Cardiac allograft vasculopathy (CAV) is one of the most significant complications after orthotopic heart transplantation. We aimed to investigate the incidence and predictors of CAV in a large cohort of orthotopic heart transplantation patients.
MethodsWe conducted a retrospective analysis on a prospective cohort of 233 patients who underwent transplantation between November 2003 and May 2014. Baseline clinical data and invasive coronary angiograms (n=712) performed as part of the follow-up program were analyzed by two independent investigators.
ResultsWe included 157 male and 45 female patients with a median age of 66 years. A third of patients had previous ischemic heart disease, 30% peripheral arterial disease, 37% hypertension and 47% dyslipidemia, and 17% were smokers. Acute moderate or severe rejection occurred in 42 patients during the first year. Over a median follow-up of 2920 days, 18% were diagnosed with CAV, with an incidence of 2.91 cases per 100 person-years. Predictors of CAV were previous ischemic heart disease (HR 2.32, 95% CI 1.21-4.45, p=0.01), carotid artery disease (HR 2.44, 95% CI 1.27-4.71, p<0.01), and donor age (HR 1.04, 95% CI 1.00-1.07, p=0.01).
ConclusionIn a single-center cohort of orthotopic heart transplantation patients, predictors of CAV were previous ischemic heart disease, carotid artery disease and donor age.
A vasculopatia do enxerto é uma das complicações mais relevantes após transplante cardíaco. O objetivo deste estudo foi investigar a incidência e os preditores de vasculopatia do enxerto numa população de transplantados cardíacos.
MétodosAnálise retrospetiva de um coorte prospetivo de 233 transplantados cardíacos no nosso centro entre novembro de 2003 e maio de 2014. As características clínicas basais e as coronariografias invasivas (n=712) realizadas no âmbito do programa de acompanhamento pós-transplante foram analisadas por dois investigadores independentes.
ResultadosForam incluídos 157 homens e 45 mulheres, com mediana de 66 anos. Um terço tinha doença cardíaca isquémica prévia, 30% doença arterial periférica, 17% fumadores, 37% hipertensos e 47% tinham dislipidemia. Verificou-se rejeição aguda moderada ou severa em 42 doentes durante o primeiro ano. Durante um período médio de seguimento de 2920 dias, 18% foram diagnosticados com vasculopatia do enxerto, com uma taxa de incidência de 2,91 casos por 100 pessoa-ano. Os preditores de vasculopatia do enxerto foram doença cardíaca isquémica [HR 2,32, 95% IC 1,21–4,45, p=0,01], doença arterial carotídea prévia [HR 2,44 95% IC 1,27–4,71, p<0,01] e idade do dador [HR 1,04, 95% IC 1,00–1,07, p=0,01].
ConclusãoNuma coorte unicêntrica de transplantados cardíacos, os preditores de vasculopatia do enxerto foram doença cardíaca isquémica e doença arterial carotídea prévias e idade do dador.
Orthotopic heart transplantation (OHT) remains the treatment of choice for refractory end-stage heart failure.1–4 It is reserved for patients with severe hemodynamic compromise, intractable ventricular arrhythmias or uncontrollable angina.5
According to data from the 2016 International Society for Heart and Lung Transplantation (ISHLT) registry, one-year and 10-year survival post-OHT exceed 85% and 50%, respectively.6 At our center, the equivalent rates are 87% and 79%.7 Survival is hampered by several factors, one of which is cardiac allograft vasculopathy (CAV), a common form of chronic rejection and one of the most limiting long-term complications of OHT.8
The aims of this study are to investigate the incidence of CAV after OHT at our institution and to identify its predictors.
MethodsStudy designWe conducted a retrospective observational analysis on a prospective single-center cohort. Patients consented to the use of anonymized data for research purposes at the time of OHT.
PatientsA total of 233 consecutive patients who underwent a first OHT at our center between November 2003 and May 2014 were identified. Recipients aged under 18 years (n=3) and those who died less than a year after OHT (n=28) were excluded. Pre-OHT baseline clinical data of recipients and donors were collected prospectively from a dedicated institutional database integrated in the national OHT registry and analyzed retrospectively. The following data were extracted for all recipients: age, gender, body mass index (BMI), hypertension, previous peripheral arterial disease, ischemic heart disease (IHD), smoking, diabetes, dyslipidemia, abnormal carotid ultrasound, positivity for cytomegalovirus (CMV) immunoglobulin G, and moderate or severe acute rejection (≥2R on the ISHLT criteria) during the first year post-OHT. Of donors’ pre-transplantation baseline characteristics, age, gender and BMI were analyzed.
Invasive coronary angiographyInvasive coronary angiography (ICA) data from April 2017 to October 2017 were extracted from two databases of the cardiology department. Patients underwent routine ICA at one, three, five, eight, 10 and 12 years after OHT and additional exams if clinically justified. A total of 712 ICAs were analyzed. The films were reviewed by two interventional cardiologists from our department (E.J. and V.M.). Finally, a total of 143 reports described coronary lesions. The reports were classified according to the ISHLT nomenclature9 presented in Table 1.
International Society for Heart and Lung Transplantation nomenclature for cardiac allograft vasculopathy.9
| ISHLT CAV0 (not significant) | No detectable angiographic lesion |
| ISHLT CAV1 (mild) | Angiographic LM <50%, or primary vessel with maximum lesion of <70%, or any branch stenosis <70% (including diffuse narrowing) without allograft dysfunction |
| ISHLT CAV2 (moderate) | Angiographic LM <50%; a single primary vessel ≥70%, or isolated branch stenosis ≥70% in branches of two systems, without allograft dysfunction |
| ISHLT CAV3 (severe) | Angiographic LM ≥50%, or two or more primary vessels ≥70% stenosis, or isolated branch stenosis ≥70% in all 3 systems; or ISHLT CAV1 or CAV2 with allograft dysfunction (defined as LVEF <45% usually in the presence of regional wall motion abnormalities) or evidence of significant restrictive physiology |
CAV: cardiac allograft vasculopathy; ISHLT: International Society for Heart and Lung Transplantation; LM: left main coronary artery; LVEF: left ventricular ejection fraction.
Categorical variables were presented as frequency and percentage, and comparisons were performed using the chi-square test or Fisher's exact test. All continuous variables had a non-normal distribution, so they were presented as median (interquartile range), and comparisons between groups were performed with the Kruskal-Wallis test. To identify CAV predictors, patients were categorized as those with (CAV+) and those without CAV (CAV-). Univariate analyses were performed using the Cox proportional hazards model. The follow-up period for each patient was calculated from the date of OHT to the date of last contact. A p-value of less than 0.05 was considered statistically significant. Data were analyzed using the IBM SPSS version 23.0 and STATA 14.0 statistical packages.
ResultsBaseline characteristicsThe recipient group included 157 males and 45 females, with a median age of 66 (57-71) years; 62 recipients were older than 70 years. Median BMI was 24.7 (23.4-26.5) kg/m2. The baseline pre-OHT characteristics are represented in Table 2. The donor group was composed of 154 males and 48 females. Median age was 35 (24-43) years and 20 (10%) donors were over 50 years old. Median BMI was 24.1 (21.9-27.0) kg/m2.
Baseline characteristics of the study population before orthotopic heart transplantation.
| Recipients | |
| Age, years | 66.0 (57.0-71.0) |
| Male gender, % | 157 (77.7) |
| BMI, kg/m2 | 24.69 (23.37-26.46) |
| Hypertension, n (%) | 74 (36.8) |
| Diabetes, n (%) | 48 (26.8) |
| Previous vascular disease, n (%) | 61 (30.3) |
| Previous IHD, n (%) | 72 (35.6) |
| Smoking, n (%) | 35 (17.3) |
| Dyslipidemia, n (%) | 94 (46.8) |
| Abnormal carotid ultrasound, n (%) | 80 (39.6) |
| Positive for CMV IgG, n (%) | 147 (80.3) |
| Acute rejection ≥2R in 1st year, n (%) | 42 (21.3) |
| Donors | |
| Age, years | 35.0 (24.0-43.0) |
| Age ≥50 years, n (%) | 20 (9.9) |
| Male gender, n (%) | 154 (76.2) |
| BMI, kg/m2 | 24.11 (21.91-27.00) |
2R: moderate or severe; BMI: body mass index; CVM: cytomegalovirus infection; IgG: immunoglobulin G; IHD: ischemic heart disease.
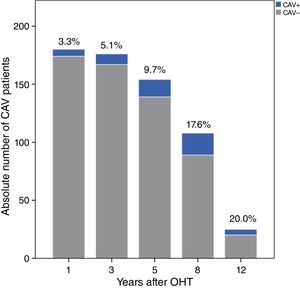
Over a median follow-up of 2920 (1825-3650) days after OHT, 37 patients (18.3%) were diagnosed with CAV. After one, three and five years, 3.3%, 5.1% and 9.7% of patients, respectively, had angiographic findings compatible with CAV (Figure 1). The prevalence increased over the rest of the follow-up period: at eight, 10 and 12 years after transplantation, 17.6%, 15.9% and 20.0% of patients, respectively, presented CAV lesions, as shown in Figure 1. The overall CAV incidence was 2.91 cases per 100 person-years.
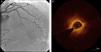
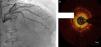
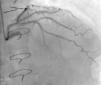
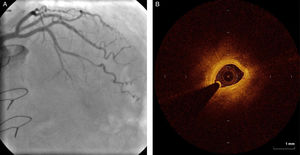
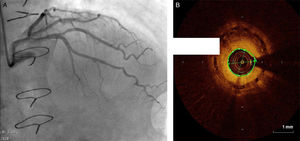
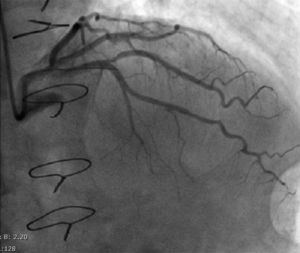
Regarding lesion type, 14 had CAV1 (38%), 12 had CAV2 (35%) and nine had CAV3 lesions (24%). During the follow-up period, six (16%) patients showed disease progression: four patients first classified with CAV1 showed progression to CAV2, and two CAV2 patients progressed to CAV3. The other patients presented a stable CAV course during follow-up. Percutaneous coronary intervention (PCI) was performed in 18 (49%) patients in the CAV+ group, in lesions classified as CAV2 and CAV3 (only focal stenosis or critical subocclusive lesions amenable to PCI). Seventeen drug-eluting stents (DES) with everolimus, sirolimus or zotarolimus, and one bioresorbable vascular scaffold (BVS), were implanted in 10 of these patients. The patient treated with BVS presented with restenosis ten months later, as illustrated in Figures 1-3, highlighting the safety issues related to these devices, which are no longer in use (Figure 4).
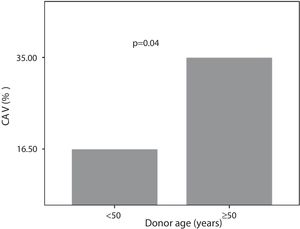
Seven (35%) patients with donors over 50 years were CAV+. Of those whose donors were younger than 50 years, 30 (16.5%) patients were CAV+ (Figure 5).
A comparison of pre-OHT baseline characteristics of the CAV+ and CAV- groups is provided in Table 3. In univariate analysis, abnormal carotid ultrasound (hazard ratio [HR] 2.44, 95% confidence interval [CI] 1.27-4.71, p<0.01), a history of IHD (HR 2.32, 95% CI 1.21-4.45, p=0.01) and donor age (HR 1.04, 95% CI 1.00-1.07, p=0.01) were significantly associated with higher CAV prevalence (Table 4). However, recipient risk factors such as hypertension (HR 1.47, 95% CI 0.75-2.86, p=0.26), diabetes (HR 1.59, 95% CI 0.79-3.25, p=0.20), dyslipidemia (HR 1.68, 95% CI 0.87-3.25, p=0.13) and smoking (HR 1.77, 95% CI 0.85-3.65, p=0.13) were not significantly associated with CAV. Multivariate analysis was not performed because of strong covariance between previous IHD and abnormal carotid ultrasound at the time of OHT (p=0.000).
Baseline characteristics before transplantation of patients with and without cardiac allograft vasculopathy.
| CAV+ group (n=37, 18.3%) | CAV- group (n=165, 81.7%) | |
|---|---|---|
| Recipients | ||
| Age, years | 65.0 (57.0-71.0) | 67.0 (57.0-72.0) |
| Male gender, % | 31 (83.8) | 126 (76.4) |
| BMI, kg/m2 | 23.53 (21.81-25.99) | 23.60 (21.50-25.51) |
| Hypertension, n (%) | 14 (37.8) | 60 (36.6) |
| Diabetes, n (%) | 12 (36.4) | 36 (24.7) |
| Previous vascular disease, n (%) | 13 (35.1) | 48 (29.3) |
| Previous IHD, n (%) | 14 (48.6) | 54 (32.7) |
| Smoking, n (%) | 18 (48.6) | 76 (46.3) |
| Dyslipidemia, n (%) | 10 (27.0) | 25 (15.2) |
| Abnormal carotid ultrasound, n (%) | 19 (51.4) | 61 (37.0) |
| Positive for CMV IgG, n (%) | 29 (85.3) | 118 (79.2) |
| Acute rejection ≥2R in 1st year, n (%) | 10 (27.8) | 32(19.2) |
| Donors | ||
| Age, years | 37 (25-46) | 34 (24-42) |
| Age ≥50 years, n (%) | 7 (35.0) | 13 (65.0) |
| Male gender, n (%) | 32 (86.5) | 122 (73.9) |
| BMI, kg/m2 | 24.38 (23.15-27.10) | 24.91 (23.44-26.31) |
2R: moderate or severe; BMI: body mass index, CAV+: with cardiac allograft vasculopathy; CAV-: without cardiac allograft vasculopathy; CI: confidence interval, CVM: cytomegalovirus infection; HR: hazard ratio, IgG: immunoglobulin G; IHD: ischemic heart disease.
Univariate analysis for predictors of cardiac allograft vasculopathy before heart transplantation (Cox regression).
| HR (95% CI) | p | |
|---|---|---|
| Recipients | ||
| Age | 0.99 (0.97-1.02) | 0.62 |
| Male gender | 1.78 (0.75-4.27) | 0.20 |
| BMI | 0.99 (0.94-1.05) | 0.84 |
| Hypertension | 1.47 (0.75-2.86) | 0.26 |
| Diabetes | 1.59 (0.79-3.25) | 0.20 |
| Previous vascular disease | 1.54 (0.78-3.02) | 0.21 |
| Previous IHD | 2.32 (1.21-4.45) | 0.01 |
| Smoking | 1.77 (0.85-3.65) | 0.13 |
| Dyslipidemia | 1.68 (0.87-3.25) | 0.13 |
| Abnormal carotid ultrasound | 2.44 (1.27-4.71) | <0.01 |
| Positive for CMV IgG | 0.93 (0.36-2.43) | 0.93 |
| Acute rejection ≥2R in 1st year | 1.40 (0.68-2.91) | 0.36 |
| Donors | ||
| Age | 1.04 (1.00-1.07) | 0.01 |
| Male gender | 2.14 (0.83-5.52) | 0.12 |
| BMI | 1.01 (0.90-1.12) | 0.88 |
2R: moderate or severe; BMI: body mass index, CI: confidence interval, CVM: cytomegalovirus infection; HR: hazard ratio, IgG: immunoglobulin G; IHD: ischemic heart disease.
CAV is defined as an accelerated fibroproliferative disease that affects epicardial and intramural arteries in OHT patients.10 It results in progressive luminal narrowing and reduced myocardial blood flow.11 CAV appears to develop first in distal vessels and to progress centripetally to the large coronary vessels,10,12 in contrast to the focal, eccentric, proximal epicardial lesions in classical atherosclerotic coronary artery disease. However, the two conditions appear to share common pathogenic factors.10
CAV results from an interaction between numerous immunologic and non-immunologic donor and recipient features that are still not well understood.13–15 Among donor features, a history of hypertension, diabetes, smoking, higher BMI, older age and male gender are under study as likely predictors of CAV.4,15–17 On the side of the recipient, previous IHD, higher BMI, dyslipidemia, hypertension, diabetes and high urinary uric acid levels have been recognized as possible pre-OHT features predicting CAV.4,18 Also, recipient CMV infection is known to be associated with CAV, because it generates a proatherogenic environment and impairs nitric oxide production, leading to immune-mediated endothelial injury.10,15
Patients with CAV are usually asymptomatic because of allograft denervation, and so ICA is the standard diagnostic technique.10 However, its sensitivity is limited by the diffuse nature of CAV, especially during the first year post-OHT.10,11 Intracoronary imaging techniques such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are therefore of growing importance in the diagnosis of CAV.10,19–21
CAV therapy focuses on modification of underlying classical cardiovascular risk factors and optimization of immunosuppression.11
Statins are recommended early after OHT, regardless of cholesterol levels.22 They slow CAV progression and improve endothelial dysfunction, as shown by landmark trials in the mid-1990s.11,23
Once a patient is diagnosed with CAV, sirolimus or its derivative everolimus is included in the immunosuppressive regimen.24,25
PCI is an option for patients with focal disease who present symptoms or evidence of ischemia.26 Coronary artery bypass grafting is rarely indicated due to the distal and diffuse nature of CAV lesions.22 Retransplantation is the only definitive treatment capable of improving survival in highly selected candidates.14,22
In our large OHT cohort, the incidence of CAV was 2.91 cases per 100 person-years, and its prevalence was 3.3%, 9.7% and 17.6% at one, five, and eight years post-OHT, respectively. CAV was in most cases grade 1 (38%) or 2 (35%).
According to the 2015 ISHLT report, the five- and eight-year prevalence of CAV is 29% and 40%, respectively.27 A single-center retrospective study, based on a prospective cohort, enrolling 495 OHT patients and using ICA for CAV diagnosis, showed a CAV prevalence of 47% 12 years after OHT.28 Another study with a similar design but a smaller population (54) using both ICA and IVUS for CAV diagnosis presented a CAV prevalence of 46.2% within 10 years of OHT.29 At our center, the incidence and prevalence were lower than previously reported, possibly because of our larger sample and the high-quality follow-up program in our center. We identified abnormal carotid ultrasound at the time of OHT, prior history of IHD and donor age as being independently associated with CAV.
Using ICA for CAV diagnosis, two single-center retrospective studies enrolling 361 and 113 OHT patients also found previous recipient IHD to be a powerful independent predictor for CAV (6- to 10-fold higher risk).16,30 Neither of these studies included abnormal carotid ultrasound at the time of OHT in their analysis, probably due to differences in pre-OHT assessment protocols. However, considering that IHD and carotid disease share similar predictors and are highly correlated, it would also likely be a predictor in these two cohorts.
Neither immunologic nor non-immunologic donor and recipient factors underlying CAV have been established, however IHD risk factors are thought to play a part in the pathophysiology of CAV.31 In our population, none of the classical cardiovascular risk factors demonstrated an association with CAV, as predicted by previous articles from our center.32 However, both IHD and abnormal carotid Doppler at the time of OHT suggest that recipients who presented CAV after OHT had some cardiovascular risk factors at that time. Therefore, even though no single classical cardiovascular factor showed an association with CAV in our cohort, our findings support the existence of a common atherosclerotic basis between CAV and classical coronary artery disease in non-transplanted patients. Both IHD and carotid disease imply persistence of classical cardiovascular risk factors that might be associated with coronary plaque progression after OHT.33,34
Donor age was the other CAV predictor in our cohort. A previous retrospective analysis of 162 OHT patients conducted in our center looking at CAV prevalence also identified donor age as a strong positive predictor, concluding that the 50-year cut-off was significant.35 Other studies have also identified age as a CAV predictor.4,28 Importantly, older recipients usually receive the hearts of older donors, both (donor and recipient) with a higher burden of cardiovascular risk factors,35 and additionally, when CAV appears early after OHT, the hypothesis of pre-existing donor disease should be considered.36 Age limits are a controversial topic in OHT, and according to the international guidelines, carefully selected patients older than 70 years may be considered.3 In our study, 62 patients were older than 70 years and interestingly, recipient age was not statistically associated with CAV development.
In view of the immunological process behind CAV, a history of acute cellular rejection assessed by endomyocardial biopsy has been suggested in previous studies of single-center cohorts as a predictor of CAV development.29,37 We did not find such an association in our population during the first year post-OHT.
Using ICA for CAV diagnosis is in agreement with recent ISHLT recommendations, but it has limited ability to detect the early stages of CAV.22 Other imaging techniques, including IVUS and OCT,20,21 may have greater sensitivity for CAV diagnosis.38 The inclusion of these techniques in follow-up programs could lead to earlier detection of abnormalities after OHT and provide more accurate identification of CAV predictors, although this would significantly increase the program cost and the time taken for each invasive exam.
Revascularization procedures are associated with poor long-term results and are considered palliative due to the diffuse and progressive nature of vascular changes.15 In the setting of three-vessel disease, for example, PCI is associated with only 27% two-year freedom from cardiac death or graft loss.26 Small single-center experiences suggest higher (90-98%) initial procedural success but a restenosis prevalence of 35-100% for PCI alone and 20-56% for PCI with stenting during the first year.39,40 The higher restenosis rate in CAV compared to IHD is explained by the lymphoproliferative response that characterizes CAV.12 Nevertheless, in our population, PCI had very good results. DES were chosen for the majority of CAV lesions described in our series. As the treatment of established CAV is disappointing, the primary effort should be directed to early diagnosis and prevention. For this, identifying CAV predictors should be a priority.
Due to the higher incidence of CAV in these subgroups (previous vascular disease and donor age over 50), we are proposing changes to our protocol. Firstly, coronary angiography will be mandatory for donors over the age of 50 years in order to confirm eligibility for heart procurement. Secondly, recipients deemed at higher risk for CAV according to the clinician's judgment (including the above subgroups) will be further studied with OCT at the time of the first routine angiography, in order to improve our diagnostic performance and to adjust immunosuppressive therapy accordingly (including initiating everolimus/sirolimus therapy on top of standard lipid-lowering treatment).
Study limitationsFirstly, due to the retrospective nature of this analysis, the comparison between the CAV- and CAV+ groups may be biased because of possible unidentified confounding factors. However, the populations are relatively similar, with only three variables being statistically different between the groups. Secondly, although we have an active transplant center, our population is relatively small. Larger populations may provide greater statistical power to demonstrate the natural course and predictors of CAV. Also, as a single-center study, its external validity is limited. Thirdly, CAV was diagnosed using ICA, which is limited by its low sensitivity. The results reported here may underestimate CAV prevalence in comparison with other techniques such as IVUS or OCT. However, strict adherence to the ICA protocol with well-defined screening intervals enabled a very low drop-out rate. Lastly, exclusion of patients who died in the early postoperative period resulted in a survival bias, so the data presented in our study cannot be applied to early post-OHT risk.
ConclusionIn this retrospective analysis of a single-center OHT cohort, the incidence of CAV (2.91 cases per 100 patient-years) was lower than that reported in other series. CAV predictors were previous ischemic heart disease, carotid artery disease and donor age.
Conflicts of interestThe authors have no conflicts of interest to declare. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.