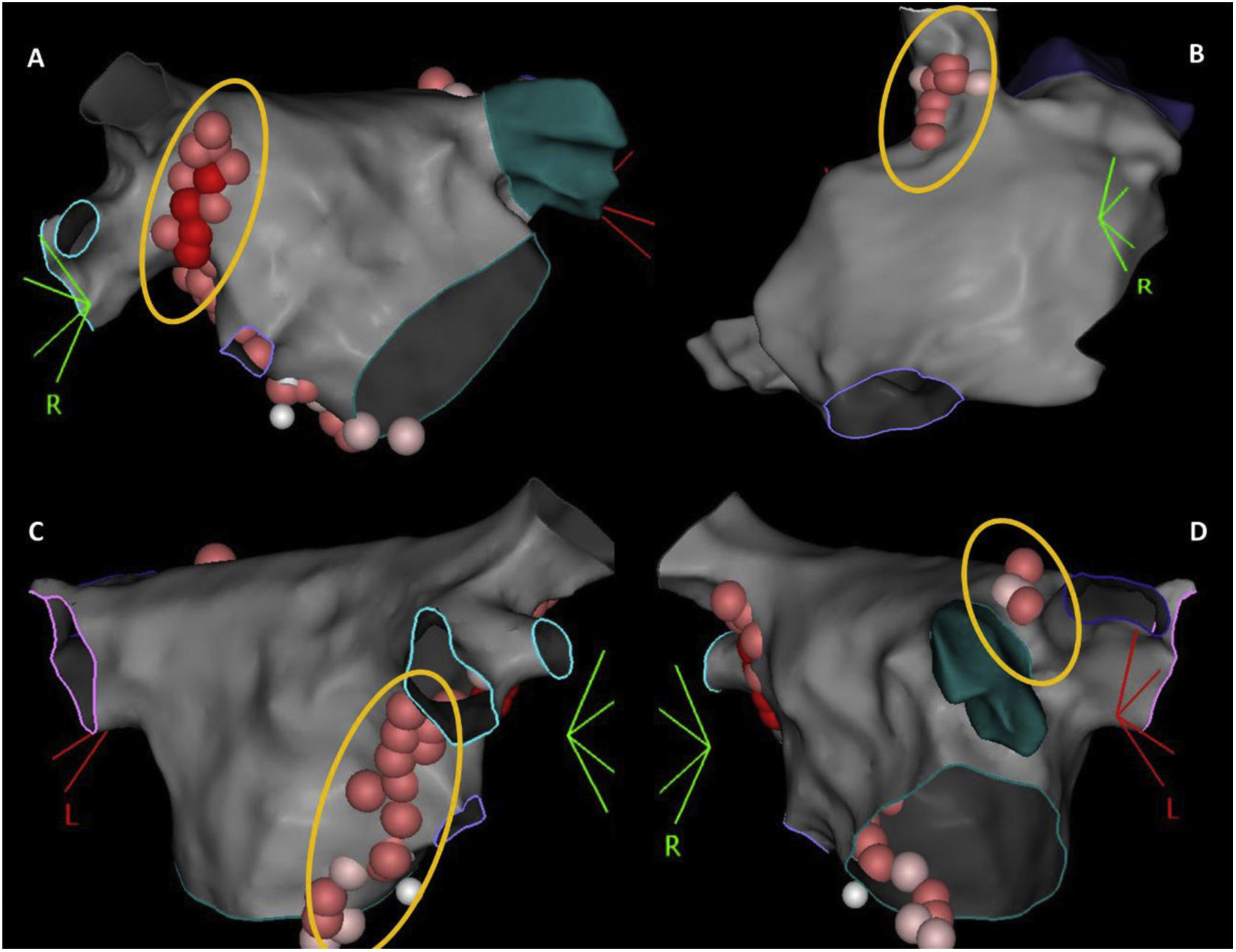
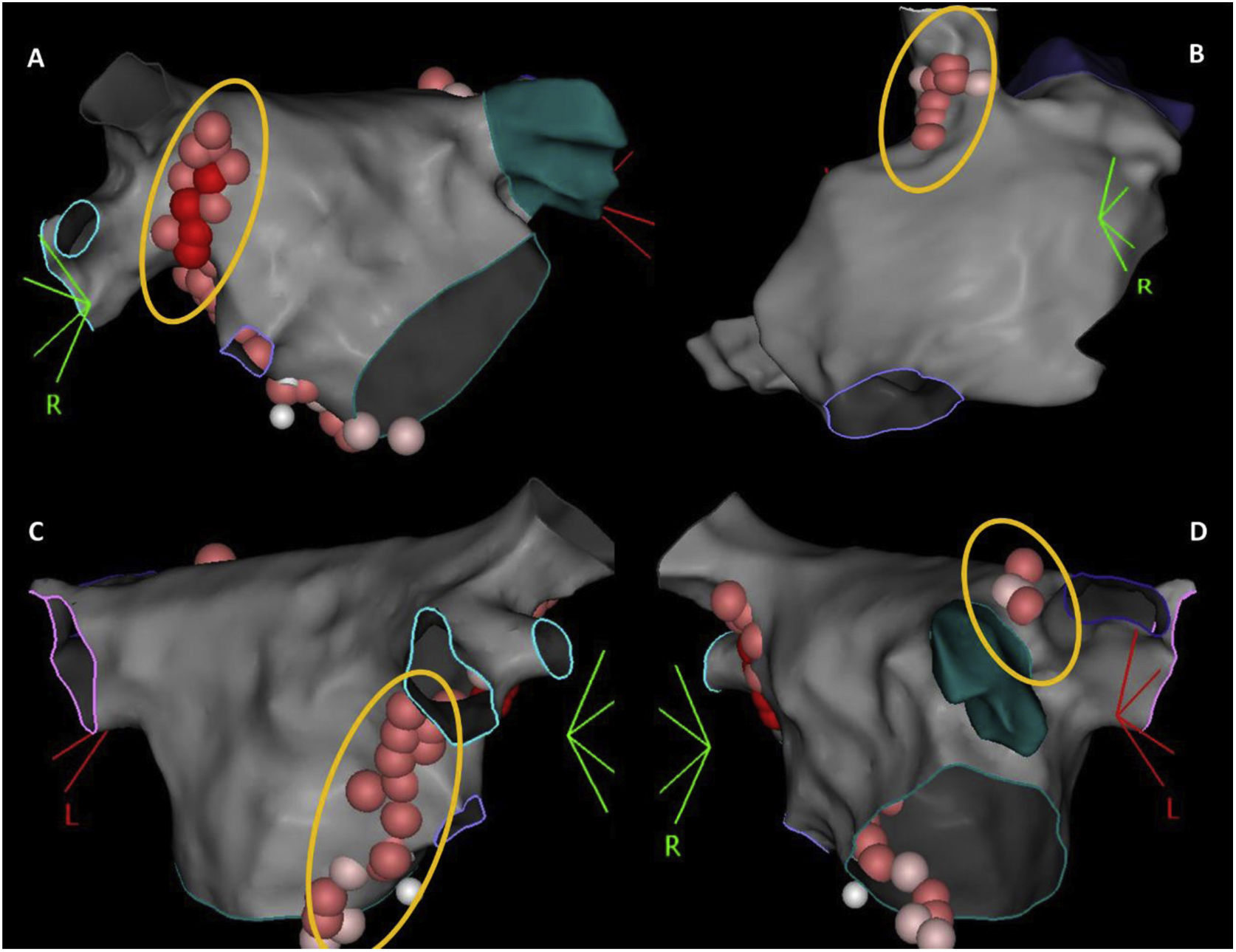
According to the type of rhythm disturbance triggered by extracardiac vagal stimulation, and also that which occurred clinically, sequential, and anatomically guided GP ablation was directed toward the ganglia most likely responsible. For patients with asystole or severe sinus bradycardia, ablation always started at the right anterior GP (RAGP), at the superoanterior area near the root of the right superior PV. If positive vagal response persisted, ablation continued, whenever necessary: (i) at the left superior GP (LSGP), located in the superolateral area around the root of the left superior PV and between the left superior PV and the left atrial appendage, and at the left lateral GP (LLGP) between the LAA and the left superior and left inferior GPs (more likely to affect AV nodal response); (ii) at the right inferior GP (RIGP) at the postero-inferior area near the root of the right inferior PV, at the postero-inferior border of the interatrial septum and posterior to the CS ostium (more likely to affect AV nodal response); (iii) at the right superior GP (RSGP) located between the superior vena cava and the aortic root just above the right superior pulmonary vein, at the postero-medial SVC-right atrial junction (more likely to affect sinus nodal response) (Figure 1). The left inferior GP area, at the infero-posterior border of the left inferior PV, was typically avoided to reduce the risk of esophageal lesion. RF ablation was performed using an irrigated catheter with contact force sensor (THERMOCOOL SMARTTOUCH™ SF Catheter) and guided by the ablation index, ranging between 380 and 500, according to the location. Extracardiac vagal stimulation was repeated after each GP group ablation to assess the degree of parasympathetic denervation. The elimination of cardiac parasympathetic response to vagal stimulation (i.e., elimination of asystole, severe bradycardia, or AV block) was the acute endpoint of the procedure.
The 3-dimensional representation of the main ganglionated plexus (GP) location. Upper panel: (A) RAGP between the right upper pulmonary vein and the right atrium; (B) RSGP between the superior vena cava and aortic root just above the right upper pulmonary vein; lower panel: (C) RIGP at the roof of the coronary sinus and medial and just under the tricuspid valve and the coronary sinus ostium; (D) LSGP at superolateral area around the root of the left superior pulmonary vein.
LSGP: left superior ganglionated plexus; RAGP: right anterior ganglionated plexus; RIGP: right inferior ganglionated plexus; RSGP: right superior ganglionated plexus.
After the procedure, patients were observed and underwent continuous ECG monitoring and transthoracic echocardiogram before discharge (24 hours).
Study endpoints and follow-upThe primary endpoint was the occurrence of syncope during follow-up. Prodromes including dizziness, fatigue or diaphoresis were documented but not considered a treatment failure.
All patients received oral anticoagulation with direct oral anticoagulants for at least one month after the procedure. Regular clinical post-ablation follow-up was performed individually by each physician. Clinical data and medical examinations were obtained through the analysis of the national clinical registry and through a phone call to the patient.
Statistical analysisContinuous data are expressed as mean±SD or median±interquartile range. Categorical data were expressed as percentages. SPSS version 26.0 (IBM) was used for statistical analysis.22
ResultsBaseline characteristicsA total of 21 ablation procedures were performed in 19 patients fulfilling the inclusion criteria (68% males, mean age 37.8±12.9 years, range 19–72 years). Baseline clinical characteristics of the study population are presented in Table 1. All patients had documented cardioinhibitory response by head-up tilt test (n=6), 24-hour Holter (n=6), implantable loop recorder (n=4) or electrocardiographic monitoring during hospitalization (n=3). The mean number of syncopal episodes per patient in the year before the procedure was 2.8 (ranging from 1 to 10), with five syncopal episodes occurring in four patients resulting in significant trauma, which included three intracranial hemorrhages requiring hospitalization, two jaw fractures requiring surgery and one rib fracture. One patient (case H) had hypertrophic cardiomyopathy with no evidence of outflow tract obstruction or ventricular systolic dysfunction, while the remaining patients had no structural heart disease. One patient (case B, 72 years old) underwent pulmonary vein isolation simultaneously as he had a history of symptomatic vagally-induced paroxysmal atrial fibrillation. Patient B had a history of recurrent VVS with documented cardioinhibition, as well as atrial fibrillation with onset either during the night or during/following an episode of vagally-induced asystole. There were no data suggesting conduction system disease (i.e., no clear indication for pacemaker implantation), based on a normal ECG and good heart rate response in a treadmill stress test and a 24-hour Holter.
Baseline clinical characteristics of the study population.
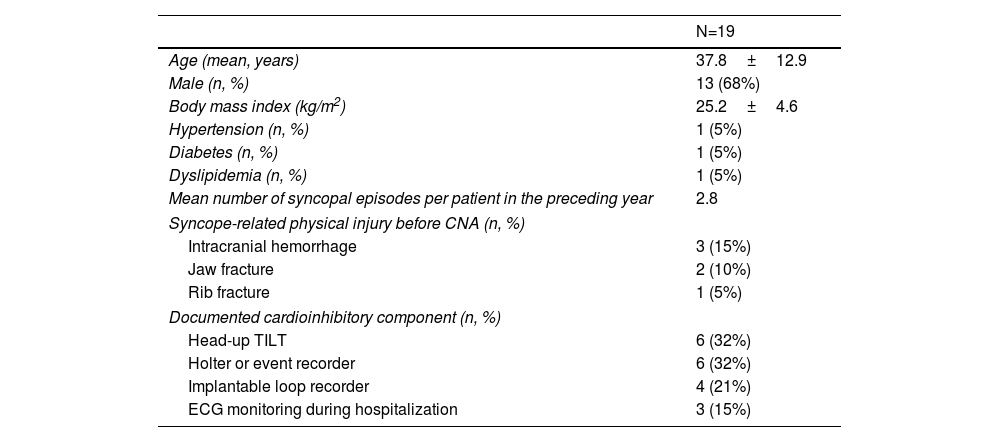
| N=19 | |
|---|---|
| Age (mean, years) | 37.8±12.9 |
| Male (n, %) | 13 (68%) |
| Body mass index (kg/m2) | 25.2±4.6 |
| Hypertension (n, %) | 1 (5%) |
| Diabetes (n, %) | 1 (5%) |
| Dyslipidemia (n, %) | 1 (5%) |
| Mean number of syncopal episodes per patient in the preceding year | 2.8 |
| Syncope-related physical injury before CNA (n, %) | |
| Intracranial hemorrhage | 3 (15%) |
| Jaw fracture | 2 (10%) |
| Rib fracture | 1 (5%) |
| Documented cardioinhibitory component (n, %) | |
| Head-up TILT | 6 (32%) |
| Holter or event recorder | 6 (32%) |
| Implantable loop recorder | 4 (21%) |
| ECG monitoring during hospitalization | 3 (15%) |
CNA: cardioneuroablation.
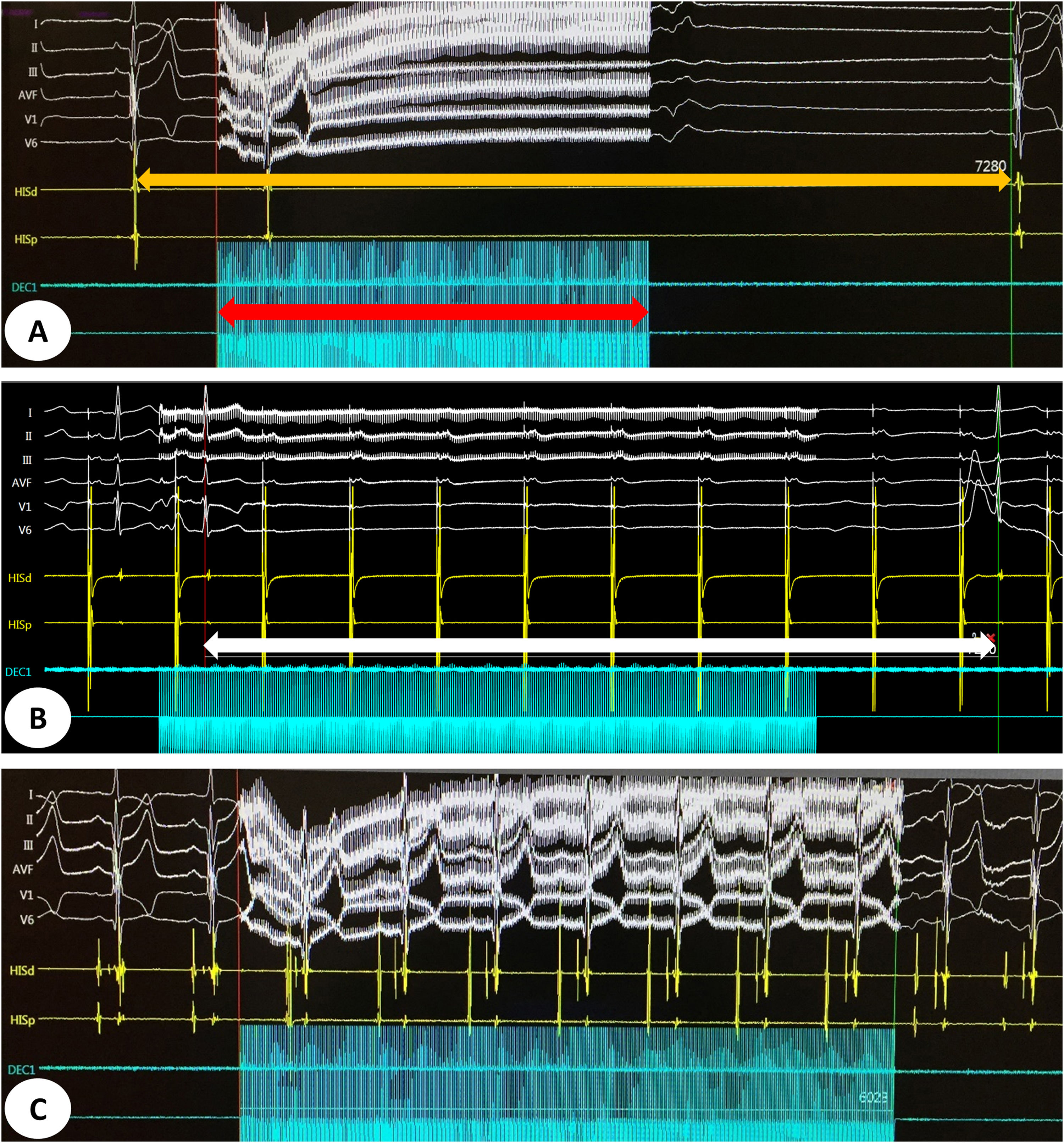
Detailed characteristics of the procedures are presented in Table 2. The mean procedure and fluoroscopy times were 115±30 min and 8.8±5.1 min, respectively. A positive mixed vagal response with extracardiac vagal stimulation was seen in all patients (asystole, and atrioventricular block when performing atrial pacing during vagal stimulation). The acute endpoint of the procedure (inhibition of vagal response after GP ablation) was achieved in all patients. Figure 2 shows an example of an initial mixed sinus and AV nodal vagal response and the result after successful CNA.
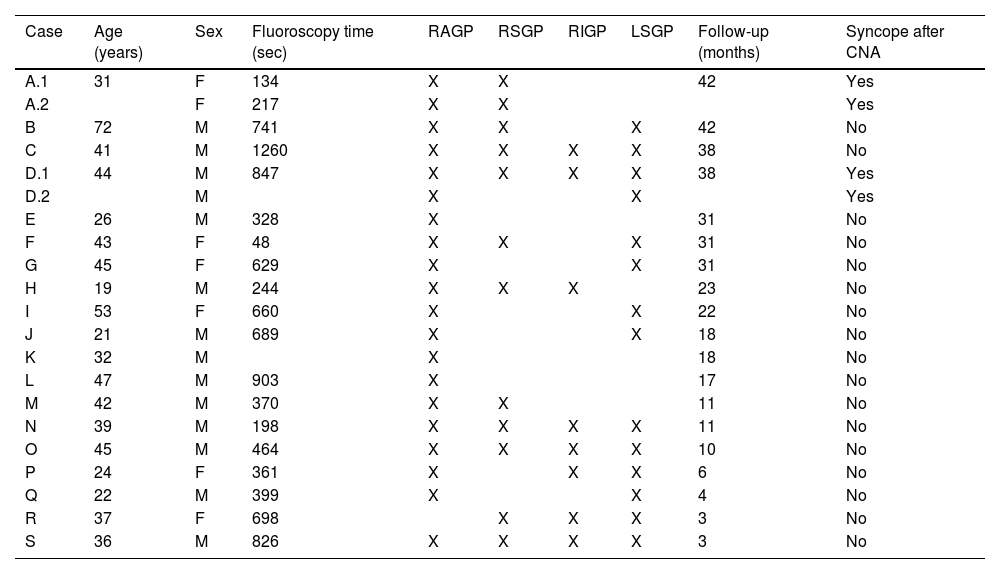
Procedural characteristics and ablation results of the study population.
| Case | Age (years) | Sex | Fluoroscopy time (sec) | RAGP | RSGP | RIGP | LSGP | Follow-up (months) | Syncope after CNA |
|---|---|---|---|---|---|---|---|---|---|
| A.1 | 31 | F | 134 | X | X | 42 | Yes | ||
| A.2 | F | 217 | X | X | Yes | ||||
| B | 72 | M | 741 | X | X | X | 42 | No | |
| C | 41 | M | 1260 | X | X | X | X | 38 | No |
| D.1 | 44 | M | 847 | X | X | X | X | 38 | Yes |
| D.2 | M | X | X | Yes | |||||
| E | 26 | M | 328 | X | 31 | No | |||
| F | 43 | F | 48 | X | X | X | 31 | No | |
| G | 45 | F | 629 | X | X | 31 | No | ||
| H | 19 | M | 244 | X | X | X | 23 | No | |
| I | 53 | F | 660 | X | X | 22 | No | ||
| J | 21 | M | 689 | X | X | 18 | No | ||
| K | 32 | M | X | 18 | No | ||||
| L | 47 | M | 903 | X | 17 | No | |||
| M | 42 | M | 370 | X | X | 11 | No | ||
| N | 39 | M | 198 | X | X | X | X | 11 | No |
| O | 45 | M | 464 | X | X | X | X | 10 | No |
| P | 24 | F | 361 | X | X | X | 6 | No | |
| Q | 22 | M | 399 | X | X | 4 | No | ||
| R | 37 | F | 698 | X | X | X | 3 | No | |
| S | 36 | M | 826 | X | X | X | X | 3 | No |
CNA: cardioneuroablation; LSGP: left superior ganglionated plexus; RAGP: right anterior ganglionated plexus; RIGP: right inferior ganglionated plexus; RSGP: right superior ganglionated plexus.
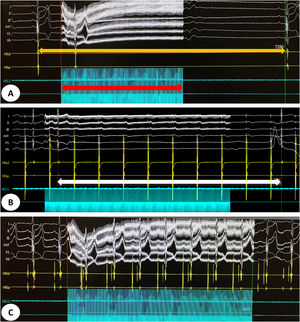
(A) Intracardiac electrogram showing a sinus pause of 7.28 s (orange arrow) induced by vagal stimulation for 4 s (red arrow) before cardioneuroablation. (B) Intracardiac electrogram showing an atrioventricular block (white arrow) induced by vagal stimulation during atrial pacing. (C) Intracardiac electrogram showing abolition of vagal response after cardioneuroablation.
Considering the division into four main groups of parasympathetic ganglia plexus, an average of 2.6 ganglia plexus were ablated per procedure: RAGP in 20 patients, RSGP in 12 patients, RIGP in 8 patients and LSGP in 14 patients.
One patient (case A.2) had a convulsive episode immediately after the second procedure, requiring admission to intensive care. A cranioencephalic computed tomography showed no abnormalities and an embolic event was excluded. This episode was interpreted as an adverse reaction to sugammadex used for rocuronium reversal, with no neurological sequelae. No other complications were observed, including the need for pacemaker implantation, pericardial effusion/tamponade, or vascular complications. All patients were discharged the day after the procedure.
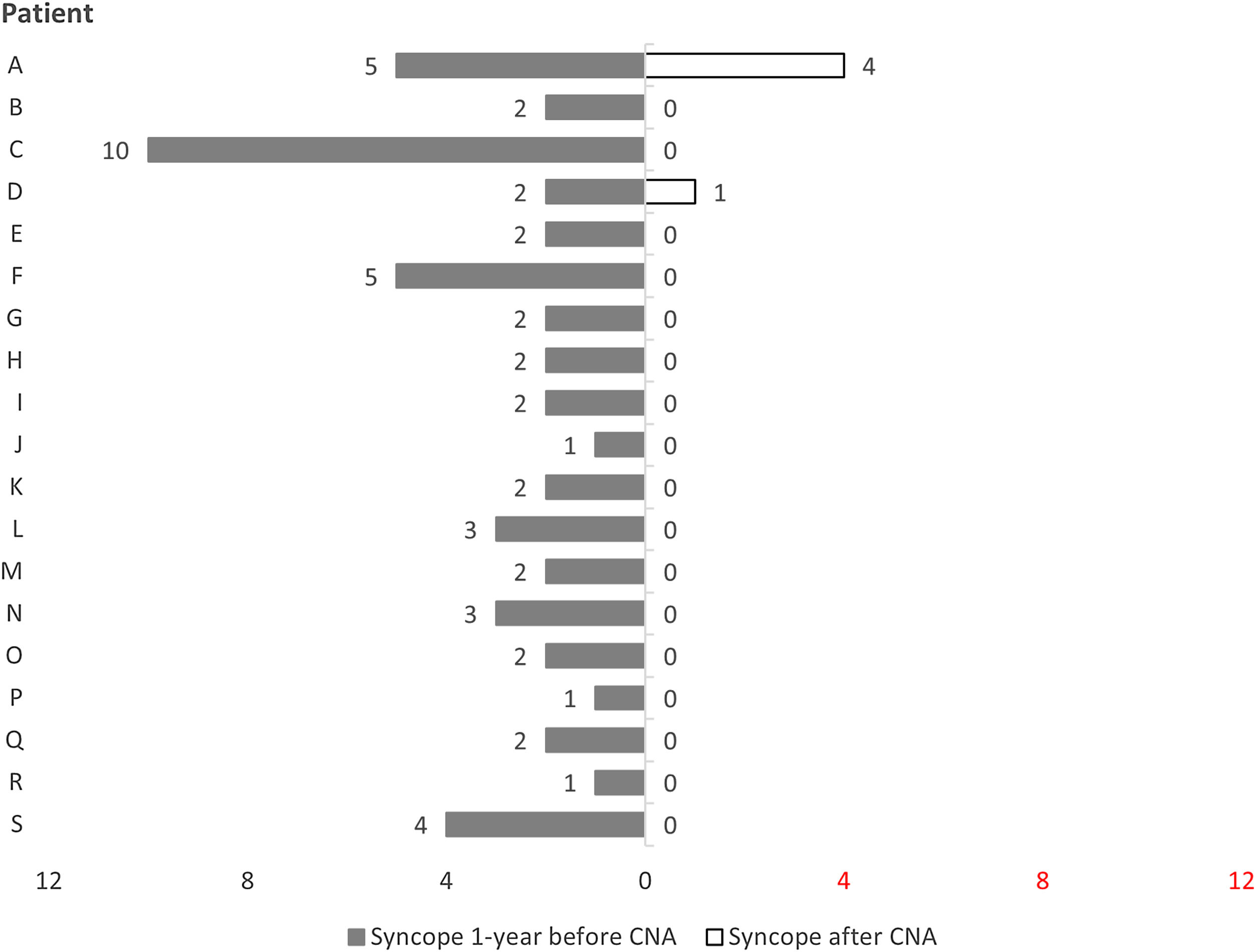
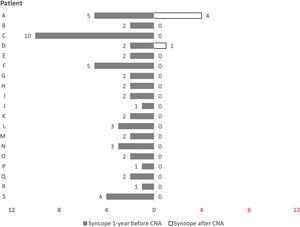
Follow-upAll patients maintained regular clinical follow-up. One-third had an implantable loop recorder. In a mean follow-up of 21.0±13.2 months (3–42 months), only two patients (11%) had recurrence of syncope. The burden of syncope was reduced following ablation compared to the year preceding it (2.8 vs. 0.3 episodes per patient, p<0.001) (Figure 3).
Patient A, who had had five syncopal episodes in the year preceding ablation, initially underwent ablation in the RAGP and RSGP. After six months free of symptoms, this patient had a recurrence of syncope, although the underlying mechanism could not be documented. Patient D was asymptomatic for nearly one year after the procedure, at which time the patient had recurrent syncope with complete atrioventricular block and a 19-second pause, documented by an implantable loop recorder. Both patients underwent a second CNA procedure and an initial positive vagal response was again observed. The same method with sequential and anatomically guided GP ablation was performed with acute success. However, both had further syncopal episodes and therefore underwent pacemaker implantation. These cases will be discussed in more detail below.
One third of patients reported being aware of a higher baseline heart rate in the first weeks after the procedure, with mild palpitations and fatigue with exertion which subsided gradually and completely over time.
DiscussionIn our prospective study of patients with recurrent vasovagal cardioinhibitory syncope, CNA confirmed by extracardiac vagal stimulation was effective in reducing the burden of syncope and seems a feasible treatment for this specific patient population.
Cardioneuroablation is a relatively recent and still developing technique which aims to promote persistent cardiac vagal denervation through endocardial ablation of parasympathetic ganglia. The first study suggesting the efficacy of GP ablation in preventing cardioinhibitory VVS was published by Pachon et al. in 2005.16 Later, in 2011, the same group published longer-term results and reported very high survival free of recurrent syncope.23
In the last decade, studies with a limited numbers of patients have been performed,17–19,24–26 and almost all these patients had a marked cardioinhibitory component. This was also the case in our study. Symptom-arrhythmia correlation is essential to select patients who may benefit from CNA properly, as patients with predominant vasodepressor syncope are very unlikely to benefit from this procedure and should be considered for alternative treatments.
One of the main difficulties has been identifying the areas in the heart where parasympathetic ganglia are located, and which should be the target of ablation.27 Three different approaches have been used: high frequency stimulation (HFS), spectral analysis and an anatomical approach. The first one involves the identification of areas containing parasympathetic nerves through the detection of a vagal response in response to HFS (e.g., significant prolongation of the PR or RR intervals).28,29 The second involves the spectral analysis of atrial potentials through a simplified mathematical tool that converts the signal into a representation of the frequency domain, enabling a differentiation between vagal innervation sites and normal atrial myocardium.16
The anatomical approach is based on the assumption that parasympathetic ganglia are typically located in similar locations in almost all patients, thus enabling an empirical ablation of these locations. Slightly different terminology has been used for the anatomical identification of these ganglionic groups. At our two centers, we use the classification of the four GP groups described above. Empirical anatomical ablation can be performed either as adjunctive to the HFS or spectral analysis methods, or as a stand-alone strategy27 as used in our center. This approach has been described and compared to HFS-guided CNA, with identical and high success rates observed in both groups, but with shorter procedure and fluoroscopy times in the anatomical approach.18 Our study adds to existing data showing the feasibility of an anatomical, empirical approach when performing CNA for cardioinhibitory syncope.
In our study, only two patients had syncope during follow-up. However, even these two patients experienced a longer symptom-free period than before CNA. It should be noted that most patients who may be candidates for CNA are young, active, and highly symptomatic (mean 2.8 syncope episodes/year in our cohort). Some of these patients may have experienced traumatic syncope, as some of those included in our study. Therefore, the possibility of eliminating or, at least, drastically reducing the syncopal burden without the need for device implantation cannot be overstated and is associated with a major improvement in the patients’ quality of life. The fact that these two recurrences occurred among the first cases in our cohort can be explained by the associated learning curve. It is important to mention that, in our study, vagal stimulation was performed from the right internal jugular vein only, including the 2 patients who had syncopal recurrence. However, particularly for patients requiring re-do ablation after syncopal recurrence, it may be advised to perform bilateral extracardiac vagal stimulation to guarantee that successful CNA occurred. Also, ablation performed in these two re-do cases was relatively limited.
Although there is still significant heterogeneity in CNA protocols between groups, results have been consistently encouraging with long-term success rates above 85%.16–18,23–25,30 This is particularly important when existing alternatives such as lifestyle modification and pharmacological therapy have such low success rates. Even dual-chamber cardiac pacing, which is suggested by the European Society of Cardiology guidelines in severe, unpredictable, and recurrent syncope, associates with conflicting results.6–12 Furthermore, pacemaker implantation may have significant implications for otherwise young or relatively young healthy adults, with a potential for long-term complications. Although CNA may still associate with a low risk of complications such as those related with left atrial access, complication rates are low in experienced centers used to performing other complex ablations. Thus, this may become an attractive option for young patients with recurrent cardioinhibitory syncope, who may wish to avoid the potential long-term implications of pacemaker implantation.
A potential limitation of CNA is the possibility of reinnervation restoring vagal hyperactivity. However, published long-term results have been consistent with the persistence of denervation. First long-term results with atropine test, tilt test and Holter showed persistence of denervation after CNA.23 In addition, no functional impairment was observed, as there were no significant changes in the maximum heart rate and in the chronotropic response during stress testing.23 More recently, Pachon et al. demonstrated there was no difference in heart rate variability after two years of follow-up, suggesting that no significant reinnervation occurs.30 Another potential concern could be an increased risk of adrenergically-mediated arrhythmias, but Pachon et al. saw no increase in arrhythmic risk, and even a possible decrease in the number of ventricular and supraventricular extrasystoles.30
LimitationsFirst, this study included a small number of patients without a control group. Still, it represents an additional source of literature suggesting that CNA may be more effective than other existing therapies. Very recently, the first randomized study documenting efficacy of CNA in patients with cardioinhibitory VVS was published.31 Second, the confirmation of cardioinhibitory syncope was made using multiple methods, including tilt table tests. However, the documentation of a spontaneous cardioinhibitory syncope might be a better way to select patients than using tilt tests. Finally, follow-up was not systematic, with some variability among patients: only one third had implantable loop recorders, and appointments at the clinic were not always carried out due to Covid-19 pandemic-related limitations. Nevertheless, data concerning our primary endpoint of syncope can be easily obtained through remote appointments.
Despite the abovementioned limitations, the results of our study are another contribution to the feasibility and safety of CNA for the treatment of VVS. Randomized controlled trials are needed to confirm the effectiveness of this procedure.
ConclusionAnatomically guided CNA, confirmed by extracardiac vagal stimulation, appears to be an effective and safe treatment option for patients with highly symptomatic, recurrent VVS with a predominantly cardioinhibitory component.
Conflicts of interestThe authors have no conflicts of interest to declare.