Acute pulmonary embolism (PE) is a major cause of morbidity and mortality in Portugal. It is the third most common cause of cardiovascular death after stroke and myocardial infarction. However, the management of acute PE remains poorly standardized, and there is a lack of access to mechanical reperfusion when indicated.
Methods and resultsThis working group analyzed the current clinical guidelines for the use of percutaneous catheter-directed treatment in this setting and proposed a standardized approach for severe forms of acute PE. This document also proposes a methodology for the coordination of regional resources in order to create an effective PE response network, based on the hub-and-spoke organization design.
ConclusionThis model can be applied at the regional level, but it is desirable to extend it to the national level.
A embolia pulmonar aguda (EP) é uma das principais causas de morbilidade e mortalidade em Portugal. É a terceira causa mais comum de morte cardiovascular após acidente vascular cerebral e enfarte agudo do miocárdio. No entanto, o manejo da EP aguda permanece mal padronizado e falta acesso à reperfusão mecânica, quando indicada.
Métodos e resultadosEste grupo de trabalho analisou as recomendações clínicas atuais para o uso de terapêutica percutânea dirigida por cateter neste cenário e propõe uma abordagem padronizada para formas graves de EP aguda. Este documento também foi redigido com o objetivo de propor uma metodologia de coordenação de recursos regionais para a criação de uma rede efetiva de resposta à EP, baseada no desenho da organização hub and spoke.
ConclusãoEsse modelo pode ser aplicado a nível regional, mas é desejável estendê-lo a nível nacional.
Pulmonary embolism (PE) is a major worldwide health issue. It is the most common cause of cardiovascular death after myocardial infarction and stroke and the leading preventable cause of death in hospitalized patients.1,2
In epidemiological studies, the annual incidence of PE ranges from 39 to 115 per 100000 population.1,3 In Portugal, the incidence of PE hospitalizations rose between 2003 and 2013, probably due to an aging population (with an exponential increase particularly after the age of 40 years; mean age of the patients was 70±16 years), as well as to greater diagnostic accuracy resulting from advances in imaging techniques. The estimated incidence of hospitalization due to PE in 2013 was 35/100000 population/year in patients aged >18 years. During the same period, in-hospital case fatality rates fell from 32% to 17%, in part due to improvements in in-hospital health care standards.4
In addition to high in-hospital mortality, acute PE is associated with high long-term mortality5 and morbidity. Venous thromboembolism (VTE) associated with hospitalizations is a leading cause of disability-adjusted life-years lost.1 In the ELOPE Prospective Cohort Study, almost half of the patients had exercise limitation with reduced maximal aerobic capacity at one year after PE.6 Klok et al. reported that up to 36% of acute PE patients complained of persistent dyspnea and/or poor physical performance.7 Finally, PE is associated with the development of chronic thromboembolic pulmonary hypertension (CTEPH) in 1–4.0% of patients after the acute event.8 Patients with this disorder have poor prognosis and varying degrees of respiratory and cardiac impairment if left untreated.9 In Portugal, in a cohort of patients with severe forms of pulmonary embolism, 12.4% of patients had persistent pulmonary hypertension in a median three-year follow-up.10
Patients with high-risk PE who present with shock or persistent hypotension are in an impending life-threatening situation due to the high mortality associated with acute right ventricular (RV) failure (mean mortality of 30% within one month).2 For this reason, they have a mandatory indication for reperfusion with systemic thrombolysis, a class I recommendation in the 2019 guidelines published jointly by the European Society of Cardiology (ESC) and the European Respiratory Society (ERS).3,11,12 Although a significant reduction in 30-day PE-related mortality is seen with reperfusion therapy,13 several registries report a high percentage of hemodynamically unstable PE patients (68–80%) who did not receive systemic thrombolysis.14–16 Furthermore, systemic thrombolysis fails in approximately 8% of high-risk PE patients.3
Considering the lack of access to emergent surgical embolectomy in Portugal, percutaneous catheter-directed treatment (CDT) is an effective alternative that broadens the spectrum of patients who can undergo reperfusion in high-risk PE. The 2019 ESC/ERS guidelines assign a class IIa recommendation for CDT for patients with high-risk PE in whom thrombolysis is contraindicated or has failed, if appropriate expertise and resources are available on-site.3,11,12
However, not all patients have access to mechanical reperfusion. This often results from a lack of awareness, scarcity of funds and resources, and a failure of coordination between all stakeholders involved. Similarly to the ST-elevation myocardial infarction (STEMI) and stroke reference networks organized effectively at national level, systems of care for high-risk PE need to be established at regional and national level.
This document aims to propose a pilot project for the coordination of regional resources in the Lisbon and Tagus Valley region, based on a hub-and-spoke model, in order to provide appropriate management, including mechanical reperfusion, for severe forms of PE, when indicated. It also describes five fundamental steps needed to implement a projected PE response network at national level.
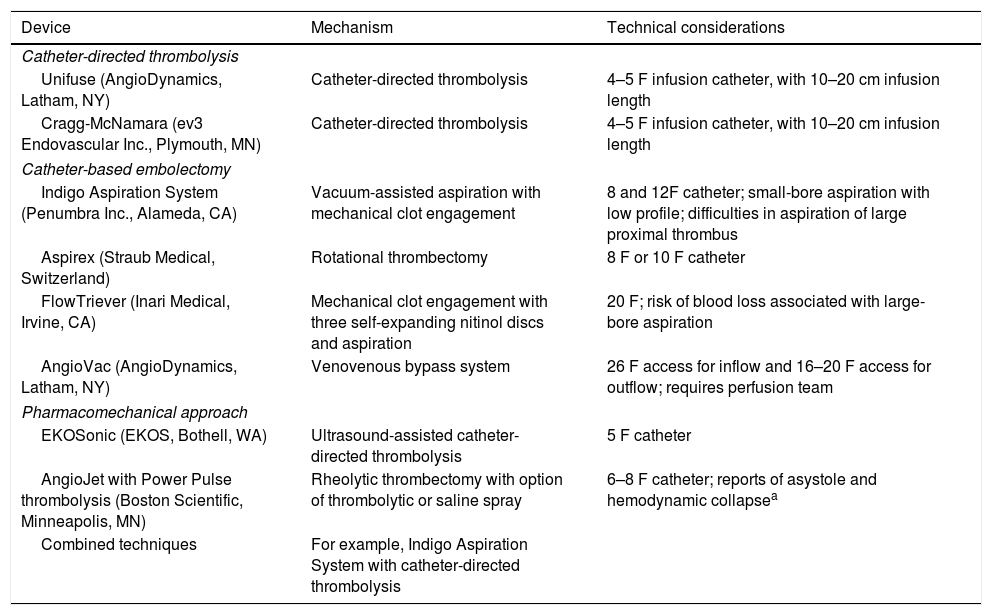
Definition of percutaneous catheter-directed treatment for pulmonary embolismCurrently, non-surgical interventional therapies for acute PE can be classified in three categories: catheter-directed thrombolysis, catheter-based embolectomy, and a pharmacomechanical approach combining mechanical or ultrasound fragmentation of the thrombus with reduced-dose thrombolysis.3,17Table 1 summarizes the technical characteristics of these devices, but a detailed description of each one is outside the scope of this document.
Percutaneous catheter-directed treatment of acute pulmonary embolism.
| Device | Mechanism | Technical considerations |
|---|---|---|
| Catheter-directed thrombolysis | ||
| Unifuse (AngioDynamics, Latham, NY) | Catheter-directed thrombolysis | 4–5 F infusion catheter, with 10–20 cm infusion length |
| Cragg-McNamara (ev3 Endovascular Inc., Plymouth, MN) | Catheter-directed thrombolysis | 4–5 F infusion catheter, with 10–20 cm infusion length |
| Catheter-based embolectomy | ||
| Indigo Aspiration System (Penumbra Inc., Alameda, CA) | Vacuum-assisted aspiration with mechanical clot engagement | 8 and 12F catheter; small-bore aspiration with low profile; difficulties in aspiration of large proximal thrombus |
| Aspirex (Straub Medical, Switzerland) | Rotational thrombectomy | 8 F or 10 F catheter |
| FlowTriever (Inari Medical, Irvine, CA) | Mechanical clot engagement with three self-expanding nitinol discs and aspiration | 20 F; risk of blood loss associated with large-bore aspiration |
| AngioVac (AngioDynamics, Latham, NY) | Venovenous bypass system | 26 F access for inflow and 16–20 F access for outflow; requires perfusion team |
| Pharmacomechanical approach | ||
| EKOSonic (EKOS, Bothell, WA) | Ultrasound-assisted catheter-directed thrombolysis | 5 F catheter |
| AngioJet with Power Pulse thrombolysis (Boston Scientific, Minneapolis, MN) | Rheolytic thrombectomy with option of thrombolytic or saline spray | 6–8 F catheter; reports of asystole and hemodynamic collapsea |
| Combined techniques | For example, Indigo Aspiration System with catheter-directed thrombolysis | |
The choice of one type of intervention over another will depend on thrombus burden, hemodynamics, bleeding risk and the presence of contraindications for systemic thrombolysis, expertise, and resources.17
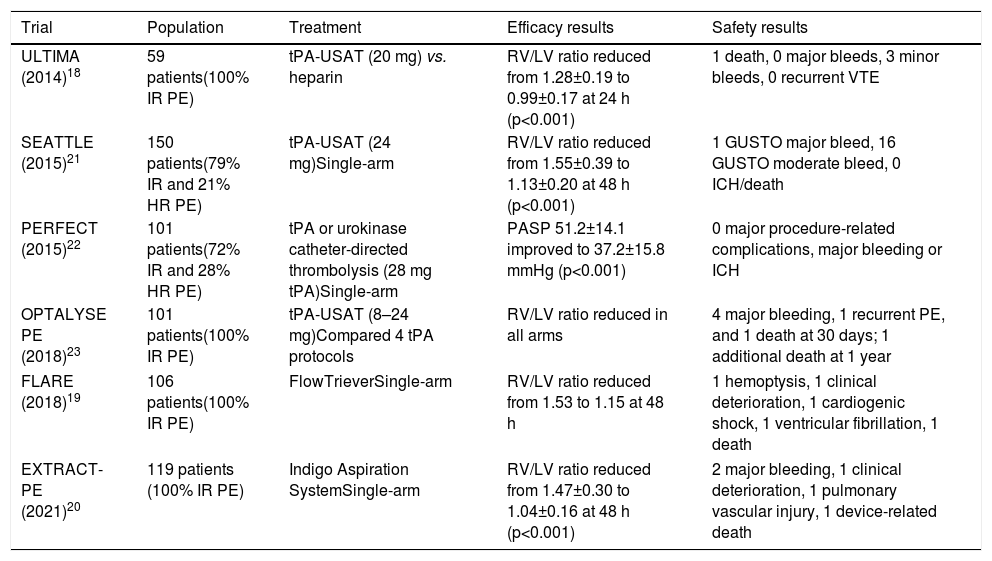
Brief review of clinical guidelines and evidence on catheter-directed treatmentTo date, no prospective study has demonstrated a mortality benefit or prevention of PE recurrence, development of CTEPH, or post-PE syndrome with the use of any interventional therapy in high- or intermediate-risk PE.17 Most data on CDT are derived from registries and prospective cohort studies. In the only randomized controlled trial with CDT to date, which included 59 patients with intermediate-risk PE, ultrasound-accelerated thrombolysis of PE plus anticoagulation more rapidly reversed RV dysfunction than anticoagulation alone.18 Studies on catheter-based embolectomy devices also demonstrated rapid improvements in RV function,19,20 although there are no data comparing their effectiveness with anticoagulation alone and almost all analyses are focused on imaging surrogate endpoints. Table 2 summarizes the most important studies on the use of CDT in PE (most of them in the setting of intermediate-risk PE).
Summary of main trials on catheter-directed treatment for pulmonary embolism.
| Trial | Population | Treatment | Efficacy results | Safety results |
|---|---|---|---|---|
| ULTIMA (2014)18 | 59 patients(100% IR PE) | tPA-USAT (20 mg) vs. heparin | RV/LV ratio reduced from 1.28±0.19 to 0.99±0.17 at 24 h (p<0.001) | 1 death, 0 major bleeds, 3 minor bleeds, 0 recurrent VTE |
| SEATTLE (2015)21 | 150 patients(79% IR and 21% HR PE) | tPA-USAT (24 mg)Single-arm | RV/LV ratio reduced from 1.55±0.39 to 1.13±0.20 at 48 h (p<0.001) | 1 GUSTO major bleed, 16 GUSTO moderate bleed, 0 ICH/death |
| PERFECT (2015)22 | 101 patients(72% IR and 28% HR PE) | tPA or urokinase catheter-directed thrombolysis (28 mg tPA)Single-arm | PASP 51.2±14.1 improved to 37.2±15.8 mmHg (p<0.001) | 0 major procedure-related complications, major bleeding or ICH |
| OPTALYSE PE (2018)23 | 101 patients(100% IR PE) | tPA-USAT (8–24 mg)Compared 4 tPA protocols | RV/LV ratio reduced in all arms | 4 major bleeding, 1 recurrent PE, and 1 death at 30 days; 1 additional death at 1 year |
| FLARE (2018)19 | 106 patients(100% IR PE) | FlowTrieverSingle-arm | RV/LV ratio reduced from 1.53 to 1.15 at 48 h | 1 hemoptysis, 1 clinical deterioration, 1 cardiogenic shock, 1 ventricular fibrillation, 1 death |
| EXTRACT-PE (2021)20 | 119 patients (100% IR PE) | Indigo Aspiration SystemSingle-arm | RV/LV ratio reduced from 1.47±0.30 to 1.04±0.16 at 48 h (p<0.001) | 2 major bleeding, 1 clinical deterioration, 1 pulmonary vascular injury, 1 device-related death |
CDT: catheter-directed thrombolysis; GUSTO: Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries trial; HR: high-risk; ICH: intracranial hemorrhage; IR: intermediate-risk; PASP: pulmonary artery systolic pressure; PE: pulmonary embolism; RV/LV: right ventricular/left ventricular; tPA: tissue plasminogen activator; USAT: ultrasound-assisted thrombolysis; VTE: venous thromboembolism.
Current clinical evidence is mostly derived from non-randomized trials focused on surrogate endpoints of improved RV function and decreased pulmonary artery systolic pressure. Nevertheless, the reported success rates of CDT are high, with hemodynamic stabilization, correction of hypoxia and survival to hospital discharge in approximately 87% of patients.24
A meta-analysis of thrombolysis trials that included patients with severe forms of PE showed a 47% reduction in all-cause mortality with the use of thrombolytics,25 highlighting the need for reperfusion therapy, particularly in the high-risk PE subgroup. In the ICOPER registry, 90-day mortality in high-risk PE patients who did not undergo thrombolysis was 55.1%.14 This finding encourages the development of alternative forms of reperfusion, such as CDT, when systemic thrombolysis is contraindicated.
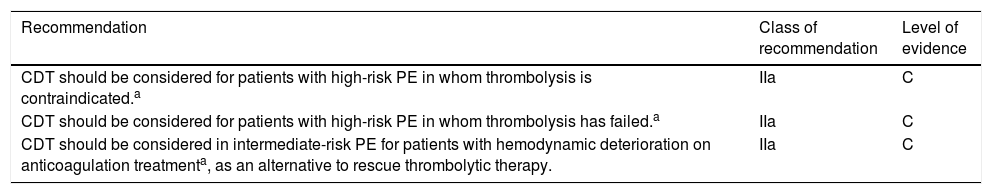
Based on expert opinion, the 2019 ESC/ERS guidelines assign a class IIa recommendation to CDT for some severe forms of PE, as shown in Table 3.12
European Society of Cardiology/European Respiratory Society guidelines for the use of percutaneous catheter-directed treatment by class of recommendation and level of evidence.
| Recommendation | Class of recommendation | Level of evidence |
|---|---|---|
| CDT should be considered for patients with high-risk PE in whom thrombolysis is contraindicated.a | IIa | C |
| CDT should be considered for patients with high-risk PE in whom thrombolysis has failed.a | IIa | C |
| CDT should be considered in intermediate-risk PE for patients with hemodynamic deterioration on anticoagulation treatmenta, as an alternative to rescue thrombolytic therapy. | IIa | C |
CDT: catheter-directed treatment; PE: pulmonary embolism.
Surgical pulmonary embolectomy can be considered in selected patients with severe forms of PE and central, surgically accessible clots located in the main bifurcation or proximal right or left pulmonary artery. However, this treatment modality has historically been carried out in large centers with surgical, perioperative and critical care expertise in this field, and there is a lack of access to it in Portugal. There are also divergences in reported outcomes regarding efficacy and safety. In a systematic review and comprehensive meta-analysis (56 studies included involving 1579 patients), in-hospital all-cause mortality was 26.3% (95% confidence interval [CI]: 22.5–30.5%) and surgical site complications occurred in 7.0% (95% CI: 4.9–9.8%).26 In a recently published study27 with a large population (58974 patients) undergoing different reperfusion modalities for acute PE between 2010 and 2014 in the National Inpatient Sample in the USA (5.2% surgical embolectomy, 37.8% CDT and 56.9% systemic thrombolysis), in-hospital mortality was 19.8%, 15.8% and 6.5% in patients treated with surgical embolectomy, CDT and systemic thrombolysis, respectively. Stroke (7.2% vs. 2.6% vs. 5.9%), cardiac arrest (15.6% vs. 3.9% vs. 11.2%) and blood transfusion (31.8% vs. 10.2% vs. 16.1%) were more frequent in the surgical subgroup compared to CDT and systemic thrombolysis. Patients in the surgical subgroup had more saddle emboli and were a higher-risk population.27
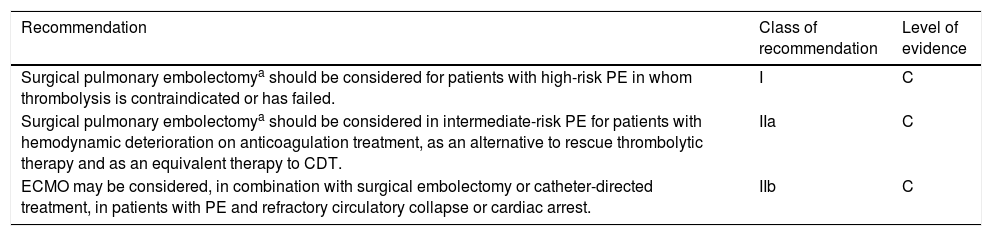
With a level of evidence C, the 2019 ESC/ERS guidelines assign a class I recommendation to surgical pulmonary embolectomy for high-risk PE with contraindication to thrombolysis or rescue, and a class IIa recommendation as an alternative to rescue thrombolysis or CDT, for some severe forms of intermediate-risk PE, as shown in Table 4.12 Surgical intervention should be an option in the above situations if there is a high-volume center available. Other indications for surgery should be the presence of thrombus in transit within the right heart chambers, patent foramen ovale or concomitant cardiac disease.28
European Society of Cardiology/European Respiratory Society guidelines for the use of surgical embolectomy by class of recommendation and level of evidence.
| Recommendation | Class of recommendation | Level of evidence |
|---|---|---|
| Surgical pulmonary embolectomya should be considered for patients with high-risk PE in whom thrombolysis is contraindicated or has failed. | I | C |
| Surgical pulmonary embolectomya should be considered in intermediate-risk PE for patients with hemodynamic deterioration on anticoagulation treatment, as an alternative to rescue thrombolytic therapy and as an equivalent therapy to CDT. | IIa | C |
| ECMO may be considered, in combination with surgical embolectomy or catheter-directed treatment, in patients with PE and refractory circulatory collapse or cardiac arrest. | IIb | C |
CDT: catheter-directed treatment; ECMO: extracorporeal membrane oxygenation; PE: pulmonary embolism.
Regarding cardiopulmonary support with venoarterial extracorporeal membrane oxygenation (ECMO), there are no randomized studies testing the safety and efficacy of this strategy in patients with high-risk PE, so it should be restricted to patients with refractory circulatory collapse or cardiac arrest. ECMO is associated with an increased risk of bleeding complications, especially those related to vascular access and in patients undergoing thrombolysis.12,29
Choice of treatment in high-risk pulmonary embolismHigh-risk PE occurs in approximately 5% of hospitalized PE patients and is associated with very high short-term mortality (18–64%).14,30,31 RV dysfunction and hemodynamic instability due to acute pressure overload are powerful predictors of poor prognosis in acute PE.32,33 Risk stratification is therefore the mandatory first step to tailor PE treatment. For high-risk PE, systemic thrombolysis is generally appropriate first-line therapy.3,12,34 However, several population-based studies report the underuse of systemic thrombolysis despite its indication in this clinical scenario.13,16,35 When there is high-risk PE and absolute or relative contraindication to systemic thrombolysis (as presented in previous ESC/ERS guidelines12), high-risk PE and failure to improve after systemic thrombolysis (rescue reperfusion therapy), CDT is recommended for rapid hemodynamic stabilization.12 In the event of an absolute contraindication for systemic thrombolysis or in rescue situations, a catheter-based embolectomy technique, without thrombolytic drug administration, should be used. In other situations, a pharmacomechanical approach could be an option. ECMO may be considered in combination with CDT in patients with PE and refractory circulatory collapse or cardiac arrest.12
Choice of treatment in intermediate-high-risk pulmonary embolismAvailable data do not support the routine use of systemic thrombolysis in intermediate-risk PE,36 although it should be considered when there are early signs of hemodynamic decompensation and acceptable bleeding risk.12 In the past decade, several randomized controlled trials and meta-analyses have helped to substantially clarify the optimal management of intermediate-risk PE. Initially, these studies focused on the use of full- or low-dose systemic thrombolysis.36,37 Overall, they demonstrated reductions in early mortality and hemodynamic deterioration. However, this benefit was offset by increases in fatal and intracranial bleeding (around 2% in clinical trials).25,38 The rationale for non-surgical interventional therapies for PE is to offer the advantages of reduced thrombotic burden in the pulmonary vasculature with less risk of systemic bleeding, thereby increasing safety.
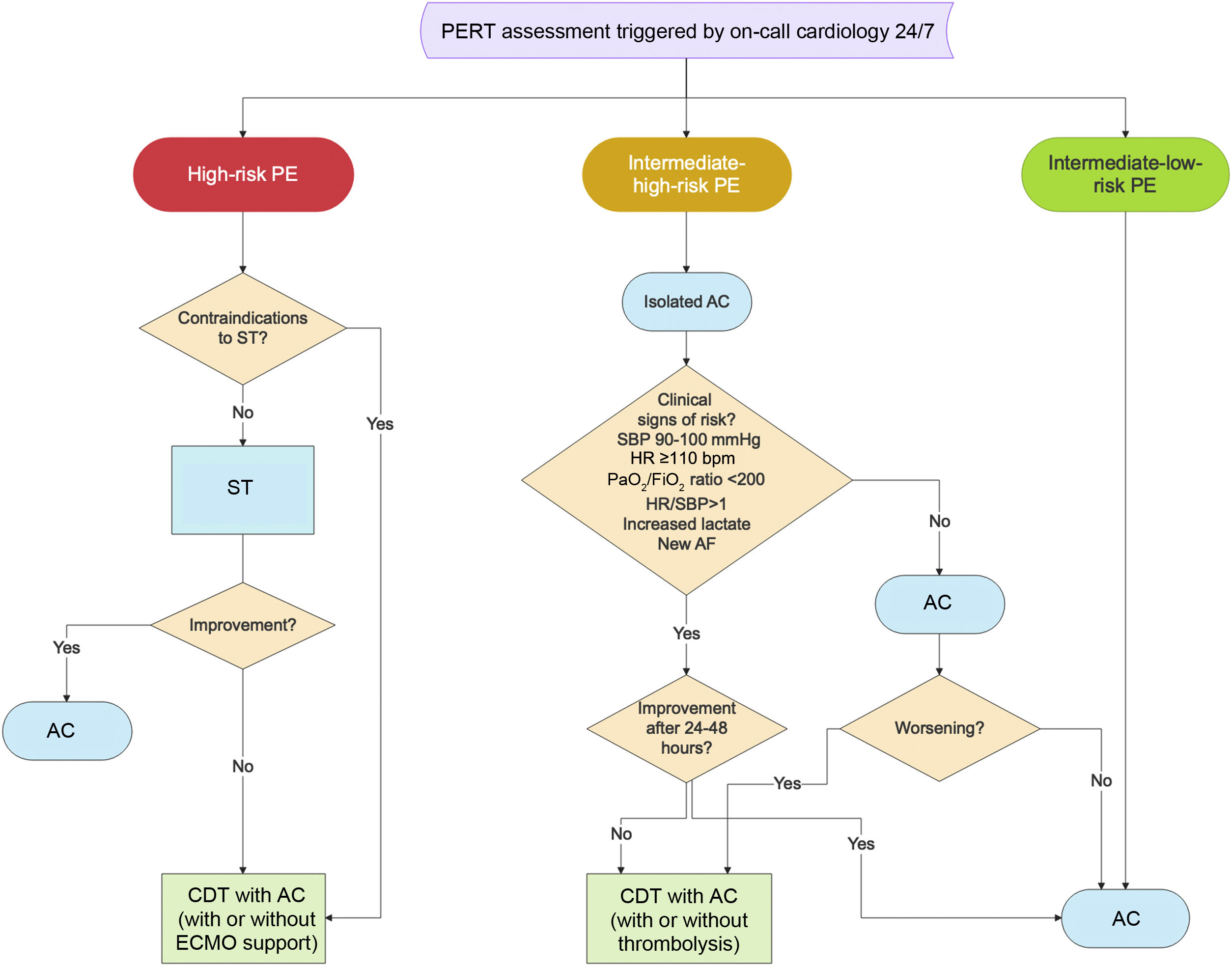
Most cases of acute PE with signs of RV dysfunction (by computed tomography pulmonary angiography or echocardiography), and myocardial necrosis as evidenced by an abnormal troponin level, evolve favorably with anticoagulation alone.12 However, they should be monitored for the first 24–48hours, and rescue reperfusion with CDT should be considered if clinical deterioration occurs. Patients are eligible for CDT if there is an additional finding of risk such as tachycardia (heart rate [HR] ≥110 bpm), arterial partial pressure of oxygen/fraction of inspired oxygen ratio (PaO2/FiO2) <200, systolic blood pressure (SPB) between 90 and 100 mmHg, shock index (HR/SBP) >1, signs of decreased peripheral perfusion (increased blood lactate levels), or new-onset atrial fibrillation.
In cases of intermediate-high-risk PE eligible for CDT, if there are no absolute contraindications for systemic thrombolysis, catheter-directed thrombolysis can be used with low-dose local thrombolysis or pharmacomechanical techniques because there is more time to relieve RV obstruction.
Characteristics of relevant parties a pulmonary embolism response networkThe following are important aspects of a PE response network.
- 1.
The primary aim of any PE network is to offer the best available percutaneous catheter-directed reperfusion treatment without delay in eligible patients with high-risk PE (emergent intervention with no delay), and in highly selected cases of intermediate-high-risk PE (urgent intervention within 72hours).
- 2.
The PE response network should offer a 24/7 service and should be organized on a regional basis in order to offer the destination therapy in all cases geographically closer to the patient and according to local resources.
- 3.
Pulmonary embolism response teams (PERTs) should be established as rapid response teams to provide individualized expert-based care for patients, coordinate multiple disciplines and efficiently mobilize resources during an acute PE.
- 4.
Standardized protocols should be implemented and applied uniformly between institutions.
- 5.
The PE response network should provide appropriate multidisciplinary follow-up of patients.
- 6.
Periodic surveillance of quality indicators should be performed as a way to improve the quality of the PE response network and as a mechanism for measuring opportunities to improve cardiovascular outcomes.
- 7.
Continuous training of all relevant parties in the PE response network should be assured, in association with the scientific societies involved.
Unlike the STEMI network, the patient does not have a primary role in reducing delay in treatment because PE is diagnosed in-hospital with the patient already in the system. There are two different ways for a PE patient to be admitted to the system: through the emergency department, or for intercurrent PE in a patient admitted to hospital for another reason (surgery, major trauma, stroke or another acute medical situation).
The hub-and-spoke model for building a pulmonary embolism response networkThe hub-and-spoke model has been used to organize STEMI39,40 and stroke networks,41 and could be adapted to plan a regional PE response network. This would enable technologies to cluster at the hubs, eliminating costly duplication of services and human resources, increasing efficiency and benefiting patients. The hub-and-spoke model would also enable standardization of performance indicators and concentration of experience in CDT techniques in its hubs, to deliver improved quality and outcomes.42,43
Like the organizational model implemented in the Regional Health Authority of the Lisbon and Tagus Valley region (ARSLVT) since January 2016 to respond to patients with cerebrovascular disease (Implementing Order no. 212-A/2018), the authors suggest that a similar model should be adopted for the treatment of severe forms of PE.
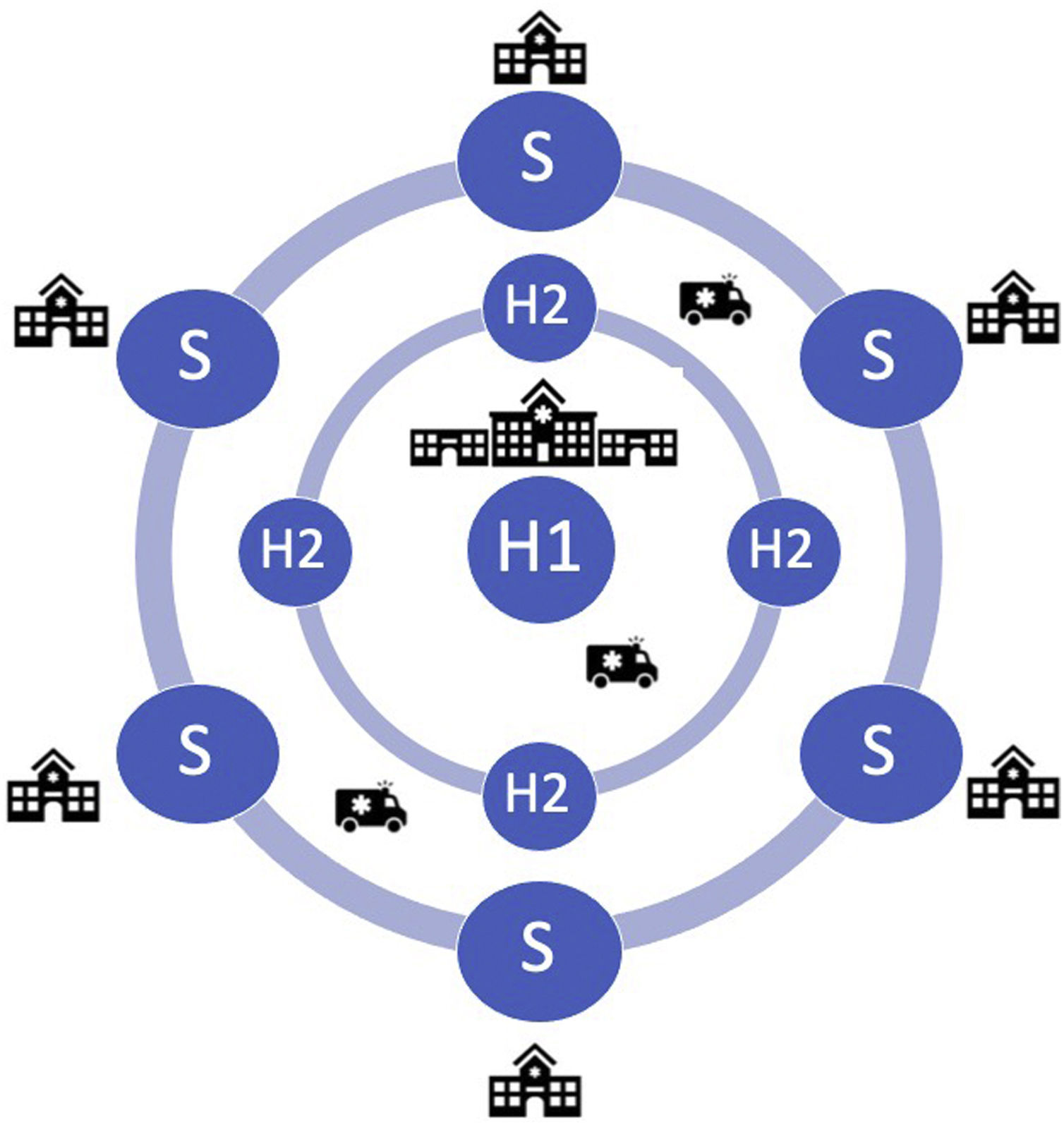
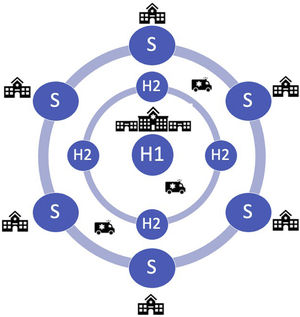
The following could be the main components of a regional PE response network in the Lisbon and Tagus Valley region (LVT) (Figure 1):
- –
Hub 1 (H1): 24/7 PE intervention hospital. This hub will periodically exchange with any Hub 2. Each H1 will be responsible for one night (8 pm to 8 am) during the week and one weekend per month (8 pm Friday to 8 am Monday);
- –
Hub 2 (H2): PE intervention hospital, but during working hours only; outside working hours these centers should act as a spoke to H1;
- –
Spoke: non-PE intervention hospital; patients are transferred routinely to H1 or H2 outside or during working hours, respectively.
Relevant parties in a PE response network are the following:
Emergency medical services (EMS): their main role is in secondary transportation. Transfer between hospitals should be coordinated with the EMS to ensure a rapid response and timely transfer to a PE intervention hospital.
Non-PE intervention hospital (spoke): protocols are to be agreed between the hub and spoke hospitals. The staff are trained and demonstrate competency in evidence-based PE care. All spokes are trained to administer fibrinolytic agents depending on the patient's hemodynamic status. The staff are also trained in when and how to activate the PERT of the nearest hub hospital in working hours and the H1 outside working hours. Standardized reperfusion and transfer protocols should be implemented based on indication and contraindication for fibrinolysis in cases of high-risk PE. Any high-risk PE diagnosed in a non-PE intervention hospital should be regarded as a high-priority situation for either immediate lysis or transfer to a hub to perform CDT. Any intermediate-risk PE could also be discussed by the PERT of the nearest hub hospital, but the decision on eligibility for reperfusion is not usually emergent and can therefore be discussed in working hours.
Physicians should assess the patient's clinical status and obtain a computed tomography pulmonary angiogram, laboratory tests and an electrocardiogram as quickly as possible. The staff then contact the on-call cardiologist at the hub hospital who will activate the institutional PERT.
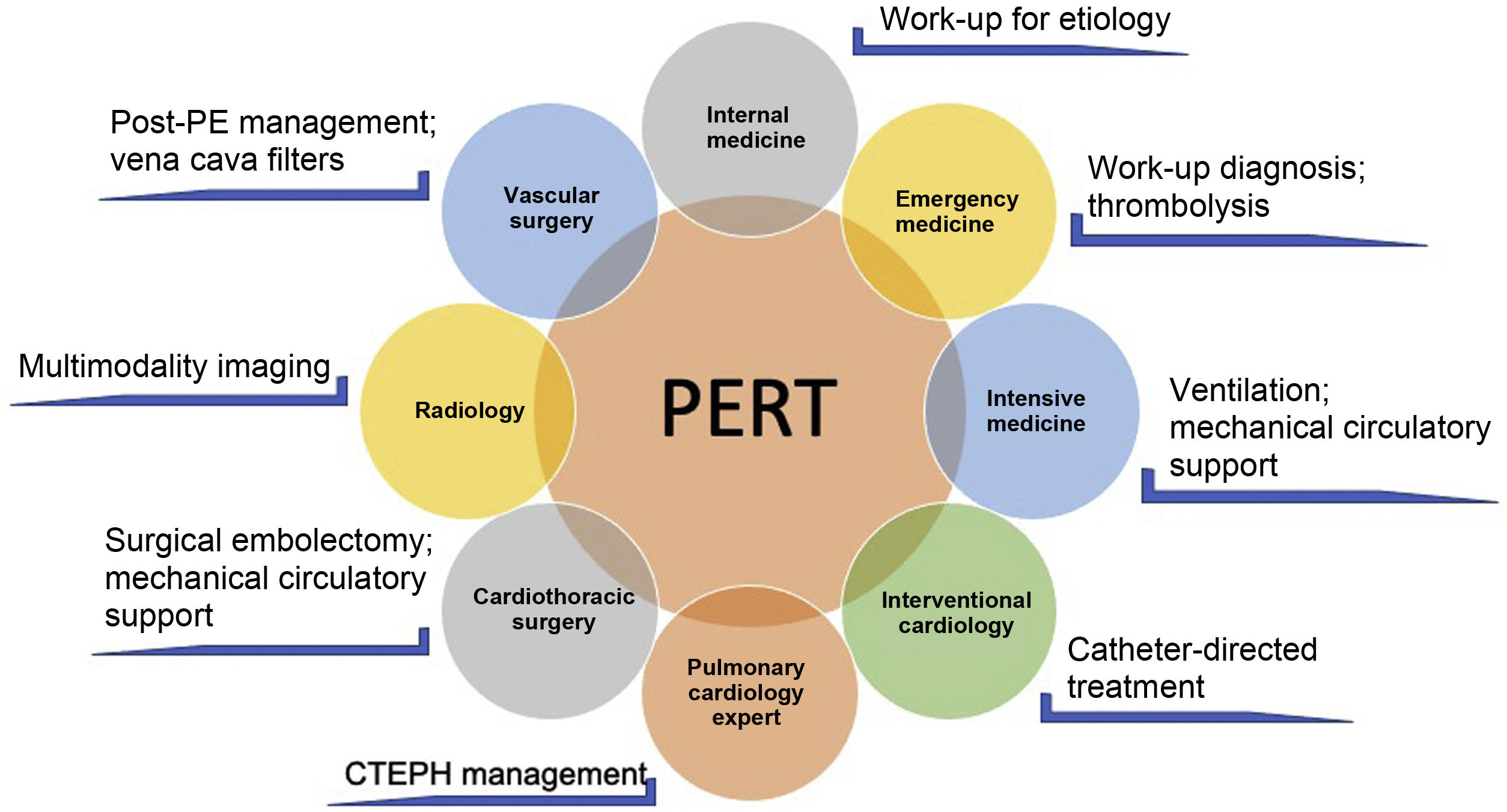
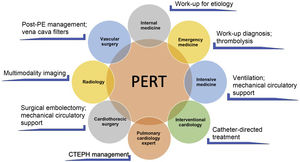
PERT: According to the PERT Consortium consensus document, a PERT is an institutionally based multidisciplinary team that has the ability to rapidly assess and provide treatment for patients with acute PE, using a full range of medical, surgical and endovascular therapies.44 The PERT's exact composition and mode of operation may differ between hospitals, depending on the resources and expertise available. It enables coordination of multiple disciplines with different knowledge and experience of PE, including internal medicine, cardiology, intensive care, interventional cardiology, cardiac surgery, emergency medicine and radiology (Figure 2). Teams can use conference calls or virtual meetings to discuss the case and the treatment decision is based on a consensus opinion of its members. The PERT must have 24-hour coverage. Its final clinical decision must be transmitted within an hour of being activated. The team must be able to provide appropriate multidisciplinary follow-up of patients and, if feasible, they should collect, assess and share data regarding the effectiveness of proposed treatments.44
Example of the multidisciplinary nature of a pulmonary embolism response team and the different skills involved (adapted from Rosovsky et al.50). CTEPH: chronic thromboembolic pulmonary hypertension; PE: pulmonary embolism; PERT: pulmonary embolism response team.
Advanced therapy (other than anticoagulation alone) in high-risk PE, in which there is hemodynamic compromise, is clearly accepted. However, in intermediate-high-risk PE, the appropriate management is less clear, due to lack of robust evidence, the heterogeneity of the population and the availability of various treatment modalities. This supports the establishment of PERTs, as they address the needs of modern system-based healthcare,45 and are associated with improved outcomes.46
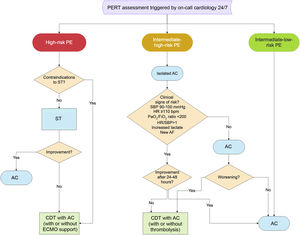
Figure 3 presents the flowchart of decision-making for CDT in PE after assessment by a PERT.
Flowchart of decision-making for catheter-directed treatment in severe forms of pulmonary embolism (adapted from Araszkiewicz et al.51). AC: anticoagulation; CDT: catheter-directed treatment; ECMO: extracorporeal membrane oxygenation; HR: heart rate; PaO2/FiO2: arterial partial pressure of oxygen/fraction of inspired oxygen; PE: pulmonary embolism; PERT: pulmonary embolism response team; SBP: systolic blood pressure; ST: systemic thrombolysis.
PE intervention hospital (hub): is a hospital with resources and expertise to perform procedures in the area of pulmonary intervention. It functions as H2 during working hours for local referral centers and as H1 (one night per week and one weekend per month) for all centers in ARSLVT.
For a center to be considered a hub, the following characteristics are essential:
- 1.
The center must have a PERT set up to correctly select patients and optimize the benefit of percutaneous treatment techniques. Representation on the PERT of both interventional and noninterventional specialties allows for appropriate discussions on the need for invasive therapies.
- 2.
The center should have experience in percutaneous treatment of pulmonary thromboembolic disease. Currently, there is insufficient evidence to determine a hub's expertise based solely on procedure volume. Having experience in percutaneous non-pulmonary intervention does not necessarily lead to success in interventions in the setting of PE, since there are differences between the pulmonary and systemic vasculature, the nature of the disease and the complications associated with the intervention. In addition to the technical proficiency acquired with the volume of procedures, clinical success for each patient also depends strongly on the correct selection of all available treatment modalities for PE within the multidisciplinary PERT.
- 3.
The center must be able to maintain the functioning of the STEMI network in parallel. Therefore, it is important to have two 24/7 cardiac catheterization laboratories (cath labs) and sufficient human resources to provide two prevention teams at the same time.
- 4.
Ideally, the center should have ventricular assist devices and/or circulatory support (ECMO) or a protocol with a reference center for this purpose. However, in cases of PE with greater hemodynamic instability, the team may move to a spoke if there is a cath lab available.
- 5.
The center must have well-established protocols with a cardiothoracic surgical center for referral of patients for emergent surgical embolectomy if indicated (high-risk PE and thrombus in transit within the right heart chambers).
- 6.
The center must have an active prospective clinical registry for monitoring quality and results.
Regional Coordination Team, the purpose of which is to coordinate all the operations of the regional network. It is responsible for defining quality measures, organizing and managing registries and conducting audits. The team's main objective is to assess the network's performance in order to implement changes that will improve it. Its members may come from spoke and/or hub centers.1,4,6,47
Quality measuresIt is essential to create quality indicators that will enable the quality of care to be quantified and will identify areas for improvement in clinical practice. The local PERT and the regional coordination team should meet periodically to analyze results and to generate changes to improve PE care. Health authorities and medical societies should be involved in the development of the PE response network.
Medical trainingIt is important to develop training actions that provide continuing medical education for all the parties in the PE response network. Medical societies of all disciplines involved in PE care (including the Portuguese societies of cardiology, internal medicine, and intensive care) will play an important role in the education of health professionals.
Pulmonary embolism response network activations in the Lisbon and Tagus Valley regionThe LVT region contains 34% of the country's population, which according to recent data amounts to 3.5 million people.48 Although epidemiological data on the incidence of PE in Portugal in recent years are not available, assuming that the incidence of hospitalization due to PE of 35/100000 population/year has remained constant, it can be estimated that there are approximately 1300 hospitalizations/year for PE in the LVT region. Considering that 35–55%2,17 of patients hospitalized with PE could be classified as intermediate-risk and 5%14,30,31 as high-risk PE, we estimate that there could be 520–780 patients/year hospitalized due to intermediate- and high-risk PE with an indication to be discussed by a PERT in order to decide the best therapeutic strategy. However, after the PERT's decision, only a few patients should have an indication for emergent CDT. We estimate there would be 65 high-risk PEs/year in the LVT region. According to a study by our group, 50% of patients with high-risk PE in a Portuguese single-center registry did not undergo reperfusion with systemic thrombolysis, mainly due to contraindications for this therapy.49 Therefore, there will be approximately 35 patients/year who may be candidates for percutaneous reperfusion as an alternative to thrombolysis. In addition to these patients, in 8% of high-risk PE there may be failure of systemic thrombolysis, which amounts to an additional five patients/year.
In addition, 5% of intermediate-risk PE patients usually suffer hemodynamic deterioration on anticoagulation treatment,36 which represents approximately 30 patients/year. If it is assumed that only 50% of these are reperfused with systemic thrombolysis, there could be another 15 patients for emergent CDT. In total then there will be around 55 patients/year who may be indicated for emergent CDT in the region. It is estimated that in coming years the incidence of high-risk pulmonary embolism will increase, similarly to what has happened in other countries.47 Therefore, these numbers are only indicative and are likely to be an underestimation.
Implementation of the pulmonary embolism response networkTo implement the PE response network, five fundamental steps are required (Table 5):
- 1.
Project design: this is the first step in the project. Firstly, an analysis of the current situation has been carried out regarding treatment of the most severe forms of PE. This document shows that at present high-risk PE is being undertreated all around Europe due to low rates of reperfusion, with serious implications in terms of disability-adjusted life years lost. Second is the establishment of the primary goals, which are to increase reperfusion rates in high-risk PE, to offer equal access to CDT as an alternative to systemic thrombolysis in selected situations, to standardize the treatment of intermediate-risk PE, to coordinate regional resources in order to create an effective PE response network based on the hub-and-spoke organizational model, and to obtain the reimbursement of dedicated teams in the PE response network, similarly to what occurs in the fully established STEMI and stroke reference networks.
- 2.
Bringing together stakeholders: the next step is to identify potential stakeholders, which will entail identifying potential hubs. Other essential stakeholders will be management teams in each hospital, and medical societies including the Portuguese Society of Cardiology (SPC) and the Portuguese Association of Cardiovascular Intervention (APIC). Finally, the media can also be an important stakeholder in publicizing the project. In this step, a kickoff meeting should be held to bring stakeholders together to discuss the project and define roles, responsibilities and performance rules.
- 3.
Submission and approval: in this step the project is presented to the regional health administration (ARSLVT), the national Directorate-General of Health and the Ministry of Health, together with an estimate of the costs involved in implementing it. If approved, steps 4 and 5 follow.
- 4.
Implementation of the pilot project for LVT and monitoring and assessment: after implementation of the PE response network at regional level, its results need to be closely monitored and assessed. Appropriate registries could be used to monitor the project and assessment would be performed by quantifying achievement of its primary goals.
- 5.
Implementation of the PE response network at national level and monitoring and assessment: this is the last step and one of the main primary goals of the project. During its implementation, the monitoring and assessment begun in step 4 is maintained but is now extended to national level.
Five steps to implement a national pulmonary embolism response network.
| 1 | Project design |
| 2 | Bringing together stakeholders |
| 3 | Submission and approval |
| 4 | Implementation of pilot project for LVT and monitoring and assessment |
| 5 | Implementation of the PE response network at national level and monitoring and assessment |
LVT: Lisbon and Tagus Valley region; PE: pulmonary embolism.
The authors propose a standardized treatment for severe forms of acute PE and the creation of multidisciplinary PERTs to rapidly assess and provide the best treatment for patients with severe forms of acute PE. This document outlines the steps needed to build a pilot project for the coordination of regional resources based on the hub-and-spoke model, in order to provide appropriate mechanical reperfusion for PE when indicated. A process of continuous care improvement through the assessment of quality indicators and continuous medical education are important ways to create a healthy PE response network. This model can be applied at the regional level, but it is desirable to extend it to the national level.
Conflicts of interestThe authors have no conflicts of interest to declare.