An implanted pacemaker is generally considered a contraindication for magnetic resonance imaging (MRI). The increasing number of indications for MRI and the rising prevalence of implanted cardiac pacemakers have prompted the recent development of MRI-conditional pacemaker systems.
We present the case of a 68-year-old woman with left ventricular hypertrophy, hypertension, aortic valve stenosis and a family history of cardiac amyloidosis, who developed complete heart block. In view of the foreseeable need for cardiac MRI, an MRI-conditional dual chamber pacemaker was implanted. The MRI scan confirmed moderate left ventricular hypertrophy and aortic valve stenosis, and showed no delayed enhancement suggestive of amyloid heart disease. This case illustrates the feasibility of cardiac MRI in this setting and the usefulness of the recently introduced MRI-conditional pacemaker systems.
A presença de um pacemaker é habitualmente considerada uma contra-indicação para a realização de ressonância magnética (RM). O número crescente de indicações para RM e de doentes portadores de pacemaker motivaram o desenvolvimento de pacemakers RM-condicionais.
Apresentamos o caso de uma mulher de 68 anos com hipertrofia ventricular esquerda, hipertensão arterial, estenose valvular aórtica e história familiar de amiloidose cardíaca, que desenvolveu bloqueio auriculo-ventricular completo. Devido à necessidade previsível de realizar uma RM cardíaca, foi-lhe implantado um pacemaker RM-condicional. A RM cardíaca confirmou a hipertrofia ventricular esquerda moderada e estenose valvular aórtica, não tendo evidenciado realce tardio sugestivo de amiloidose cardíaca. Este caso ilustra a exequibilidade da RM cardíaca neste contexto e a utilidade dos pacemakers RM-condicionais actualmente ao nosso dispor.
The presence of implantable cardiovascular electronic devices (pacemakers, implantable cardioverter-defibrillators and cardiac resynchronization devices) is generally considered a contraindication for magnetic resonance imaging (MRI) due to safety issues.1,2 Potential adverse interactions between pacemakers and MRI include heating, induction of ventricular fibrillation, rapid atrial or ventricular pacing, reed switch malfunction, asynchronous pacing, inhibition of pacing output, alteration of programming with potential damage to the pacemaker circuitry, and movement of the device.3 This contraindication is particularly important due to the parallel exponential growth in both the use of MRI and the number of patients with such devices,2 50–75% of whom are expected to need an MRI during their lifetime.4 The recent introduction of MRI-conditional pacemakers represents an important step in overcoming one of the major limitations of MRI.
Case reportA 68-year-old woman with a history of longstanding hypertension presented to the emergency department with pre-syncope and heart rate <30 bpm. The ECG revealed complete heart block, which persisted after the washout time of heart rate-lowering drugs, thus establishing the need for pacemaker implantation. Her echocardiogram showed good left ventricular systolic function, moderate septal hypertrophy with a speckled appearance, and moderate aortic stenosis. The patient's older sister had died recently from biopsy-proven amyloid heart disease and it was noted that the echocardiograms of both patients (performed four years apart) were remarkably similar. Her sister's differential diagnosis between senile amyloidosis vs. familial amyloidosis restricted to the heart was very difficult to achieve since all other organs seemed to be spared. She did not have signs of neuropathy or nephropathy, rectal and abdominal fat biopsies were negative, as were studies for AL and AA amyloidosis. Endomyocardial biopsy was only performed after a cardiac MRI that was highly suggestive of cardiac amyloidosis.
In view of the foreseeable need for cardiac MRI in our patient, an MRI-conditional dual chamber pacemaker was implanted (Ensura DR MRI™ SureScan™ EN1DR01 with 5086 leads, Medtronic®). Subsequently, cardiac MRI was requested to assess for amyloid heart disease as a possible concurrent cause for this patient's left ventricular hypertrophy. Prior to scanning on a 1.5 T MRI system, the device was interrogated, lead integrity checked and the pacemaker was switched to DOO mode at 60 bpm at 5 V @ 1 ms. The exam was well tolerated and completely uneventful. After scanning, all parameters were reviewed to verify that they were unaffected, and normal operation was resumed.
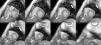
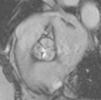
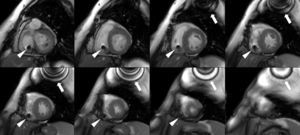
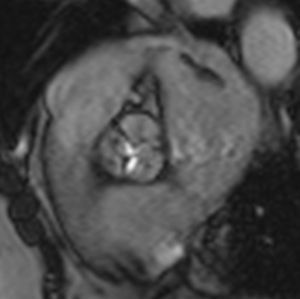
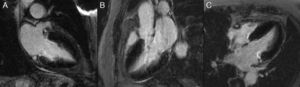
Cardiac MRI showed predominantly septal left ventricular (LV) hypertrophy, maximal end-diastolic wall thickness of 16 mm, and an estimated LV mass of 78 g/m2 (normal values for women >35 years of age: 34–70 g/m2).5 LV volumes and systolic function were normal, with no regional wall motion abnormalities. Metallic artefacts from the pacemaker lead and generator were visible but did not hinder image analysis and interpretation (Figure 1). Cine imaging of the aortic valve confirmed the presence of moderate aortic stenosis (Figure 2). Delayed enhancement imaging (10 minutes after intravenous injection of gadopentetate dimeglumine) showed no areas of hyperenhancement (Figure 3). Overall, these findings are consistent with LV hypertrophy secondary to hypertension and aortic stenosis. Nonetheless, since the absence of delayed enhancement is insufficient to rule out cardiac amyloidosis in its early stages,6 diagnostic workup continues and a follow-up cardiac MRI is scheduled in one year.
The first MRI-conditional pacemaker was introduced in 2010 and was initially approved for use in 1.5 T MRI scanners to image all body regions except the chest. The latest systems (such as the one our patient received) have recently been approved for imaging all body regions (chest included), thus making cardiac MRI possible.
MRI-conditional devices differ from standard pacemakers in several aspects. The amount of ferromagnetic material is minimized, the pacemaker leads are insulated (minimizing increases in temperature), and the gradient and radiofrequency fields do not interfere with the pacing function (so long as MRI mode is activated). Even though MRI-conditional pacemakers are specially designed for safe use in the MRI environment, it should be emphasized that scanning patients with such devices is only safe if a certain number of conditions are fulfilled. Prior to scanning, the MRI-conditional nature of both pacemaker generator and leads should be confirmed (this can be done by looking for specific markers on a chest radiograph), and the absence of other cardiac leads or electromagnetic cardiac devices must be ensured. A special programming mode (MRI mode) must be set on the MRI-conditional pacemaker before the scan, and turned off immediately afterwards. Close cooperation between cardiologist and radiologist is therefore mandatory. So far, scanning can only take place in MRI scanners with a field strength of 1.5 T, and limits in specific absorption rate and gradient slew rate should be observed.
Despite these constraints, the introduction of MRI-conditional devices overcomes an important limitation of MRI, allowing clinicians to take full advantage of this imaging method in the growing number of patients with a cardiac pacemaker. For this reason, it is very likely that MRI-compatible pacemakers will become standard of care in the near future. Meanwhile, at least those patients requiring a pacemaker who also have pre-existing comorbidities of an oncological, neurological, orthopedic or cardiovascular disease should be proposed for implantation of an MRI-conditional device.
This case illustrates the feasibility of cardiac MRI in this setting and the usefulness of the recently introduced MRI-conditional pacemaker systems. To the best of our knowledge, this was also the first cardiac MRI performed in Portugal on a patient with an MRI-conditional pacemaker. We welcome it as the first of many to come.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Conflicts of interestThe authors have no conflicts of interest to declare.