Balloon pulmonary angioplasty (BPA) has emerged as a promising therapeutic option for patients with chronic thromboembolic pulmonary hypertension (CTEPH) who are not eligible for pulmonary thromboendarterectomy (PEA) or who have recurrent or persistent pulmonary hypertension after surgery. There is no standardized technique for BPA and, its complexity and high risk of severe complications, requires skills and appropriate training and should be reserved for expert CTEPH centers, as a complementary intervention to medical and surgical therapy.
ObjectiveThe purpose of this document is to describe the BPA protocol used at a high-volume center nationwide, validated by its results.
MethodsThe present protocol includes technical details, definition of outcomes and complications, as well as patient full diagnostic work-up and treatment algorithm, before and after BPA.
ResultsThe technical, hemodynamic, and clinical results of the application of this protocol will be subject of a later publication where they will be described in detail. In conclusion, we present a percutaneous intervention protocol in the treatment of pulmonary hypertension in the context of chronic pulmonary thromboembolism, validated by its clinical, hemodynamic, and technical results.
A angioplastia da artéria pulmonar com balão (APB) é uma opção terapêutica promissora para doentes com hipertensão pulmonar tromboembólica crónica (HPTEC), não elegíveis para tromboendarterectomia pulmonar ou que apresentam hipertensão pulmonar recorrente ou persistente após cirurgia. Não existe uma técnica padronizada para APB, a sua complexidade e seu elevado risco de complicações severas requerem competências e um treino apropriado e deve ser reservada para centros especializados em HPTEC e como uma intervenção complementar à terapêutica médica e cirúrgica.
ObjetivoDivulgar um protocolo de APB usado num centro com elevado volume a nível nacional, validado pelos seus resultados.
MétodosO presente protocolo inclui detalhes técnicos, definição atual de resultados e complicações, bem como o algoritmo de avaliação diagnóstica e tratamento do doente antes e depois da APB.
ResultadosOs resultados técnicos, hemodinâmicos e clínicos da aplicação deste protocolo serão alvo de uma publicação posterior, na qual serão descritos em detalhe.
ConclusãoApresentamos um protocolo de intervenção percutânea no tratamento de hipertensão pulmonar em contexto de tromboembolismo pulmonar crónico, validado pelos seus resultados clínicos, hemodinâmicos e técnicos.
Pulmonary hypertension (PH) is a condition characterized by an increase in pulmonary artery pressure (PAP), which can be found in multiple clinical situations.1 Currently, a revised threshold for pre-capillary PH – a combination of mean PAP>20 mmHg, pulmonary arterial wedge pressure<15 mmHg and pulmonary vascular resistance (PVR)>3 Wood unit – has been proposed by the 6th World Symposium on Pulmonary Hypertension.2 Chronic thromboembolic pulmonary hypertension (CTEPH), within group four PH, has a cumulative incidence reported between 0.1 and 9.1% in the first two years after a symptomatic pulmonary embolism (PE) event.3 Following persistent obstruction of pulmonary arteries by organized thrombi, flow redistribution and secondary remodeling of the pulmonary microvascular bed lead to progressive increase in PVR, PH and right heart afterload, ultimately progressing to right ventricular (RV) failure and death,1,4 if left untreated. CTEPH is associated with poor prognosis when treated only medically.
Pulmonary thromboendarterectomy (PEA) is the first-line treatment. At experienced centers, it is associated with an in-hospital mortality rate below 5% and confers hemodynamic and functional improvement with good long-term survival.5 However, less than 60% of patients are eligible for surgery.6 For inoperable cases, PH-specific medical therapy with soluble guanylate cyclase (sGC) riociguat,7 in addition to chronic anticoagulation therapy, should be the standard medical treatment. Balloon pulmonary angioplasty (BPA), with sequential dilation of stenotic or obstructed pulmonary arteries, appears as an emerging therapeutic option for patients with CTEPH who are not eligible for surgery, or who have recurrent or persistent pulmonary hypertension after PEA.8 Interventional BPA is already mentioned in the European guidelines for pulmonary hypertension for non-operable patients with CTEPH (recommendation class IIb, level of evidence C).1
Although there is no standardized technique for BPA, it requires appropriate training. Patient selection and management of complications remains a challenge. For these reasons, BPA as a complementary intervention to medical and surgical therapy, should be reserved for expert CTEPH centers, with experience in the evaluation and care of this unique patient population.9
The purpose of the current document is to describe the BPA protocol used in our center, which includes procedure details, current definition of outcomes and complications, as well as the patient screening and treatment algorithm before and after BPA.
Evaluation before balloon pulmonary angioplastyThe diagnosis of CTEPH is based on findings obtained after three months of effective anticoagulation, to distinguish this condition from acute PE.
As recommended by the current guidelines,1 the initial imaging test used for CTEPH screening in our center is the V/Q scan. It has the ability to exclude the disease with a sensitivity and specificity of 90-100% and 94-100%, respectively.10
In a patient with mismatching perfusion defects on lung ventilation/perfusion scintigraphy (V/Q scan),3 CTEPH diagnosis requires invasive confirmation of PH through right heart catheterization (RHC).
Prior to the multidisciplinary CTEPH team meeting, a full clinical evaluation and investigation is required (see Table 1).
Assessment at baseline and timing for the follow-up of patients treated with balloon angioplasty for chronic thromboembolic pulmonary hypertension.
| At baseline | 3-6 months after change in vasodilator therapy | Before each BPA session | After 3 months of completed BPA procedures | After 6 months of completed BPA procedures | In case of clinical worsening | |
|---|---|---|---|---|---|---|
| Clinic visit and WHO functional class | X | X | X | X | X | X |
| Lab evaluation/NT-proBNP | X*1 | X | X | X | X | X |
| Blood gas analysis | X | X | X | X | X | |
| Pulmonary function tests | X*2 | |||||
| ECG | X | X | X | X | X | X |
| Echocardiogram | X | X | X | X | ||
| 6MWT/Borg dyspnea score | X | X | X | X | ||
| CPET | X*3 | X | X | |||
| Right heart catheterization | X | X | X | X | X | |
| V/Q scan | X | X | ||||
| DSA | X | X | ||||
| CTPA | X | X |
Table adapted from Galiè N, et al.1
BPA: balloon pulmonary angioplasty; BNP: brain natriuretic peptide; CPET: cardiopulmonary exercise testing; CTEPH: chronic thromboembolic pulmonary hypertension; CTPA: computed tomography pulmonary angiography; DSA: digital subtraction angiography; V/Q scan: lung ventilation/perfusion scintigraphy; WHO: World Health Organization; 6MWT: six-minute walking test.
*1 At baseline include thyroid function, viral serologies (hepatitis B and C and human immunodeficiency virus); screening for hypercoagulability syndromes when indicated.
*2 Including spirometry, lung volumes, diffusion capacity of carbon monoxide.
*3 For those who can perform a maximal six-minute walking test.
Biplanar digital subtraction angiography (DSA)11 and electrocardiography (ECG)-gated computed tomography pulmonary angiography (CTPA)12 are both used to characterize vessel morphology, the types of lesions (ring-like stenosis, web, subtotal, total occlusions or tortuous lesions – Figure 1) and location of thrombi in the main, lobar, or segmental pulmonary arteries.13 CTPA gives us complementary information about the presence of bronchial collateral arteries (which can correlate with more central disease14), lung parenchyma and mediastinum.12
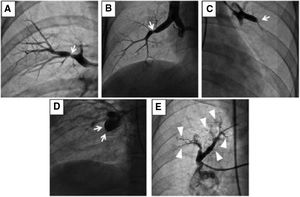
Angiographic classification of CTEPH lesions.
(A) Type A: ring-like stenosis lesion. (B) Type B: web lesion. (C) Type C: subtotal lesion. (D) Type D: total occlusion lesion. (E) Type E: tortuous lesion. From A to E increases the complexity of BPA; decreasing the success rate and increasing the likelihood of complications.
Reprinted with permission from Kawakami et al. Circ Cardiovasc Interv 2016;9:e003318.
For biplanar DSA (Figure 2), femoral vein access is usually used, but the jugular is also possible. A pigtail catheter 5 or 6 Fr is used to perform angiography. Low osmolarity ion contrast injection, and an automatic injector, with a volume of 20 to 30 ml, flow rate of 12 to 15 ml/s (depending on the severity of pulmonary hypertension), pressure limit of 1000 psi and injection delay of 2 seconds is used. The acquisition of images should be in apnea, two to three seconds after deep inspiration, in digital subtraction angiography mode, six to ten frames/s, with the field of view not enlarged.
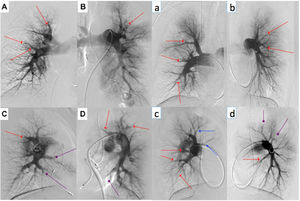
Digital subtraction angiography of two BPA patients (represented by upper and lowercase letters, respectively). We perform selective angiography of the right and left pulmonary arteries, acquiring images in anteroposterior (A and B; a and b) and profile (C and D; c and d) using a biplane angiography equipment. Different types of CTEPH lesions are indicated: web lesion (red arrows), ring-like stenosis (blue arrows) and occlusion-type lesion (purple arrows).
RHC is performed at baseline and repeated three to six months after change in vasodilator therapy, before each BPA session and after six months of BPA procedures. Table 2 presents the directly invasive and non-invasive measurements and calculated parameters obtained in RHC for these patients at our institution.15
Right heart catheterization recording protocol.
| Directly invasive measured parameters | Directly non-invasive measured parameters | Calculated parameters |
|---|---|---|
| mRAP (mmHg) | SBP, DBP, MAP (mmHg) | PVR (WU) |
| sPAP, dPAP, mPAP (mmHg) | Heart rate (beats/min) | PVRi (WU/m2) |
| mPCWP (mmHg)*1 | Weight (Kg) | SVR (WU) |
| CO (L/min)*2 | Height (cm) | SVRi (WU/m2) |
| Arterial and SvO2 (%) | BSA (m2) | |
| CI (L/min/m2) | ||
| SV (ml) | ||
| SVi (ml/m2) | ||
| PVR/SVR ratio |
BSA: body surface area (Dubois formula); CI: cardiac index; CO: cardiac output; DBP: diastolic blood pressure; HR: heart rate; MAP: mean arterial pressure; mRAP: mean right atrial pressure; sPAP: systolic pulmonary artery pressure; dPAP: diastolic pulmonary artery pressure; mPAP: mean pulmonary artery pressure; mPCWP: mean pulmonary capillary wedge pressure; SvO2: mixed venous oxygen saturation rate.
SBP: systolic blood pressure;
PVR: pulmonary vascular resistance; PVRi: pulmonary vascular resistance index; SVR: systemic vascular resistance; SVRi: systemic vascular resistance index; SV: stroke volume; Svi: stroke volume index; WU: Wood Units.
*1 or end-diastolic ventricular pressure when pulmonary capillary wedge pressure measured is not reliable.
*2 average of three measurements, performed and calculated using cardiac output device/thermodilution methodology and indirect Fick method.
After the full diagnostic work-up is complete, all cases are discussed in a multidisciplinary CTEPH team meeting, including surgeons experienced in PEA, interventional radiologists or cardiologists, expert radiologists in pulmonary vascular imaging, and clinicians with expertise in PH, to access potential eligibility for surgery. The CTEPH team confirms the diagnosis, assesses the surgical accessibility of thrombotic lesions (surgical operability), and considers comorbidities that may increase surgical morbidity and mortality risk (‘medical operability’).3Table 3 lists anatomical and clinical features that can predict an unfavorable outcome after surgery.9
Predictable features for risk-benefit assessment for pulmonary thromboendarterectomy.
| Characteristics | Favorable risk/benefit | Unfavorable risk/benefit |
|---|---|---|
| History | History of DVT/PE | No history of DVT/PE |
| Examination | No signs of right heart failure | Signs of right heart failure |
| Comorbidity | None | Concomitant lung or left heart disease |
| Functional limitation | Functional class II or III | Functional class IV |
| Type of disease | More proximal diseaseLower lobe disease | More distal diseaseNo disease in lower lobes |
| Hemodynamics | PVR<12.5 WU, in proportion to site and number of obstructions in imaging; higher PA pulse pressure. | PVR>15 WU, out of proportion to site and number of obstructions on imaging; higher PA diastolic pressure. |
Table adapted from Kim et al.4
DVT: deep venous thrombosis; PE: pulmonary embolism; PEA: pulmonary thromboendarterectomy; PVR: pulmonary vascular resistance.
BPA should be considered in patients who are non-operable or carry an unfavorable risk/benefit ratio for PEA, if the anatomical lesions appear suitable to BPA.1 BPA may also be an alternative therapy for patients with persistent or recurrent pulmonary hypertension after PEA.16
Prior to any BPA, a patient-based program is tailored. All CTEPH patients are subject to clinical stability and optimal supportive treatment including appropriate anticoagulant regimen, volume overload management with judicious diuretic use and long-term oxygen therapy if deemed necessary to maintain PaO2>60 mmHg.1
Specific targeted pulmonary vasodilator therapy, including monotherapy with the sGC stimulator riociguat in low-risk patients, or add-on combination therapy with off-label endothelin-receptor antagonists and/or prostanoids in high risk patients is implemented and titrated in order to optimize the pulmonary vascular hemodynamic profile prior to the BPA program.6
A set of clinical evaluation, of each patient, are established at the time of CTEPH diagnosis, before the first baseline BPA session, before each subsequent treatment session and three and six months after the last BPA session, as outlined in Table 1.
Balloon pulmonary angioplasty techniqueThe BPA technique used is similar to others previously described in the literature.17,18
The procedure is performed while maintaining chronic outpatient therapy, particularly anticoagulant therapy (vitamin K antagonists or direct oral anticoagulants) and PH-specific pulmonary vasodilators. The patient is awake, with continuous monitoring of oxygen saturation. Under local anesthesia, venous access is obtained in the femoral vein using the Seldinger technique followed by administration of 50 units/kg of weight of unfractionated heparin in patients receiving vitamin K antagonists and International Normalized Ratio (INR)<3.0. Patients under treatment with direct oral anticoagulant therapy receive 2000 units of unfractionated heparin per hour of procedure. Oxygen supplementation may be given to ensure peripheral oxygen saturation (SpO2) greater than 95%. RHC is performed at the beginning of each procedure for baseline hemodynamic assessment, followed by selective catheterization of the right or left pulmonary artery and replacement of the short introducer with a 6-French long sheath (Flexor Check-Flo 70 cm, Cook Medical LLC, IN, USA; or Destination 65 cm, Terumo, Tokyo, Japan).
Subsequently, the pulmonary arterial segment is catheterized using a 6-French 110-cm guide catheter (Judkins, Amplatzer or Multipurpose, CardinalHealth, Dublin, OH, USA). Segmental pulmonary angiography is performed while patients are taking a deep breath and injection of 1:1 diluted contrast at 7.5 to 15 frames/s without digital subtraction (Figure 3).
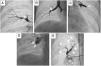
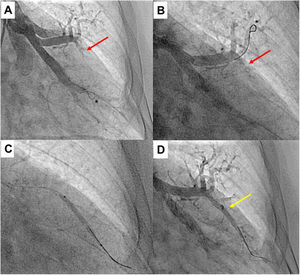
Upper segment of lingula BPA (left lung). A) Selective pulmonary angiography showing total occlusion of one vessel in lingula (red arrow); B) Selective angiography of lingula and passage of Gladius guide wire (Asahi Intecc Co., Aichi, Japan) through the vessel; C) Vessel dilation of the occluded lesion with a 3.0/30 mm semi-compliant balloon; D) Selective pulmonary angiography showing a good final result with increased arterial flow in the previous occluded vessel (yellow arrow); * shows the positioning of the distal wire with knuckle-wire technique which gives security of the procedure, decreasing the risk of perforation.
Conventional angiography images are acquired from anteroposterior, lateral or oblique 45° views. Subsequently, a 0.014 guidewire with soft, nontraumatic tip is placed in the distal vessel. Most target lesions for BPA are crossed with the Whisper MS wire (Abbot Vascular, Santa Clara, CA, USA) to avoid the risk of vascular injury associated with the use of polymer-jacketed guidewires. For total occlusions, an antegrade wire escalation supported by a microcatheter (Finecross 130 cm, Terumo, Tokyo, Japan) and increasing guidewire tip stiffness can be used (Pilot 50-150, Abbot Vascular, Santa Clara, CA, USA; Gaia First or Second or Gladius, Asahi Intecc Co., Aichi, Japan). After crossing the target lesion, we perform the balloon inflation of the target lesion with 2.0 to 9.0 mm diameter semi-compliant coronary or peripheral balloons depending on vessel diameter (Trek or Viatrac 14 Plus, Abbot Vascular, Santa Clara, CA, USA; Tazuna or Ryurei, Terumo, Tokyo, Japan; Emerge, Sterling or Mustang, Boston Scientific, Marlborough, MA, USA; Pantera Pro, Biotronik SE & Co KG, Berlin, Germany).
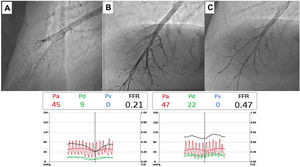
Complementary invasive tools for balloon pulmonary angioplastyContemporary imaging modalities, including intravascular ultrasound (IVUS, Eagle Eye Platinum, Philips, North Ryde, NSW, Australia; Figure 4)17 and optical coherence tomography (OCT, Dragonfly Optis; Abbot Vascular, Santa Clara, CA, USA; Figure 5),19,20 have been applied in BPA for a better characterization of the lesion, its extension and diameter. These imaging modalities are not widely used as they prolong procedure time. Nevertheless, IVUS is particularly useful in determining the vessel diameter in total occlusion BPA (Figure 4), increasing procedure safety.17 Pressure-wire-guided BPA using a microcatheter-based manometer (Navvus rapid exchange FFR micro-Catheter, Acist, Eden Prairie, Minnesota, USA) for distal pressure measurement can be used to guide vessel dilatation and reduce the risk of lung injury in patients with severe baseline hemodynamics (mean PAP>40 mmHg and/or PVR>7 WU).21 In the severe patient subgroup, the dilation of each vessel is stopped before the distal mean PAP (Pd value) reaches 20-25 mmHg,1,2 assessed by pressure wire after each dilation (Figure 6). In previous published data, the optimal cut-off varies between 19.5 to 35 mmHg.22,23 However, since the mean PAP is normally <25 mmHg, this is the cut-off used by our group. In some other cases, the pressure wire could be useful to identify abnormal lesions that are of uncertain significance when assessed by angiography. A Pd/Pa ratio of less than 0.80 could be used to guide treatment.21
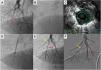
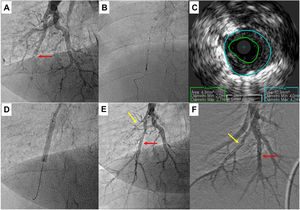
Pulmonary balloon angioplasty of a total occluded vessel from a segment of inferior right lower lobe, guided by intravascular ultrasound (IVUS). A) Selective pulmonary angiography showing occlusion of one of the A10 subsegments (red arrow); B and C) IVUS using an Eagle Eye® Platinum ST catheter (Philips, Amsterdam, the Netherlands) enabling the accurate determination the real size of the vessel and, thus, size the balloons to be used during angioplasty; D) Dilatation of the vessel with a 4.0-mm diameter balloon; E) Selective pulmonary angiography showing a good final result of chronic total occlusion (CTO) balloon plastic angioplasty (BPA) (red arrow). There is another CTO in segment A9 (yellow arrow) that was proposed to intervene later; F) Two months later, the final angiography revealed good BPA result in the previous CTO intervention and A9 CTO lesion successfully treated.
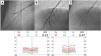
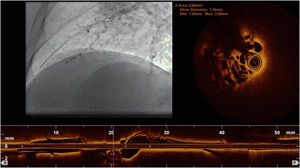
Pressure-wire-guided balloon plastic angioplasty of right medium lobe A5 segment, measuring distal pressure using a microcatheter-based manometer (Navvus rapid exchange FFR micro-Catheter, Acist, Eden Prairie, Minnesota, USA). This was the first session of a severe chronic thromboembolic pulmonary hypertension patient with systolic pulmonary artery pressure (PAP) around 80mmHg and mean PAP 40 to 50 mmHg. A) Subocclusion of two A5 subsegments in selective angiography. Intravascular hemodynamics (bottom left) revealed a significantly dampened distal pressure waveform (green line) and resting Pd/Pa=0.21; B) Improved angiographic appearance of one A5 subsegment after successive dilation with 2.0-mm and 3.0-mm balloons. Intravascular hemodynamics (bottom right) revealed improved distal pressure waveform with a Pd value of 22mmHg (green line); C) Final angiographic result with improved vessel perfusion in both subsegments.
Unlike coronary revascularization, clinical improvement after BPA is only observed after sequential dilation of several segments of the pulmonary circulation. For this reason, successful treatment of BPA requires multiple staged procedures with repeat catheterizations and dilatation to increase the pulmonary artery circuit cross-sectional area and total pulmonary blood flow. In other words, hemodynamic improvement is usually not observed after a single BPA session; additional sessions are needed so that clinical and hemodynamic benefit can be observed.17
The target lesions for each session are selected by matching the perfusion defects on V/Q scan, and lesion morphology in DSA and CTPA (Figure 7), which are later confirmed through segmental pulmonary angiography. In the first sessions, the lobe with the worst perfusion is often the target. If there are multiple perfusion defects, the treatment of lower lobe lesions where perfusion is greater and, consequently, with higher potential to reduce mean PAP, are selected for first BPA procedures. The treatment of less complex lesions such as ring-like stenosis, webs and subtotal lesions are favored. Total occlusions and tortuous vessel injuries are less frequently addressed during the first sessions, due to high risk of vascular injury. As mentioned by Matsubara et al.,24 total occlusions although more difficult to intervene and more likely to cause vascular injury, are also related to superior hemodynamic improvement when compared to less stenotic lesions. Increasing the number of treated segments, as well as addressing difficult lesions are both needed to improve the efficacy of BPA.24 Therefore, the treatment of total occlusions can be carried out in later sessions, when pulmonary hemodynamics have improved.
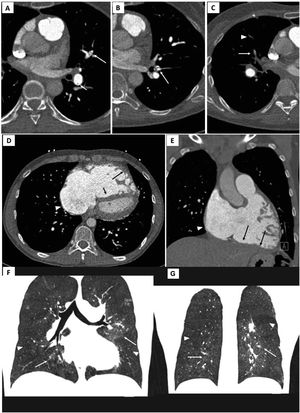
Electrocardiogram-gated pulmonary computed tomography (CT) angiography of patients with chronic thromboembolic pulmonary hypertension. Upper panel: A) and B) axial images showing hypodense linear structures forming webs and bands, inside the lumen of arteries, consistent with residual thrombus; C) demonstrates luminal opacities and marked caliber reduction of segmental vessels, suggesting subtotal lesion, as well as marked stenosis of more distal, subsegmental vessels (arrowhead).
Middle panel: D) Pulmonary CT angiography depicting right atrial dilation (arrowheads) and leftward deviation of the interventricular septum (short arrow) yielding a “D-shaped” left ventricle. D and E) right ventricular dilation and hypertrophy (long arrows show free wall thickening) with marked myocardial trabeculation.
Lower panel: F and G) Mosaic pattern of lung attenuation. The hypoperfused (oligemic) lung appears lower in attenuation (arrowheads) than adjacent normal perfused lung (arrows).
Pulmonary flow grade23 is estimated before and after each dilation of a treated segment or subsegment (Table 4). Semi-compliant balloon dilation using a relatively small balloon size is performed first (2.0-mm balloon), and then balloon size is sequentially increased to obtain larger vascular diameter and increased pulmonary flow in the target vessel. The most important when performing BPA is not so much the morphological result in the dilated area as the hemodynamic result (whether visually assessed on the quality of the antegrade flow and venous return or by measuring pressures downstream of the dilated area).
Pulmonary flow grade score.
| Grade 0 | No perfusion or penetration with minimal perfusion of pulmonary arteries |
| Grade 1 | Partial perfusion of pulmonary arteries and no perfusion or penetration with minimal perfusion of pulmonary veins |
| Grade 2 | Complete perfusion of pulmonary arteries and partial perfusion of pulmonary veins |
| Grade 3 | Complete perfusion of both pulmonary arteries and veins |
Information from Inami et al.24
Due to recognized correlation between pre-BPA mean PAP over 40 mmHg25 and the severity of reperfusion lung injury, adequate balloon sizing depends on hemodynamics, angiographic lesion type and estimated vessel diameter (derived from eyeballing subjective analysis of angiogram in most of the lesions) (Table 5).26 Under sizing balloon to vessel diameter (i.e. a low balloon/vessel ratio) is needed more in patients with severe hemodynamics (mean PAP>40 mmHg and/or PVR>7 WU), especially in the first sessions. The balloon is inflated until the fluoroscopic waist disappears or until 5 to 8 atmospheres is reached. The aim for each target lesion should be the presence of grade 3 final flow according to the Pulmonary Flow Grade (Figure 3). Lesions with residual stenosis (pulmonary flow grade <3) can be re-intervened in subsequent sessions with balloons of appropriate diameter to the vessel size. The total number of segments treated at each session varies according to the patient's hemodynamic severity, procedure time, and the amount of iodinated contrast used. If the patient's hemodynamics are severe, preferably only two segments on the same side are treated, avoiding intervention on both the right and left pulmonary arteries in the same procedure, to restrict the treatment area at risk. If pre-procedural pulmonary hemodynamics is not severe, there are no limitations on the number of segments or lobes targeted in one session. Nevertheless, any single BPA session should be limited to <2 Gy of radiation exposure, 60 minutes of fluoroscopy and maximal load of administered contrast adjusted to renal function (<300 ml).
Balloon sizing according to hemodynamics severity and type of lesion.
| Type of lesion | Balloon-vessel ratio | |
| mPAP<40 mmHg and/or PVR<7 WU | mPAP>40 mmHg and/or PVR>7 WU | |
| Ring-like stenosis | 1.0 | 0.8 |
| Web | 0.8 | 0.6 |
| Total occlusion | 0.6 | 0.5 |
Table adapted from Ahn et al.27
mPAP: mean pulmonary artery pressure; PVR: pulmonary vascular resistance.
As multiple procedures are required to finalize the BPA program, in order to decrease the total number of patient admissions, two BPA sessions are performed two to four days apart at each hospital admission. RHC is repeated three to four weeks after each BPA session. Additional BPA sessions are performed until PVR and/or mean PAP drops below 4 WU and 25 mmHg, respectively, under pulmonary vasodilator therapy, and/or all accessible lesions are intervened.
Definition of balloon pulmonary angioplasty-related complicationsIn an attempt to standardize reports of BPA complications at various centers, a classification of complications was proposed by the task force on CTEPH at the 6th World Symposium on Pulmonary Hypertension, and was published recently (Table 6).9 The most common complication of BPA is lung injury, characterized by lung opacities on chest radiograph or CTPA. Initially, it was thought that the mechanism was similar to the reperfusion edema that can occur after PEA. However, after carefully reviewing CTPA images, Ejiri K. et al.25 found that the so-called “reperfusion pulmonary edema” was a focal opacification located only at the site of BPA-targeted lesions. And for that reason, currently, the most frequently accepted cause for lung injury is hemorrhage caused by iatrogenic vascular injury due to trauma caused by the guidewire, balloon overdilation or high-pressure contrast injection.25 The lung injury can be classified according to five degrees using a classification system previously published in the literature (Table 7).23 An angiographic vascular injury (perforation) is usually detected by the following signs: extravasation of contrast, hypoxemia, cough, tachycardia or increased pulmonary arterial pressure. Other complications related to BPA were the vascular dissection, vascular access site complications and contrast nephropathy defined as a 25% or 0.5 mg/dl increase compared to the pre-procedure creatinine value.27 Major complications related to the procedure are moderate and severe lung injury (Table 7) or other complications that require bail-out intervention, noninvasive positive-pressure ventilation, mechanical ventilation, extracorporeal membrane oxygenation, urgent surgery or death.
Balloon pulmonary angioplasty complications.
| During the procedure | After the procedure |
|---|---|
| Vascular injury (with/without hemoptysis)Vascular dissectionAllergic reaction to contrastAdverse reaction to sedation/local anesthesia | Lung injury (with/without hemoptysis; with/without hypoxemia)Contrast nephropathyAccess site complicationsRadiation injury |
Table adapted from Kimet al.9
Classification of lung injury.
| Grade | Definition | Oxygen needs |
|---|---|---|
| 1 | No significant recognition of lung injury on chest x-ray, only detected by CTPA | No need |
| 2 | Mild small lung injury on chest x-ray | Increase in oxygen achievable with nasal cannula (maximum 4l/min) |
| 3 | Moderate lung injury on chest x-ray | Elevated concentration of oxygen with need of facial mask |
| 4 | Moderate to severe lung injury on chest x-ray | Non-invasive positive pressure ventilation with high-concentration oxygen inhalation |
| 5 | Severe lung injury on chest x-ray | Mechanical ventilation |
Table adapted from Inami, et al. article.24
CTPA: computed tomography pulmonary angiography.
Besides being aware of potential BPA complications, one must learn how to prevent and treat them. Before starting a procedure, the operator should confirm that all the devices and resources necessary to control the most serious complication, pulmonary artery perforation, are readily available for use. Some measures that can decrease the risk of pulmonary perforation are careful wire positioning; using workhorse guidewires with soft, nontraumatic tips, whenever possible; cautious use of polymer-jacketed guidewires; using the knuckle-wire technique (* in Figure 3); appropriate balloon sizing and avoiding the treatment of total occlusions with no visible runoff. If a perforation or rupture occurs, immediate balloon tamponade of the vessel should be performed. Other bailout techniques are covered stent implantation, bioabsorbable gelatin injection or coil embolization. In severe forms of lung or vascular injury, noninvasive positive-pressure ventilation with supplemental oxygen, mechanical ventilation or extracorporeal membrane oxygenation may be needed.9,28
Definition of clinical outcomes of balloon pulmonary angioplastyTo date, there is no standardized definition of short or long-term clinical outcomes in literature.
Technical success for each lesion is a successful delivery and balloon expansion at the intended target lesion, and an increase of >1 grade in pulmonary flow grade score, which represents the increase in the perfusion of pulmonary arteries (Table 4). The end point for each target lesion should be a pulmonary flow grade of 3.
The acute procedural success of each BPA session must meet the angiographic criterion of technical success for at least two targeted lesions plus absence of severe complications (requiring mechanical ventilation, extracorporeal membrane oxygenation or urgent surgery) and survival at hospital discharge.
Clinical success of BPA should be evaluated after six months of the BPA program have been completed. The first goal of a BPA process is the relief of PH, invasively measured, with a decrease in mean PAP and PVR (to reach values below 25 mmHg and 4WU, respectively).8,17,18 The second goal is an improvement of: a) clinical parameters: exercise tolerance, assessed through improvement in WHO functional class, 6-minute walking test (6MWT) and/or cardiopulmonary exercise testing; (CPET)8,17,18 and; b) right heart overload parameters: right ventricular reverse remodeling,29 improvement of right ventricular systolic function30 and decreased levels of N-terminal pro-B-type natriuretic peptide (NT pro-BNP).31 In addition to relief of PH and symptoms, an important aim of the BPA program is the reduction in chronic targeted vasodilator therapy (especially prostanoids) and the withdrawal of long-term oxygen therapy.32Table 8 summarizes the definitions of clinical outcomes of BPA, proposed by our group.
Definition of clinical outcomes of balloon pulmonary angioplasty.
| Definition of technical success |
| Technical success of each lesion is defined by all of the following conditions:- successful delivery and balloon expansion at the intended target lesion- increase of >1 grade in pulmonary flow grade score |
| Definition of procedure success |
| Procedure success is defined by all of the following conditions:- angiographic criterion of technical success*1- absence of severe complications during the hospital stay*2- survival at hospital discharged |
| Definition of clinical success*3 |
| Primary outcome measures:- To reach PVR<4 WU- To reach mPAP<25 mmHgSecondary outcome measures:- Change from baseline in WHO functional class- Change from baseline in 6MWD- RV reverse remodeling and change from baseline in RV systolic function- Change from baseline in NT-proBNP or BNP- Change from baseline in the number and doses of vasodilator therapy- Withdrawal of long-term oxygen therapy |
BNP: brain natriuretic peptide; mPAP: mean pulmonary artery pressure; PVR: pulmonary vascular resistance; RV: right ventricle; WHO: World Health Organization; 6MWT: six-minute walking test.
*1 of at least two targeted lesions.
*2 severe lung injury (as described in Table 7) requiring mechanical ventilation, extracorporeal membrane oxygenation or urgent surgery.
*3 evaluated after 6 months of completed BPA staged multiple procedures.
The main long-term treatment goal is to decrease mortality risk. Data from a multicenter registry from Japan reported a three-year survival rate of 94.5% in 308 inoperable CTEPH patients treated with BPA (from 2004 to 2016; 1408 procedures),8 which is higher when compared to the poor prognosis of patients treated only medically (3-year survival rate described as 78.4%).33
Evaluation after balloon pulmonary angioplastyOur protocol comprises three phases of post-procedure follow-up: Immediate post-procedure care (0-24 hours), post-procedure care (24-72 hours) and outpatient clinical follow-up, with specific care at each stage, as described below:
Immediate post-procedure care (0-24 hours)In the first 24 hours after the procedure, the patient must be admitted to an intermediate care unit bed or equivalent, capable of continuous electrocardiographic monitoring and pulse oximetry. Chest X-ray is requested in all patients. If opacification is visible on the chest X-ray, high-resolution chest computed tomography without contrast is performed for early detection of lung injury.
Oxygen therapy is provided if oxygen saturation (SpO2) is below 92% or partial pressure of oxygen (PaO2) is below 60 mmHg, delivered through nasal cannula or face mask up to 100% fraction of inspired oxygen, to guarantee SpO2 greater than 95%. Non-invasive ventilation is implemented if there is signs of respiratory distress and/or respiratory failure despite O2 supplementation (respiratory rate>25 cycles/min or SpO2≤92%), in the bilevel mode with a minimum expiratory pressure of 5mmHg (can be increased to 8 mmHg if there is no right ventricular failure) and target tidal volume of 4-8 ml/kg of ideal weight.
During hospital stay, patients keep their previously prescribed specific pulmonary vasodilators and supportive therapy, including oral anticoagulant therapy.
Post-procedure care (24-72 hours)Chest X-ray, laboratory tests (which include renal function) and arterial blood gas are repeated every 24 hours. If there are no complications, clinical discharge is scheduled at 48-72 hours, depending on the patient's hemodynamic profile.
Outpatient clinical follow-upAfter completing the BPA process, all patients are asked to contact our PH center if any deterioration in clinical status is perceived. The first reassessment appointment is scheduled three months after conclusion of the BPA program, when patients are evaluated by PH expert physicians. Clinical assessment at this time includes evaluation of functional capacity, blood analysis including NT pro-BNP and 12-lead electrocardiogram. Six months after the last BPA session, reassessment includes all the above and Doppler echocardiography, 6MWT or CPET and RHC. Pulmonary vasodilator therapy is maintained from the moment of inclusion in the BPA program until the 6-month follow-up assessment. After this period, if risk stratification is low with achievement of normal or near-normal hemodynamics, weaning of pulmonary vasodilator therapy can be cautiously undertaken based on expert opinion followed by close follow-up. Anticoagulation is provided lifelong for preventing recurrent pulmonary embolism episodes. The need of long-term oxygen therapy is reassessed every BPA session with blood gas analysis and can be withdrawn if PaO2>60 mmHg without oxygen supplementation.
Lifelong follow-up is warranted to all patients included in our BPA program.
The clinical evaluation and exams for the evaluation at 3 and 6 months are presented in Table 1.
Final commentsA successful BPA program requires extensive training and should be reserved for expert centers. As Brenot et al.18 demonstrated in their experience, there is a learning curve, even after the technical refinements introduced by the Japanese experience.17 Thus, programs must be started with a proctor with recognized expertise in the technique and appropriate training standards. In addition, correct diagnostic assessment, management of vasodilator therapy and global clinical care, before and after the BPA, is one of the pillars dictating the success of the technique in this complex and unique patient population. For these reasons, this procedure should be performed at centers with expert CTEPH teams. These multidisciplinary teams should be capable of providing all current treatment modalities, and for that, be closely linked to a surgical center with experience in PEA that can determine the operability of each patient.34
Conflicts of interestThe authors have no conflicts of interest to declare.