Heart failure (HF) is a high prevalent syndrome with significant burden worldwide. B-type natriuretic peptide (BNP) and N-terminal proBNP are the gold standard biomarkers in HF management. Although useful in clinical practice, they have limitations as their expression can be influenced by ventricular function, aging, obesity, renal failure and atrial arrhythmias. MicroRNAs have recently emerged as potential diagnostic and prognostic biomarkers, given that they are related to cell growth, proliferation, differentiation, and metabolism. An increasing amount of research has highlighted some microRNAs for their potential as HF biomarkers. However, different study designs, methods and study groups have led to inconsistent results.
Methods and resultsWe performed a systematic search of available literature on Pubmed and Scopus reporting the prognostic value of microRNAs in HF, followed by a review of risk of bias, according to Quadas Group Standards. Simultaneously, microRNAs’ potential as differential diagnosis and severity biomarkers was also analyzed. Studies have described circulating microRNA as potential diagnostic, prognostic, and severity markers. Mir-622, -519 and -499 were significantly related to HF with reduced ejection fraction, whereas miR-22-3p revealed greater ability as a severity biomarker. Let-7i-5p, miR-223-5p, miR-423-5p, miR-21, miR-1306-5p and miR-122 serum expressions presented a consistent correlation with HF prognosis. Furthermore, identified miR targets were associated with signaling pathways already known to be involved in HF progression.
ConclusionSeveral miRs were related to HF pathophysiology and demonstrated potential as biomarkers for disease progression. MicroRNAs have a promising role in HF, and although unquestionable, we require a deeper and broader understanding of their role and function for future research.
A insuficiência cardíaca é uma síndrome com elevada prevalência mundial e impacto significativo na qualidade de vida e sobrevida dos doentes. Os peptídeos natriuréticos (BNP e NT-proBNP) são biomarcadores gold standard no diagnóstico, tratamento e prognóstico de insuficiência cardíaca. A sua utilização na prática clínica, embora útil, apresenta limitações. A sua expressão pode ser influenciada por diversos fatores, como idade, obesidade, insuficiência real e arritmias auriculares. Os microRNAs surgiram recentemente como potenciais marcadores de diagnóstico e prognóstico, dado estarem intimamente relacionados com crescimento, proliferação, diferenciação e metabolismo celular. Investigação crescente destaca a potencialidade dos microRNAs como biomarcadores de insuficiência cardíaca. No entanto, diferentes protocolos e critérios de inclusão nos grupos em estudo originaram resultados inconsistentes.
Métodos e resultadosFoi realizada uma pesquisa sistemática da literatura disponível na Pubmed e Scopus de artigos que relatam o valor prognóstico dos microRNAs em insuficiência cardíaca. Seguiu-se uma análise de risco de viés de acordo com Quadas Group Standards e simultaneamente foi analisado o potencial dos miRs como marcadores de diagnóstico e gravidade. Os miR-622, -519 e -499 foram significativamente relacionados com HF com fração de ejeção reduzida, enquanto miR-22-3p revelou maior capacidade como marcador de gravidade. As expressões séricas de Let-7i-5p, miR-223-5p, miR-423-5p, miR-21, miR-1306-5p e miR-122 apresentaram uma correlação consistente com o prognóstico de insuficiência cardíaca. Para além disso, foi possível concluir que os principais alvos dos mesmos correspondem a vias de sinalização já identificadas na progressão da insuficiência cardíaca.
ConclusãoVários microRNAs foram associados à fisiopatologia da insuficiência cardíaca e demonstraram potencial como biomarcadores de diagnóstico e para a progressão da doença. Embora seja inquestionável o papel promissor dos microRNAs em insuficiência cardíaca, é necessário um entendimento mais profundo e amplo sobre seu papel e função para pesquisas futuras.
Heart failure (HF) affects 38 million people worldwide, impacting the lives of more than 10% of population over 70 years contributing significantly to hospitalization rate increase, challenging economic and healthcare systems.1–4 HF syndrome is characterized by a complex interplay among genetic, neurohormonal, inflammatory and biochemical changes that affect cardiac cells and the interstitium and perpetuate cardiac injury.5–7
Currently, natriuretic peptides (NP) are the gold standard serum biomarkers in HF.8,9 Atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) are essential in the diagnosis and prognosis of HF, and may serve as a therapy guide.10 The prognostic efficiency of BNP and NT-proBNP is reported in the literature.9 The ADHERE study found a linear relationship between BNP expression and in-hospital mortality in acute HF patients11 and a meta-analysis by Doust et al. also found that a 100 pg/ml increased in BNP was associated with a 35% increase in the relative risk of death.12 However, their clinical use has relevant limitations. Ventricular function, aging, obesity, renal failure, atrial arrhythmias may influence clinical interpretation of natriuretic peptides.9
MicroRNA (MiRs) are small-non-coding ribonucleic acids (RNA)13 produced by all cell types which, ultimately, are secreted in the blood.14 Their main role is to regulate the output post-transcriptional proteins; they are, therefore, related to cell growth, proliferation, differentiation, and metabolism.15,16 Small non-coding RNA dysregulation was initially associated with cancer17 but, recently, several variations in miRs expression have been linked to HF.2,18 Further research has highlighted miRs for their potential as HF biomarkers (miR-423, let-7i-5p, miR-223-5p, miR-1306-5p and miR-22-3p) but with inconsistent results. While some studies have shown a positive association, others have found a negative association or no associations between miRs expression and prognosis. These disparities have hampered a clear assessment of miR biomarker potential, and demonstrate the need for systematic research.
In this study, we performed a systematic review of the studies investigating the diagnostic and prognostic value of miRNA in HF to provide a summary of the literature on how specific miRNA can help in the differential diagnosis between HF with preserved or reduced ejection fraction and predicting the incidence of all-cause death, cardiovascular hospitalization, and especially rehospitalization for HF decompensation.
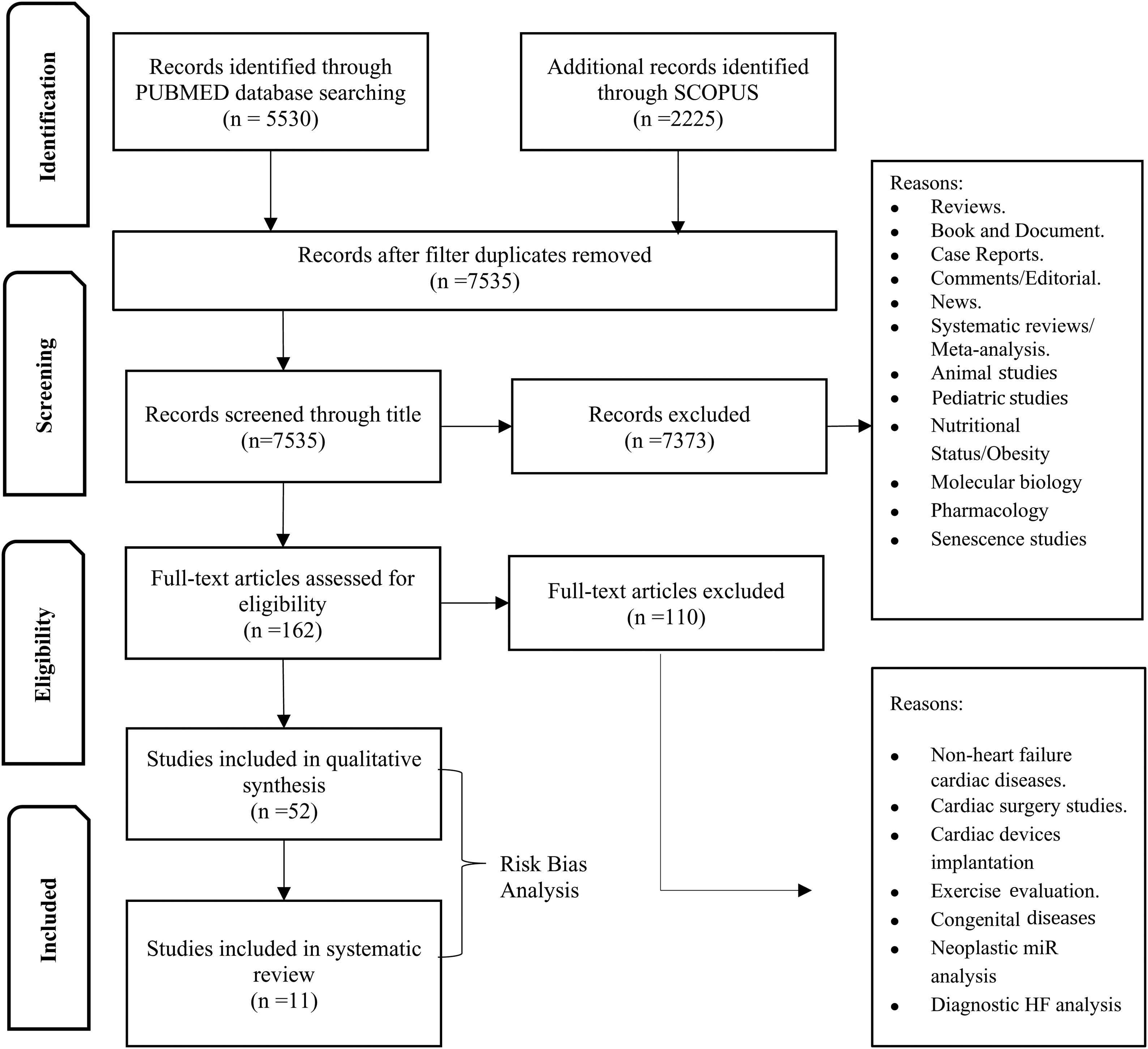
MethodsData sources and search strategyWe performed a systematic search of PUBMED and SCOPUS for all studies with prognostic evaluation of microRNAs in HF patients, including all stages of HF and clinical settings (Figure 1).
Publications were limited between 1 January 2010 and 31 July 2020. The search strategy on PUBMED included a MESH search: ((“Biomarkers”[Mesh]) OR (“MicroRNAs”[Mesh] OR “Circulating MicroRNA”[Mesh])) AND (“Heart Failure”[Mesh] OR “Heart Failure, Diastolic”[Mesh] OR “Heart Failure, Systolic”[Mesh]), and the filter English was not used. The search strategy for SCOPUS encompassed key-words search: ALL (“microRNAs”) OR (“circulating microRNAs”) AND (“heart failure”) AND (Limit-To (DOCTYPE, “ar”)) AND (Limit-To (SUBJAREA, “medi”)). Authors were contacted when relevant for missing prognostic performance data. All publications of interest were collected in EndNote. After removing duplicates, publication titles and abstracts were reviewed by one assessor (R.F.) to check they met the inclusion criteria, before full-text screening. Secondly, studies were assessed for risk of bias by R.F., R.A., C.S. PRISMA was used to include all items that are part of a systematic review19 (Figure 1). Tools recommended for producing a systematic review were adopted (QUADAS-220 and Cochrane Handbook for Systematic Reviews for Diagnostic Test Accuracy21).
Inclusion criteriaTime period. Studies published from 1 January 2010 to 31 July 2020 in English to focus on recent evidence written in English concerning human participants.
Biomarker types and sample types. Studies focusing on microRNA as an individual biomarkers or multiple biomarkers were included. Studies that did not report statistical significance or quantitative prognostic measures (hazard ratio (HR), odds ratio (OR), or relative risk (RR)) of the biomarker's prognostic performance were excluded. Studies measuring miR as a biomarker in medium other than serum or plasma samples were excluded. Studies using methods other than RT_PCR for miR quantification or studies that used RNU6 as a normalizer were excluded.
Study population. Studies in adult subjects with HF diagnosis were included. To minimize the risk of excluding promising early-stage research studies, the inclusion cut-off was studies with >50 human participants in total. There were no criteria for the number of participants per group.
Study types. Studies with any cohort design, including cross-sectional studies were included. As recommended by the Cochrane Handbook for Systematic Reviews for Diagnostic Test Accuracy, we excluded all case-control design studies.21 Reviews, systematic reviews, meta-analysis, books and documents, case reports, comments, news, and editorials were excluded.
Data collection and quality controlTo assess the true potential of miRs in HF, we analyzed miRs expression as: (i) The potential to discern between HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF); (ii) a marker for HF severity (including associations between echocardiographic measurements, New York Heart Association (NYHA) class and NP).
Lastly, to assess the capacity of miRs as prognostic biomarkers, three outcomes were established: (i) Cardiovascular hospitalization (CV hospitalization) (including the risk of CV patients, focusing on HF patients being admitted or re-admitted to hospital), in order to comprise one endpoint without death; (ii) Cardiovascular hospitalization and/or death (including the risk of patients with HF diagnosis being hospitalized/re-hospitalized or death after HF diagnosis) and (iii) all-cause death (including HF patients’ risk of death, regardless of the cause).
The data collection from included articles comprised: the study design; study population; number of subjects; female and male percentage; mean age±SD; initial diagnosis; variables included in multivariate models (Table 1) and the associated measures (HR, OR and RR) for different outcomes and statistical analysis performed (univariate or multivariate).
Overview of articles included in study, organized per outcome.
| Authors | Study design | Study population | No. of subjects | Male (%) | AgeMeanSD (years) | Outcomes | Main findings | Variables included in the multiple models |
|---|---|---|---|---|---|---|---|---|
| A. General population-based studies | ||||||||
| i) Prospective associations – CV hospitalizations | ||||||||
| Vegter et al., The Netherlands, 201731 | Prospective cohort | Subset of random HF patients from COACH | 114 | 66 | 71.1±10.4 | CV hospitalization | Median follow-up of 18 months; univariate analysis found miR-106a-5p, miR-223-3p, miR-27a-3p, miR-16-5p, miR-30e-5p and let-7i-5p as significantly predictive. Multivariate analysis resulted in 5 miRNAs significantly predictive (miR-106a-5p, miR-223-3p, miR-27a-3p, miR-16-5p, and let-7i-5p; all HR>1.4; p<0.05). | Age, sex, BNP and eGFR. |
| Masson et al., Italy, 201734 | Prospective cohort | GISSI-HF trial | 953 | 80 | 67.1±10.76 | CV hospitalization | Median follow-up was 46.2 months, in univariate analysis miR-132 was associated with HF hospitalization. After adjustment, miR-132 remained associated with outcome (HR 0.79; p=0.001). | Demographic, clinical echocardiographic risk factors, and baseline NT-proBNP concentrations. |
| Seronde et al., France, 201532 | Prospective cohorts – test | AHF patients from Leicester hospitals | 236 | 60.6 | 76 (65.5-84.5) | CV hospitalization | Multivariate analysis of miR-21, miR-126, miR-423-5p, miR-1 and miR-23; only miR- 423-5p was associated with hospital readmission (HR 0.70 [CI 0.53-0.93], p=0.01). | Age, gender, heart rate, systolic and diastolic blood pressure, history of AF and of HF, LVEF, plasma levels of BNP, sodium, creatinine, proteins, and haemoglobin. |
| Seronde et al., France, 201532 | Prospective cohorts – validation cohort | AHF patients from Leicester hospitals. | 711 | 64.2 | 77 (68.6-83) | CV hospitalization | Multivariate analyses, miR-423-5p was not a significant predictor of 1-year readmission in this cohort. | Age, gender, heart rate, systolic and diastolic blood pressure, history of AF and of HF, LVEF, plasma levels of BNP, sodium, creatinine, proteins, and haemoglobin. |
| Boven, The Netherlands, 201737 | Prospective | TRIUMPH | 456 | 63.4 | 73 (64-80) | HF hospitalization | Multivariate analysis evidenced miR-1306-5p as predictor of HF hospitalization (HR: 1.22, p<0.05) | Age, sex, systolic blood pressure, diabetes mellitus, atrial fibrillation, BMI, previous hospitalization for HF during the last 6 months, ischemic HF, baseline eGFR, and baseline NT-proBNP level. |
| Van Boven, The Netherlands, 201727 | Prospective | Bio-SHIFT study | 263 | 72 | 67±13 | CV hospitalization | None of the baseline miR values were associated with the secondary endpoint comprising HF hospitalizations. | Age and gender. |
| Zhang Jianghua, China, 201728 | Prospective | Hospitalized HF patients. | 120 | 71.25 | 59.68±10.24 | Re-hospitalization | Follow-up for 24 months concluded that miR-21 samples from coronary sinus were related with re-hospitalization (OR 1.160, p=0.0021). In samples from PV miR-21 was not related with the outcome. | EF, BNP-PV, creatinine, CK-MB, alanine aminotransferase, CRT. |
| ii) Prospective outcomes - heart failure rehospitalization and/or death | ||||||||
| Vegter et al., The Netherlands, 201731 | Prospective cohort | Subset of patients from COACH | 114 | 66 | 71.1±10.4 | HF hospitalization and/or death | OnlymiR-106a-5pwasunivariate predictive (HR 1.38 CI (1.017-1.882), p=0.039). No significant association was found after adjustment. | |
| Boven, The Netherlands, 201737 | Prospective cohort | TRIUMPH | 456 | 63.4 | 73 (64-80) | All-cause mortality and readmission for HF | MiR-1306-5p levels were associated with outcome. (HR 1.13 CI [1.03-1.23]). After adjustment, miR-320a, miR-378a-3p and miR-423-5p were positively associated with outcome. MiR-1254 displayed a borderline significant association with all-cause mortality and HF hospitalization, when adjusted to variables. | Age, sex, systolic blood pressure, diabetes mellitus, atrial fibrillation, BMI, previous hospitalization for HF during the last 6 months, ischemic HF, baseline eGFR, and baseline NT-proBNP level. |
| Boven, The Netherlands, 201727 | Prospective | Bio-SHIFT study | 263 | 72 | 67±13 | Hospitalization for management of HF and mortality | The temporal pattern of miR-22-3p was inversely associated with the primary endpoint after adjustment of age and gender (HR per doubling of miR-22-3p level, 0.64; CI 0.47-0.77; p=0.001). After adjustment, the association of miR-22-3p remained present (HR per doubling of miR-22-3p level at any given time point, 0.61; CI 0.51-0.73; p=0.001). | Age, gender, ICM and NYHA class. |
| Bayés-Genis et al., Spain, 201733 | Prospective cohort | Cohort I (Barcelona) | 834 | 71 | 68.1±12.7 | All-cause mortality and HF hospitalization | MiR-1254 and miR-1306- 5p were significantly associated with outcome. (HR of 1.21 [95% CI 1.0-1.39] and a HR of 1.13 [95% CI 1.0-1.25] respectively). | Age, gender, hemoglobin, creatinine and NT-proBNP |
| Bayés-Genis et al., Spain, 201733 | Prospective cohort | Cohort II (Detroit) | 1369 | 58 | 68.8±12.1 | All-cause mortality and HF hospitalization | MiR-1254 andmiR-1306-5pwere significantly associated with outcome (HR of 1.14 [95% CI 1.04-1.25] and a HR of 1.11 [95% CI 1.0-1.19] respectively). | Age, gender, haemoglobin, creatinine and NT-proBNP |
| iii) Prospective outcomes – all cause death | ||||||||
| Vegter et al., The Netherlands, 201731 | Prospective cohort | Subset of randomly selected patients from COACH | 114 | 66 | 71±10 | Mortality | No significant associations were identified for any of the miRNAs with all-cause mortality within 18 months. | Age, sex, BNP and eGFR. |
| Boven, The Netherlands, 201737 | Prospective cohort | TRIUMPH | 456 | 63.4 | 73 (64-80) | All-cause mortality | Positive associations were found between miR-499a-5p (HR: 2.04, CI 0.96-4.43) and miR-1306-5p (HR: 1.03, CI 0.90-1.17) | Age, sex, systolic blood pressure, diabetes mellitus, atrial fibrillation, BMI, previous hospitalization for HF during the last 6 months, ischemic HF, baseline eGFR, and baseline NT-proBNP level. |
| Seronde et al., France, 201532 | Prospective cohorts – validation cohort | AHF patients from Leicester hospitals | 711 | 64.2 | 77 (68.6-83) | All-cause mortality | In 20 months of follow-up, miR-423-5p significantly predicted mortality (OR 0.54, CI [0.36-0.829, p=0.004]). Patients within the lowest quartile of miR-423-5p levels had a higher risk of mortality compared to patients with low levels of miR-423-5p. This association was evident for 2 years. | Age, gender, heart rate, systolic and diastolic blood pressure, history of AF and of HF, LVEF, plasma levels of BNP, sodium, creatinine, proteins, and haemoglobin. |
| Bayés-Genis et al., Spain, 201733 | Prospective cohort | Cohort I (Barcelona) | 834 | 71 | 68.1±12.7 | All-cause mortality | MiR-1254, miR-133b, miR-622 and miR-208a-3p were predictive of outcome. (HR of 1.19 CI [1.03-1.38]; HR 1.20 CI [1.06-1.36]; HR 1.18 CI [1.00-1.38]; HR 1.32 CI [1.05-1.42] respectively). | Age, gender, haemoglobin, creatinine and NT-proBNP. |
| Bayés-Genis et al., Spain, 201733 | Prospective cohort | Cohort II (Detroit) | 1369 | 58 | 68.8±12.1 | All-cause mortality | MiR-1254, miR-133b, miR-622, miR-208a-3p were predictive of outcome. (HR of 1.31 CI [1.13-1.52]; HR 1.18 CI [1.03-1.34]; HR 1.12 CI [1.03-1.22]; HR 1.32 CI [1.02-1.34] respectively). | Age, gender, haemoglobin, creatinine and NT-proBNP. |
| OvchinnIkova et al., The Netherlands, 201530 | Prospective cohort | PROTECT trial (AHF patients) | 100 | 50 | 68.9±11.4 | All-cause mortality | A univariate analysis concluded that 7 miRNAs (let-7i-5p, miR-18a-5p, miR-18b-5p, miR-223-3p, miR-301a-3p, miR-423-5p, miR-652-3p) were predictive for 180-day mortality (all HR 1.5, p<0.05). | |
| Zhang Jianghua, China, 201728 | Prospective | Hospitalized HF patients between March 2013 and October 2013 | 120 | 71.25 | 59.68±10.24 | All-cause mortality | Samples from PV and coronary sinus were significantly correlated to miR-21 (RR 1.936 and 1.125, p=0.001 respectively). | HF. EF, BNP-PV, creatinine, CRT |
| Klenke et al., Germany, 201824 | Prospective cohort | ICM | 91 | 83.5 | 56.1±13.9 | All-cause mortality | MiR-192 low expression is associated with survival in univariate analysis (p=0.03) and multivariate analysis (p=0.014). | NYHA status, EF, and BNP concentration. |
| Masson et al., Italy, 201734 | Prospective cohort | GISSI-HF trial | 953 | 80 | 67.1±10.76 | All-cause mortality | Median follow-up was 46.2 months, in univariate analysis miR-132 was associated with outcome, but not after multivariate analysis (HR 0.95, 95% CI 0.85-1.07 for 1 unit increase in miR-132, p=0.41). | Demographic, clinical echocardiographic risk factors. |
| Xiao et al.35 | Prospective cohort | Patients admitted to the cardiac care unit. | 95 | 62.5 | 61.5±16.5 | All-cause mortality | Higher hemoglobin, serum sodium and miR-30d level were associated with a reduced risk of death caused in AHF patients. Patients with higher serum miR-30d levels had significantly lower mortality (p=0.001). Death prediction of miR-30d with OR of 0.610 CI (0.409-0.911) p=0.016. | Heart rate, serum sodium, blood urea nitrogen, haemoglobin, cystatin, uric acid, and serum. |
| Stojkovic et al.36 | Prospective cohort | HFrEF patients | 234 | 81.6 | 65.1 | All-cause mortality | Circulating miR predictive value was assessed by Cox proportional Hazard regression models. Both miR-122 and mR-423 predicted the outcome with HR per 1−SD of 1.15 (95% CI: 1.02-1.29; p=0.021) and HR per 1−SD 1.27 (95% CI: 1.10-1.46; p=0.001) respectively. | Age, gender, NYHA classes, DM II, eGFR, BMI, NP-proBNP, right ventricular dysfunction, cholinesterase, gamma-GT, and previous myocardial infarction. |
HF: heart failure; COACH: Coordinating Study Evaluating Outcomes of Advising and counselling in Heart Failure; CV: cardiovascular; AF: atrial fibrillation; DM II: Type 2 Diabetes Mellitus; BMI: body mass Index; AHF: acute heart failure; LVEF: left ventricular ejection fraction; PROTECT: Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function; BNP: b-type natriuretic peptide; eGFR: estimated glomerular filtration rate; NT-proBNP: N-terminal pro-B-type natriuretic peptide; Gamma-GT: gamma-glutamyl transferase; HR: hazard ratio. OR: Odd ratio; CI: confidence interval; RR: relative risk; CK-MB: creatine kinase, muscle and brain; BNP-PV: brain natriuretic peptide from peripheral vein; CRT: Cardiac-resynchronization Therapy; ICM: Ischemic Heart failure; Methodological quality of all studies was assessed using the revised and validated version of the Quadas-2.
To assess the quality of the studies and identify potential bias, we applied the QUADAS-2 tool. Considering the risk of bias analysis, study design, patient selection, miR index test and standard reference, studies were scrutinized for each outcome of interest.
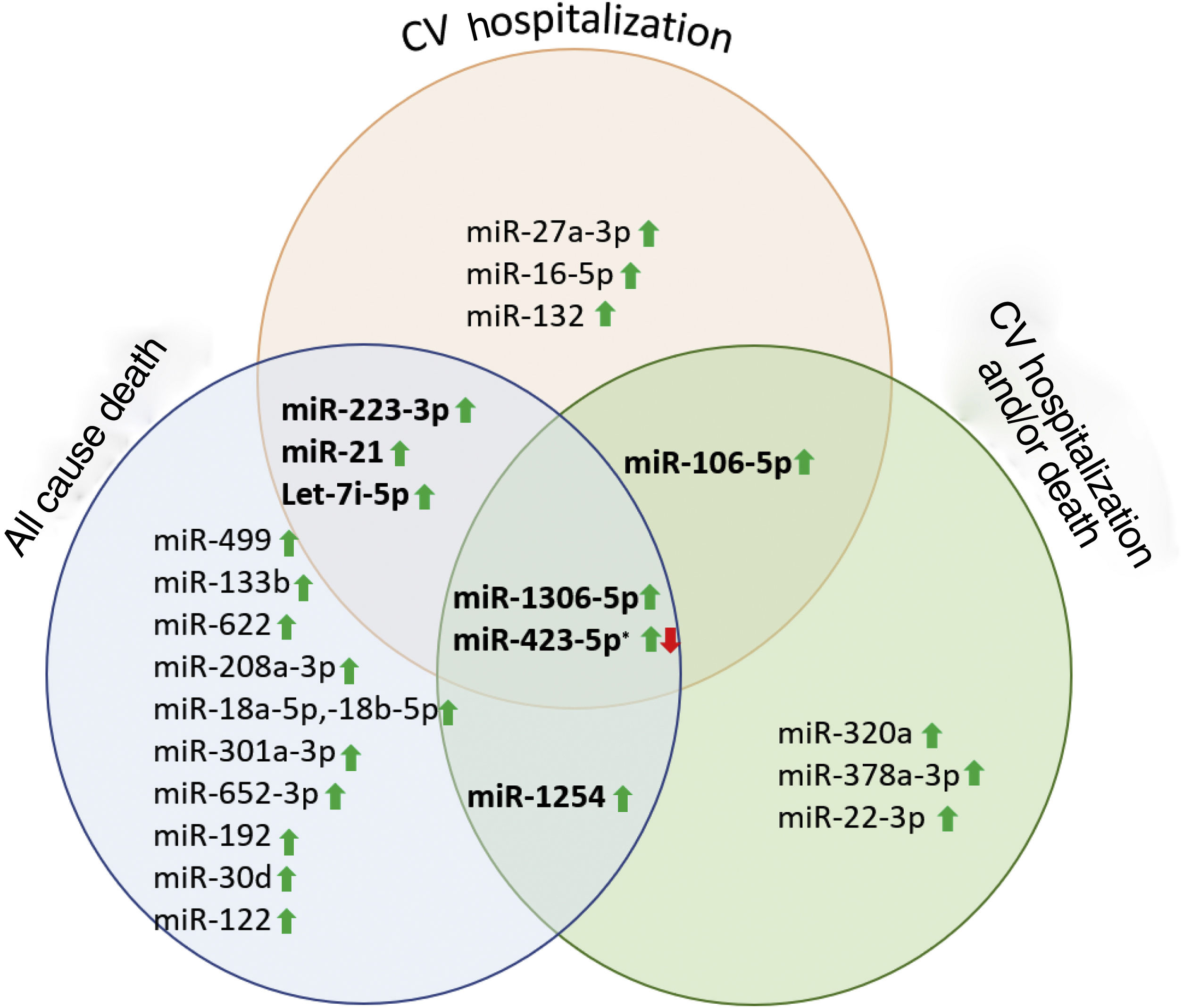
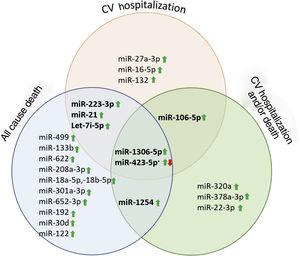
ResultsA total of 52 articles were identified, of which 11 reported the association between miRNA and HF prognosis. The diseases studied and the miR tests were similar across the included articles and the risk of bias was low. Thus, we classified the selected studies per outcome, as presented in Table 1. Furthermore, several identified miR were described as prognostic markers of more than one outcome (Figure 2).
Few studies analyzed miRs as potential biomarkers in a differential diagnosis between HF with preserved or reduced ejection fraction. Watson et al. demonstrated that serum expression of miR-30c, miR-221, miR-328, and miR-375 were able to distinguish HFrEF from HFpEF (all area under curve (AUC) >0.7).22 Wong et al. performed a similar analysis and identified four miRs that are able to differentiate between the two HF entities (miR-125a-5p, -190a, -550a-5p, and -638) (AUC miR panel 0.8).23
MiRNAs and heart failure severityPrevious studies suggest that specific miR could help in HF severity evaluation. From the articles included in our review, only Klenke et al. did not find any association between BNP or NYHA status.24 Vogel et al. in order to test if miR expression might correlate with disease severity, compared systolic function (mild-moderate and severe) and concluded that miRNAs expression patterns in control groups are different from HF group and significant different between the severity groups. To summarize miRs expression might correlate with different HF stages or severities. Also, significant correlations between left ventricular (LV) EF and miR-622, miR-520d, miR-519, miR-200b, miR-122, and miR-588 were found (miR expression levels increased with lower values of (LV)EF), but not with NYHA functional class.25 MiR-150-5p expression was significantly correlated with symptoms severity and adverse remodeling degree. Also, miR-150-5p was inversely correlated with NYHA classes (p=0.004) and log NT-proBNP).26 MiR-21 and miR-132 expression levels increase alongside NYHA grade and consequently so does HF severity.27,28 On the other hand, negative correlations between miR-145 and cardiac function were found and lower levels of miR-22-3p and Ln_miR-145 (p<0.0001) were associated with HF worsening.27,29 Ovchinnikova et al. found that acute HF is associated with the analyzed miR (let-7i-5p, miR-18a-5p, miR-18b-5p, miR-223-3p, miR-301a-3p, miR-423-5p miR-652-3p) shown by a consistent pattern of decreased miR expression levels with increased HF severity, thus reaching the conclusion that miR expression levels were higher at discharge than at admission.30 In 2017, Vegter et al. discovered that miRNA levels decreased in parallel with clinical manifestations of atherosclerotic disease, therefore contributing to HF severity.31
PrognosisAll-cause deathAll-cause death was analyzed in 10 articles.24,28,30–37 Five articles found a positive correlation between miR expression and outcome,28,30,33,36,37 four found a negative correlation,24,32,34,35 and two did not find any association between the analyzed miR and all-cause mortality (Table 2).
Overview of significant miR – all-cause death.
| Study | No of patients | Diagnosis | Tested miR | Significant miRs | Statistical analysis |
|---|---|---|---|---|---|
| Outcome: all-cause death | |||||
| Vegter et al., The Netherlands, 201731 | 114 | HF | let-7i-5p, miR-16-5p, miR-18a-5p, miR-26b-5p, miR-27a-3p, miR-30e-5p, miR-106a-5p, miR-199a-3p, miR-223-3p, miR-423-5p, miR-652-3p | -- | Univariate analysis (p>0.05) |
| Boven, The Netherlands, 201737 | 456 | HF | miR-486-5p, miR-320ª, miR-1254, miR-22-3p, miR-378a-3p, miR-423-5p, miR-345-5p, miR-1306-5p, miR-133a-3p, miR-499a-5p, miR-133b, miR-622, miR-208a-3p | Mir-1306-5p, miR-499ª-(+) | Multivariate analysis (p<0.05) |
| Seronde et al., France, 201532 | 711 | AHF | miR-423-5p | miR-423-5p(−) | Multivariate analysis (p=0.004) |
| Bayés-Genis et al., Spain, 201733 | 834 | HF | miR-133b, miR-1254, miR-378a-3p, miR-423-5p, miR-320ª, miR-345-5p, miR-22-3p, miR-1306-5p, miR-133a-3p, miR-622, miR-499a-5p, miR-208a-3p | miR-1254, miR-133b, miR-622, miR-208a-3p(+) | Multivariate analysis (p<0.05) |
| Bayés-Genis et al., Spain, 201733 | 1369 | HF | miR-133b, miR-1254, miR-378a-3p, miR-423-5p, miR-320ª, miR-345-5p, miR-22-3p, miR-1306-5p, miR-133a-3p, miR-622, miR-499a-5p, miR-208a-3p | miR-1254, miR-133b, miR-622, miR-208a-3p(+) | Multivariate analysis (p<0.05) |
| OvchinnIkova et al., The Netherlands, 201530 | 100 | AHF | let-7i-5p, miR-16-5p, miR-18b-5p, miR-18a-5p, miR-26b-5p, miR-27a-3p, miR-30e-5p, miR-106a-5p, miR-128, miR-199a-3p, miR-223-3p, miR-301a-3p, miR-423-3p, miR-423-5p, miR-652-3p | let-7i-5p, miR-18a-5p, miR-18b-5p, miR-223-3p, miR-301a-3p, miR-423-5p, miR-652-3p(+) | Univariate analysis (p<0.05) |
| Zhang Jianghua, China, 201728 | 80 | HF | miR-21 | miR-21(+) | Multivariate analysis (p=0.001) |
| Klenke et al., Germany, 201824 | 91 | HF | miR-192 | miR-192(−) | Multivariate analysis (p=0.014) |
| Masson et al., Italy, 201734 | 953 | HF | miR-132 | -- | Multivariate analysis (p=0.41) |
| Xiao et al., China, 201735 | 95 | HF | miR-30d | miR-30d(−) | Multivariate analysis (p=0.016) |
| Stojkovic et al., Austria, 202036 | 234 | HFrEF | miR-122, miR-423, miR-126 | miR-122, miR-423(+) | Multivariate analysis (p<0.021) |
HF: heart failure; AHF: acute heart failure; miR: microRNAs; No: number.
(+): positive correlation; (−): negative correlation; (--): no correlation was found.
Despite several analysis, Vegter et al. did not find any significant miR correlated with all-cause death.31 MiR-423-5p, miR-192, and miR-30d were individually analyzed in HF patients and, after multivariate analysis, a negative correlation was found24,32 (Table 2). In contrast, Stojkovic found positive correlations between the outcome and miR-423 and miR-122 in HFrEF patients (HR: 1.15, p=0.021 and 1.27, p=0.001, respectively).36 Also, Bayés-genis et al. conducted a multicentered study where a set of miR with a positive correlation and outcome (miR-1254, miR-133b, miR-622, miR-208a-3p) were identified (HR: ≥1.19, ≥1.18, ≥1.12 and ≥1.32, p≤0.05, respectively).33 Additionally, Ovcchinnikova et al. used a univariate analysis to study several miRs in 100 acute HF patients, identifying seven miRs (let-7i, -18a-5p, -18b-5p, -301a-5p-423-5p-652-3p) with mortality prediction potential (all HR >1.5, p≤0.05). Also, Vegter et al. and Van Boven et al. identified miR-21 and miR-499a as a predictor of mortality (relative risk (RR): 1.936, p=0.001 and HR: 2.04, p≤0.05, respectively).37,38
Heart failure rehospitalization and/or deathFour articles provided the value of miRs to predict HF rehospitalization and/or death (Table 3).27,31,33,34,37 Among these, three articles estimated a positive correlation31,33,37 and one achieved a negative correlation.27 MiR-22-3p was inversely associated with the outcome (HR 0.61, p=0.001).27 On the other hand, miR 106a-5p, miR-1306-5p, miR-320a, miR-378a-3p, miR-423-5p and miR-1254 were described by the included articles as positively correlated with the outcome (HR: 1.38; >1.11; 1.10; 1.03; 1.05; and >1.1, respectively.31,33,37
Overview of significant miR – cardiovascular hospitalization and/or death.
| Study | No of patients | Diagnosis | Tested miR | Significant miRs | Statistical analysis |
|---|---|---|---|---|---|
| Outcome: cardiovascular hospitalization and/or death | |||||
| Vegter et al., The Netherlands, 201731 | 114 | HF | let-7i-5p, miR-16-5p, miR-18a-5p, miR-26b-5p, miR-27a-3p, miR-30e-5p, miR-106a-5p, miR-199a-3p, miR-223-3p, miR-423-5p, miR-652-3p | miR-106a-5p(+) | Univariate analysis (p=0.039) |
| Boven, The Netherlands, 201737 | 456 | HF | miR-486-5p, miR-320ª, miR-1254, miR-22-3p, miR-378a-3p, miR-423-5p, miR-345-5p, miR-1306-5p, miR-133a-3p, miR-499a-5p, miR-133b, miR-622, miR-208a-3p | miR-1306-5p, miR-320a, miR-378a-3p and miR-423-5p(+) | Multivariate analysis (p<0.05) |
| Boven, The Netherlands, 201727 | 263 | HF | miR-1254, miR-22-3p, miR-423-5p, miR-486-5p, miR-320ª, miR-345-5p, miR-378a-3p | miR-22-3p(−) | Multivariate analysis (p=0.001) |
| Bayés-Genis et al., Spain, 201733 | 834 | HF | miR-133b, miR-1254, miR-378a-3p, miR-423-5p, miR-320ª, miR-345-5p, miR-22-3p, miR-1306-5p, miR-133a-3p, miR-622, miR-499a-5p, miR-208a-3p | miR-1254, miR-1306-5p(+) | Multivariate analysis (p<0.05) |
| Bayés-Genis et al., Spain, 201733 | 1369 | HF | miR-133b, miR-1254, miR-378a-3p, miR-423-5p, miR-320ª, miR-345-5p, miR-22-3p, miR-1306-5p, miR-133a-3p, miR-622, miR-499a-5p, miR-208a-3p | miR-1254, miR-1306-5p(+) | Multivariate analysis (p<0.05) |
HF: heart failure; miR: microRNAs; No: number.
(+): positive correlation; (−): negative correlation.
A total of six articles analyzed the association between cardiovascular hospitalization and miR plasma expression,27,28,31,32,34 as shown in Table 4. Three articles demonstrated that higher levels of miRs were associated with higher risk of hospitalization (miR-106-5p, -223-3p, -27a-3p, -16-5p, let-7i-5p, - 1306-5p and -21 with HR of 1.694, 1.478, 1.482, 1.763, 2.058, 1.22 and OR: 1.16, p<0.05 respectively, Table 4).28,31,37 Vegter et al studied several miR for 18 months and found, after a multivariate analysis, a set of 5 miR that are significantly predictive of CV hospitalization.31 Zhang et al. explored the association between miR-21 level coronary sinus and peripheral venous blood in a follow-up period of 24 months, showing that higher levels of miR-21 in coronary sinus serum were associated with an increased risk of hospitalization (OR:1.16, p=0.0021).38 In contrast, Masson et al. found a negative correlation between CV hospitalization and miR-132 blood expression in 953 patients over 46.2 months (HR: 0.79, p=0.01).34 Also, Seronde et al. demonstrated that miR-423-5p might have a protective role in CV hospitalization in a test cohort with acute HF patient, however, these data were inconclusive using a larger validation cohort (236 patients in a test cohort vs. 711 patients in a validation cohort) (OR:0.82, p=0.48, respectively).32
Overview of significant miR – cardiovascular hospitalization.
| Study | No of patients | Diagnosis | Tested miR | Significant miRs | Statistical analysis |
|---|---|---|---|---|---|
| Outcome: cardiovascular hospitalization | |||||
| Vegter et al., The Netherlands, 201731 | 114 | HF | let-7i-5p, miR-16-5p, miR-18a-5p, miR-26b-5p, miR-27a-3p, miR-30e-5p, miR-106a-5p, miR-199a-3p, miR-223-3p, miR-423-5p, miR-652-3p | miR-106a-5p, miR-223-3p, miR-27a-3p, miR-16-5p, let-7i-5p(+) | Multivariate analysis (p<0.05) |
| Masson S. et al., Italy, 201734 | 953 | HF | miR-132 | miR-132(-) | Multivariate analysis (p=0.001) |
| Seronde et al., France, 201532 | 236 | AHF | miR-423-5p, miR-126, miR-23, miR-21, miR-1 | miR-423-5p(−) | Multivariate analysis (p=0.01) |
| Seronde et al., France, 201532 | 711 | AHF | miR-423-5p | -- | Multivariate analysis (p=0.48) |
| Boven et al., The Netherlands, 201737 | 456 | HF | miR-486-5p, miR-320ª, miR-1254, miR-22-3p, miR-378a-3p, miR-423-5p, miR-345-5p, miR-1306-5p, miR-133a-3p, miR-499a-5p, miR-133b, miR-622, miR-208a-3p | miR-1306-5p(+) | Multivariate analysis (p<0.05) |
| Van Boven N.v, The Netherlands, 201727 | 263 | HF | miR-1254, miR-22-3p, miR-423-5p, miR-486-5p, miR-320ª, miR-345-5p, miR-378a-3p | -- | Multivariate analysis (p>0.38) |
| Zhang Jianghua, China, 201728 | 80 | HF | miR-21 | miR-21(+) | Multivariate analysis (p=0.0021) |
HF: heart failure; AHF: acute heart failure; miR: microRNAs; No: number.
(+): positive correlation; (−): negative correlation; (--): no correlation was found.
In this systematic review, several miRs were identified as potential biomarkers for HF diagnosis, prognosis and disease severity, supporting miR's pivotal role in cardiac physiology.
Several miR were related with HF. MiR-21, miR-22 and miR-132 were described as important biomarkers in HF pathophysiology and progression. They are also clinically correlated to NYHA classes, volume status and fluid overload. Although none of the studied miR were revealed to have a better potential as a biomarker, after combining miR expressions with BNP and NT-proBNP, their potential to distinguish HFrEF from HFpEF was prominent, and even better than NP. MiR-423, let-7i-5p, miR-223-5p, miR-1306-5p and miR-22-3p were analyzed in more than one article and described as potential biomarkers for HF prognosis (Table 5).
Correlations found on included articles per outcome.
| miR | Outcome: cardiovascular hospitalization | Outcome: HF hospitalization and/or death | Outcome: all-cause death | |||
|---|---|---|---|---|---|---|
| Correlation [HR or OR or RR) | p | Correlation (HR or OR or RR) | p | Correlation (HR or OR or RR) | p | |
| miR-423-5p | Negative (OR: 0.7032[a]) | 0.01 | Positive (HR: 1.05[a]37) | 0.05 | OR: 0.54[a]32Positive (HR: 1.681[b]30)Positive (HR: 1.27[a]36) | 0.001 |
| miR-1254 | – | – | Positive (HR: 1.14[a]33)Positive (HR: 1.21[a]33) | 0.05 | Positive (HR: 1.19[a]33)Positive (HR: 1.31[a]33) | 0.05 |
| miR-133b | – | – | – | – | Positive (HR: 1.20[a]33)Positive (HR: 1.18[a]33) | 0.05 |
| miR-622 | – | – | – | – | Positive (HR: 1.18[a]33)Positive (HR: 1.12[a]33) | 0.05 |
| miR-208a-3p | – | – | – | – | Positive (HR: 1.32[a]33)Positive (HR: 1.32[a]33) | 0.05 |
| let-7i-5p | Positive (HR: 2.05831[a]) | 0.002 | Positive (HR: 1.958[b]30) | 0.007 | ||
| miR-18a-5p | – | – | – | – | Positive (HR: 1.616[b]30) | 0.014 |
| miR-18b-5p | – | – | – | – | Positive (HR: 1.851[b]30) | 0.013 |
| miR-223-3p | Positive (HR: 1.47831[a]) | 0.039 | – | – | Positive (HR: 1.557[b]30) | 0.034 |
| miR-301a-3p | – | – | Positive (HR: 1.782[b]30) | 0.04 | ||
| miR-652-3p | – | – | – | – | – | – |
| miR-21 | Positive (OR: 1.16028[a]) | 0.0021 | Positive (RR: 1.93628) | 0.001 | ||
| miR-499a | Positive (HR: 2.04[a]37) | 0.05 | ||||
| miR-192 | – | – | – | – | *24 | 0.014 |
| miR-132 | Negative (HR: 0.7934[a]) | 0.01 | – | – | HR: 0.95[a]34 | 0.41 |
| miR-30d | – | – | – | – | Negative (HR: 0.6135[a]) | 0.016 |
| miR-122 | – | – | – | – | Positive (HR: 1.15[a]36) | 0.021 |
| miR-1306-5p | Positive (HR: 1.2237) | 0.05 | Positive (HR: 1.13[a]37)Positive (HR: 1.13[a]33)Positive (HR: 1.11[a]33) | 0.05 | ||
| miR-22-3p | – | – | Positive (HR: 0.61[a]27) | 0.001 | Positive (HR: 1.03[a]37) | 0.05 |
| miR-320a | – | – | Positive (HR: 1.10[a]37) | 0.05 | – | – |
| miR-378a-3p | – | – | Positive (HR: 1.03[a]37) | 0.05 | – | – |
| miR-106a-5p | Positive (HR: 1.69431[a]) | 0.012 | Positive (HR: 1.38[a]31) | 0.039 | – | – |
| miR-27a-3p | Positive (HR: 1.48231[a]) | 0.027 | – | – | – | – |
| miR-16-5p | Positive (HR: 1.76331[a]) | 0.005 | – | – | – | – |
[a] Multivariate analysis; OR: odd ratio; HR: hazard ratio; RR: relative risk; [b] univariate analysis; * missing information.
Heart failure with preserved ejection fraction and HFrEF cannot be differentiated on clinical grounds and imaging tests are essential for a correct diagnosis. HFpEF is not a straightforward matter because it is diagnosed by a constellation of clinical signs and symptoms, most of which relate to the fact that the ventricle operates at a higher filling pressure.39,40
The distinction between HFpEF vs. HFrEF is based on echo derived left ventricular ejection fraction (LVEF). However, LVEF has limitations in detecting subtle abnormalities of systolic function. Previous studies using strain showed a reduction of radial and longitudinal despite normal ejection fraction. Furthermore, echography tests involve well trained sonographers and equipment. A serum-based biomarker that could help distinguis between HFpEF vs. HFrEF would facilitate easy and fast differential diagnosis in clinical practice. Studies have tried to use NP levels to distinguish HF entities, but the use of NP lacks specificity and does not allow a correct differentiation of HF subtypes.10,22,41,42
Natriuretic peptides are directly related to ventricular function; they are produced in response to ventricular diastolic stretch. The main trigger for their release in the bloodstream is left ventricular (LV) end-diastolic wall stress, present in a dilated LV in HFrEF but not necessarily in HFpEF.43,44
MiRs have been described as being able to distinguish HFrEF and HFpEF, and several correlated with echocardiographic measurements.25,26,29 Several articles have stated that miR expression has a similar but not superior performance to NP. However, when the same analysis combined miR expressions with BNP and NT-proBNP, the potential for distinguishing HFrEF from HFpEF was prominent, and even better than NP used alone.22,23,37
The articles under study compared diagnostic potential for both NP and miRs. To assess miR diagnostic potential in HFrEF, Vogel et al. compared NT-proBNP accuracy and sensitivity with miR and found a better performance of miR -519, -520d and -622 (miRs AUC 0.81; NT- pro-BNP sensitivity 78% and specificity 44%).25 Wong et al. compared NT-proBNP and miR as diagnostic biomarker between HFrEF and HFpEF and although NT-proBNP showed potential, the combination of miR with NT-proBNP improved efficacy to distinguish between them (AUC NT-proBNP alone 0.83 to AUC NT-proBNP with miR 0.91).23 Watson et al. assessed miR capacity to improve the diagnostic utility of existent BNP and concluded that miR could improve diagnostic performance of BNP in HF (AUC of BNP alone 0.87 vs. AUC of BNP with miR 0.90), demonstrating superiority in distinguishing HF entities (AUC BNP alone 0.66 vs. AUC BNP with miR 0.85).22
MiR-622, -519 and -122 were significantly related with HFrEF. High expression levels of miR-622 and-519 were found in granulocyte cells by Vogel et al., reinforcing the inflammatory processes involvement in HF development and progression. MiR-122 is originated in hepatic cells and has been related to HF due to diminished cardiac output, resulting in liver congestion.25,45,46 Nevertheless, only miR-328, miR-375 and miR-499 expression levels were significantly different between HFrEF and HFpEF.22,33
MicroRNAs and New York Health Association class and heart failure severityThe articles under study made reference to a gradual increase in specific miR levels in more stabilized HF, chronic HF (CHF) and healthy controls, when compared to acute HF patients.30,31,47 Indeed, low levels of miRs were associated with increased levels of biochemical markers of inflammation, angiogenesis and endothelial dysfunction, thus supporting previous data.31
Negative correlations between miR-22 and the outcome have also been described.27 Scientific research has stated that miR-22-3p has a protective role in the myocardium due to its negative regulation of angiotensin II.27,48 It is mostly expressed by striated muscle tissues and is essential in normal cardiac remodeling after environmental stress.27,47 In fact, among the included studies, several severity-related miRs were described as upregulated28,34 or downregulated26,30 regardless of HF etiology. This suggests that different factors may influence miR serum expression. Volume status variations and fluid overload due to renal impairment and proteinuria (once they are bound to plasma proteins such as albumin, and low filtration rate may lead to the loss of carrier plasma proteins).34,47
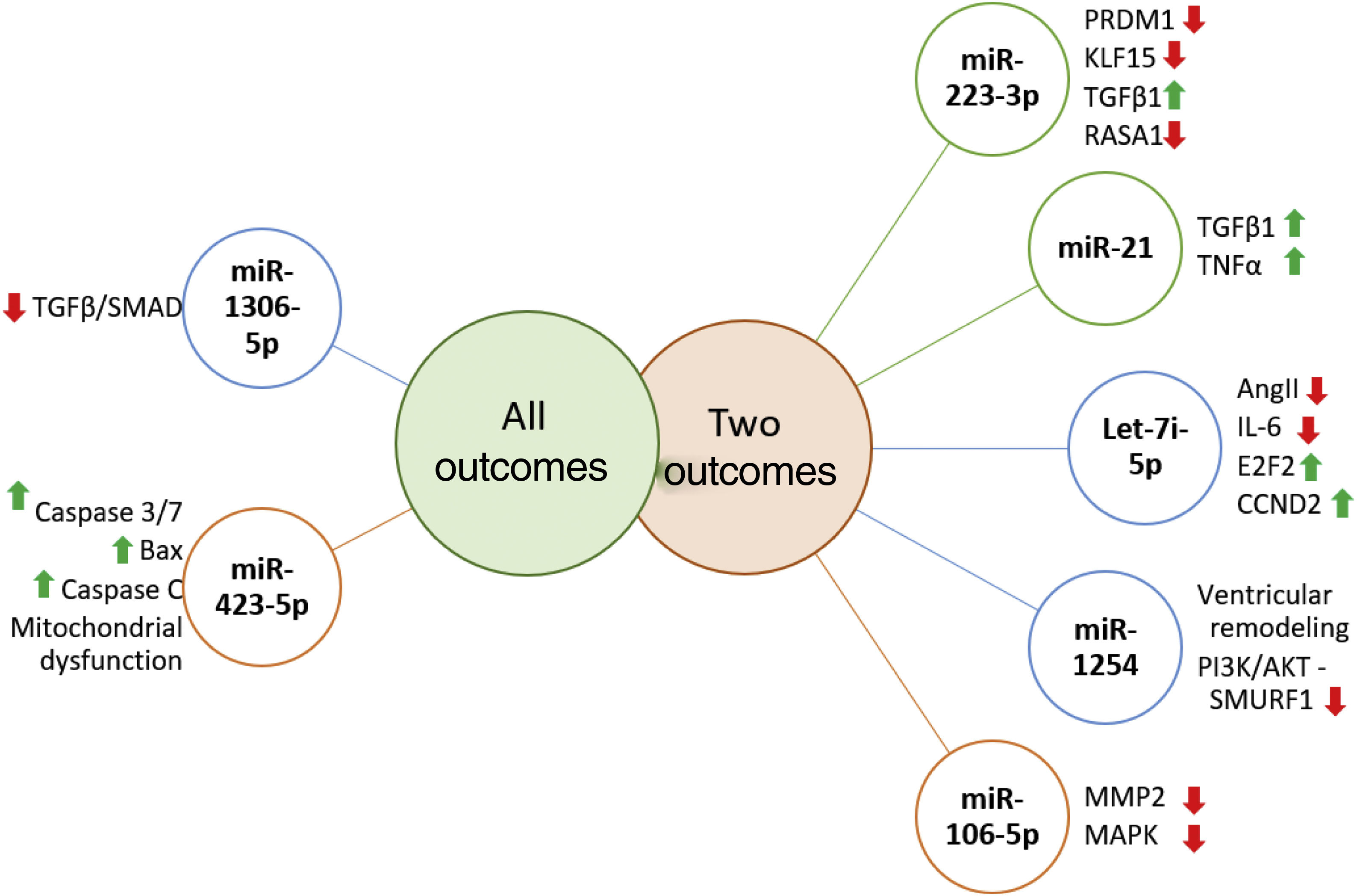
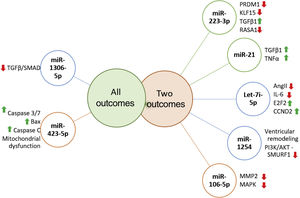
Prognostic role of miR in heart failureRecent literature reports the ability of miRs to predict independently the outcome in HF patients. Several miR were highlighted as prognostic markers and whose targets are signaling pathways already known to be involved in vascular and cellular processes related with HF (Figure 3).
MicroRNAs targets associated with more than one outcome. MiR-223-3p has been associated with several carcinomas, and is involved in cell proliferation, growth, migration and invasion, furthermore it negatively regulates PRDM1 involved in immune and inflammatory processes. KLF15 promotes cellular growth, regulating negatively TGFβ1 and RASA1, promoting cell migration.67–70 MiR-21 is negatively related to TGFβ1/SMAD7 and TNFα1, enhancing fibrotic processes, thus promoting ventricular remodeling.71–73 Let-7i-5p is known for negatively regulating inflammation and fibrosis processes, partly due to its effect on Ang-II and IL-6 and on E2F2 and CCND2 expression (regulators of cell cycle) and cardiac recovery.49,74 Few studies analysed miR-1254 targets, which is thought to be involved in cardiac remodeling, playing a protective role by inhibiting the PI3K/AKT pathway and SMURF1 expression (important in cell motility, signaling and polarity processes).75 MiR-106-5p might have a protective role, since it is suggested that it affects stress response due to MAPK signaling pathway inhibition and consequently oxidative stress.76,77 MiR-1306-3p inhibits the TGFβ/SMAD pathway, and is involved in pro-apoptotic processes. MiR-423-3p expression is triggered by hypoxia/reoxygenation processes, positively regulating Caspase 3/7, Bax, Caspase C promoting mitochondrial dysfunction.
Let-7i-5p and miR-223-5p are well known inflammation and fibrosis related miRs.31,49 Authors have suggested that the Let-7 family might have an active role in HF pathogenesis, and it has been related to poor outcomes in dilated cardiomyopathy.50,51 Interestingly and similar to miR-22, recent literature states that let-7i-5p negatively regulates angiotensin II, attenuating cardiac inflammation and fibrosis,49 thus contradicting previous literature and our findings.
Recently identified for the first time in HF patients, miR-1306-5p had been considered a possible biomarker in two large cohorts.33,37 Its function might be related to numerous processes in cells, including proliferation, differentiation and cell cycles.52
Previous literature states that miR-208 and miR-499 overexpression promotes LV hypertrophy, and ultimately HF.53 Positive correlations with HF have been found in miR-208 but not in miR-499, respectively.33
MiR-122, as previously stated, is a liver-specific miR associated with the risk of developing metabolic syndrome and has been linked to HF prognosis.45 Stojkovic et al. pointed to miR-122 as an independent predictor of all-cause mortality after adjustment for NT-proBNP in HFrEF patients. These data might suggest liver involvement in HF pathophysiology.36
Several of the included studies analyzed miR-423-5p. Positive, negative and no association with disease progression were described30–32,36,37,54 MiR-423-5p has been associated with chemotherapy resistance in cancer55–58 and, more recently, with cardiovascular diseases. The literature has pointed to a link between miR-423, vascular endothelial growth factor and nitric oxide (NO), concluding that miR-423 may serve as a biomarker of vascular growth/proliferation or inhibition, depending on the pathological process and target organ.59 These contradicting conclusions could be related to different miR expression in HF patients during an acute phase. In fact, most of the literature describes an association between miR-423-5p and a poor outcome. Cells and blood are the major source of miR suggesting that they might have a paracrine function that could justify miRs serum dysregulation. This indicates that HF may not be the only source of prognostic information, but it may derive from other damaged organs or cells.60
Curiously, negative associations were found between miR-423-5p and CV hospitalization and all-cause death,32 implying a protective role of miR-423. This unexpected result can be explained for different reasons: miR can be diluted and, also, different expression values in collected blood samples can be detected.38 Indeed, some miR can be undetectable in a large portion of samples.37,61
MiR-21 has also been linked to cell proliferation, migration and apoptosis processes and it plays an important role in the pathophysiology of hypertension.62 Despite its apparent role in heart disease, only one study has clinically referred to an association between miR-2163 and HF prognosis.32,38
Study limitationsThe studies subject to analysis revealed the need for a common approach to miR quantification and uniformization of methods. The standardization of methods and an adequate normalization of miR levels could clarify disparities in the conclusions.
MiR quantification method. The small noncoding RNA RNU6 genes are the reference genes most used as normalizers. Nonetheless, RNU is not a miR and, thus, it is not able to reflect the biological and chemical features of miRs, among other limitations.64 Several miR have been described and suggested as normalizers (i.e., miR-16) because of their increased blood expression and uniformity across several samples.64 Currently, there is no standardized normalizer miR in the scientific community, however, several studies have demonstrated that the use of more than one reference normalizer increases quantification accuracy. Unfortunately, all the studies included in this review only used one miR as a normalizer. Proportionally, different miRs were identified as altered in serum along with the study groups, making it impossible to measure and compare. This implies that using different normalizers may be responsible for distinct miR detection.
Study population definition criteria. An additional identified limitation was the lack of uniformity regarding a definition of HF and functional class criteria among the included studies. Also, study group selection in analyzed articles was distinct. Indeed, several groups included numerous definitions such as: hospitalized acute HF patients, hospitalized and outpatient chronic HF patients, unspecified HF, among others.
ConclusionThe involvement of MiRs in HF is unquestionable. The increasing interest in miRs has led to the identification of new pathways and mediated HF-related and miR potential as a HF biomarker.65 There are therefore questions related to miR that need to be unraveled, such as: how miR are released into circulation and what role do they play and to what extent can circulating miR reflect heart tissue expression levels.66
In the future, improved study designs with analogous miRs and larger cohorts will encourage the design of more clinically representative studies. Nevertheless, evidence shows the potential of miRs as a prognostic biomarker, as they are one of the most promising elements for future application in HF.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was financed by national funds through FCT Fundação para a Ciência e a Technology, I.P., within the scope of the Cardiovascular R&D Center (UIDB/00051/2020 and UIDP/00051/2020), RISE (LA/P/0053/2020) and project IMPAcT (PTDC/MED-FSL/31719/2017;POCI-01-0145-FEDER-031719).