Orthostatic intolerance (OI) syndromes are a confusing topic and determining a specific diagnosis to achieve optimal treatment can be troublesome. We sought to assess biomarker, hemodynamic and autonomic variables in OI patients (autonomic dysfunction [AD], postural orthostatic tachycardia syndrome [POTS] and neurally mediated syncope [NMS]) and healthy controls during supine and head-up tilt position in order to achieve a better diagnosis.
ResultsIn response to head-up tilt, patients with AD presented a marked decrease in systolic blood pressure (SBP) (p=0.002), and a blunted increase in heart rate (HR) (p=0.04). Baroreceptor gain was almost absent in supine position and did not change in response to tilt. Patients with POTS had lower values of atrial natriuretic peptide (p=0.03) but similar neurohormonal biomarkers and hemodynamic and baroreceptor function in supine position compared to healthy subjects. However, in response to head-up tilting greater reductions in stroke volume (p=0.008) and baroreceptor gain (p=0.002) and greater rises in HR (p=0.001), total peripheral resistance (p=0.008), low frequency component of SBP variability (LF-SBP) (p=0.003) and plasma noradrenaline (p=0.03) were observed. Patients with NCS had similar biomarkers and autonomic indices to healthy subjects in supine position, but a larger decrease in baroreceptor gain (p=0.007) and a greater rise in LF-SBP (p=0.004) and plasma adrenaline (p=0.003) response to head-up tilting.
ConclusionAlthough different OI syndromes share similar symptoms, including blurred vision, syncope and dizziness particularly during orthostatism, they differ markedly regarding biochemical, autonomic and hemodynamic parameters. Assessment of these differences may be helpful for better diagnosis and management.
As síndromes de intolerância ortostática (IO) continuam a ter uma avaliação difícil e um diagnóstico específico para se obter o melhor tratamento é frequentemente problemático. Para melhor esclarecimento destas patologias avaliamos os biomarcadores, parâmetros hemodinâmicos e atividade autonómica em doentes com diversos tipos de OI (disfunção autonómica (DA), síndrome de taquicardia postural ortostática (POTS) e com síncope neuromediada (SNM)) e comparamos com controles saudáveis durante a posição supina e após ortostatismo passivo (teste de tilt – TT).
ResultadosEm resposta ao TT doentes com DA, tiveram uma grande diminuição na pressão arterial sistólica (PAS, p = 0,002) e um aumento muito atenuado da frequência cardíaca (FC, p = 0,04). O ganho dos barorrecetores era quase residual na posição supina e não mudou em resposta ao TT. Doentes com POTS comparados com normais apresentaram menores valores de ANP (p = 0,03), mas valores de catecolaminas, parâmetros hemodinâmicos e função dos barorrecetores semelhantes na posição supina. No entanto, em resposta ao TT observou-se maior redução no volume de ejeção (SV, p = 0,008), e do ganho dos barorreceptores (p = 0,002), e um maior aumento da frequência cardíaca (FC, p = 0,001), da resistência periférica total (TPR, p = 0,008), do componente de baixa frequência de PAS (LF_SBP, p = 0,003) e da noradrenalina plasmática (p = 0,03). Doentes com SNM tinham biomarcadores e índices autonómicos similares aos controlos em supino, mas uma redução maior no ganho dos barorreceptores (p = 0,007), um maior aumento da LF_SBP (p = 0,004) e da adrenalina plasmática (p = 0,003) em resposta ao TT.
ConclusãoApesar das diferentes síndromes de IO apresentarem sintomas semelhantes, como visão turva, tonturas e síncope especialmente durante o ortostatismo, eles marcadamente diferem quanto ao comportamento dos parâmetros bioquímicos, autonómicos e hemodinâmico ao ortostatismo passivo. A avaliação destas diferenças pode ser útil para um melhor diagnóstico e abordagem terapêutica.
Orthostatic intolerance (OI) is a confusing topic due to the different clinical conditions it describes and also the lack of an uniform nomenclature.1
The term orthostasis literally means standing upright. OI may be defined as “the development of symptoms while standing that are relieved by recumbency”.2 However, special equipment is usually required to detect these abnormalities.
Standing involves an interplay of blood volume, physical, neurologic, humoral, and vascular factors which compensate for the effects of gravity on venous pooling.1,2
OI is not always due to dysfunction of autonomic or other compensatory mechanisms, but can also be due to inadequate responses of compensatory mechanisms to environmental stressors. For example, someone who is dehydrated may be unable to stand up without dire consequences, but autonomic dysfunction is not present; instead, the autonomic nervous system and other compensatory systems cannot adequately compensate for the loss of extracellular volume.3 On the other hand, pure autonomic failure induces OI because compensatory factors governed by the autonomic nervous system are inadequate. Patients with this condition not only cannot easily stand but clearly have detectable autonomic abnormalities in all positions. Therefore, OI encompasses any condition with inadequate regulation of blood flow, heart rate (HR), and blood pressure that is most easily demonstrable during orthostatic stress but may be present in all postures.
In order to help clarify OI syndromes we compared neurohormonal, hemodynamic and autonomic nervous system features in patients with different OI syndromes to determine the different pathophysiological behavior of these variables in orthostatic stress.
MethodsSubjectsWe studied 12 patients with autonomic insufficiency and orthostatic hypotension (OH), all with familial amyloid polyneuropathy (FAP). FAP was diagnosed on the basis of clinical findings, a family history of FAP, the presence of the TTR Met30 mutation in plasma, and a positive skin biopsy for amyloid. These patients with autonomic insufficiency were classified as the OH group. Twelve patients without orthostatic hypotension, but with frequent, typically repetitive neurally mediated (NM) syncope episodes and with a positive head-up tilt test that induced symptoms, were classified as the NM group. The same protocol was also performed in 10 patients with florid postural tachycardia syndrome (POTS), with orthostatic tachycardia at more than 120 bpm2; these were classified as the POTS group.
Twelve apparently healthy normotensive volunteers were used as controls and classified as the control group.
Patients and controls underwent the tests in a syncope clinic with autonomic laboratory facilities.
All participants had a normal electrocardiogram, were non-smokers and were not on medication, with the exception of birth control pills. All patients and controls were asked not to consume food or caloric beverages in the eight hours preceding the study or to drink coffee in the 24 hours before the study. Subjects with heart disease, diabetes or any other disease that could influence the results were excluded. The study protocol was approved by the Ethics Committee of Porto University Hospital and all patients gave their written informed consent.
Study protocolIn accordance with our protocol, each subject's assessment started at 10 am in a temperature-controlled room. After 15 min of bed rest, data were recorded for 10 min in the supine position, and subsequently during tilting in a head-up position at 70° with foot-board support for a maximum of 40 min. Continuous beat-by-beat recording (ECG and finger BP) was performed. The 10 min supine recording and the initial 10 min after tilting were used for comparison of autonomic, hemodynamic and neurohormonal parameters between groups.
Non-invasive blood pressure and electrocardiographic monitoringFinger BP was obtained non-invasively with the Ohmeda 2300 device (Finapres®, Englewood, CO), which uses a photoplethysmographic technique. With this method, pressure waves from finger recordings with excellent correlation with intra-arterial pressure values4 are generated continuously. The ECG signal was acquired after careful preparation of the skin, to keep impedance <5 kΩ. A CM5-type lead was used to obtain a high-amplitude QRS complex in order to decrease R-wave recognition errors.5,8
Analysis of heart rate variability and systolic blood pressure variabilitySpectral analysis of HR (RR interval) and systolic blood pressure variability (SBVP) was performed with software developed in the Matlab® environment (The MathWorks, Inc., South Natick, MA), to provide a flexible analysis system. Spectral analysis was performed by the non-parametric Welch method.5,6,8
The spectrum was decomposed in the three classical bands: the high frequency component (HF), between 0.15 and 0.40 Hz equivalent (Hz eq)2; the low frequency component (LF), between 0.04 and 0.15 Hz eq; and the very low frequency component (VLF), between 0.01 and 0.04 Hz eq. All data were calculated in absolute values by the area under the curve of the respective spectra.
Calculation of spontaneous arterial baroreceptor gainAssuming that variations in arterial pressure in the band centered around 0.10 Hz, obtained by spectral analysis of SBVP, represent rhythmic fluctuations of vasomotor activity mediated by the arterial baroreflex (Mayer waves), we calculated the spontaneous arterial baroreceptor gain (BRG) by spectral coherence.5 This band in the spectrum of heart rate variability (HRV) seems to correspond to baroreflex-mediated sympathetic and vagal adjustments.5,7,8 Baroreflex sensitivity was calculated from the modulus of the cross spectrum of RR interval and SBP for frequencies between 0.04 and 0.15 Hz.5,8
Calculation of non-invasive hemodynamicsStroke volume (SV), cardiac output and total peripheral resistance (TPR) were calculated by the method developed by Wesseling et al.9 because of its simplicity, low cost and non-invasive nature. This method analyzes the finger arterial pressure wave, as obtained by Finapres or Portapres devices or intra-arterial recordings, and calculates several hemodynamic parameters after applying the beat-to-beat model flow interpretation. An application of this three-element model has been published.10 Wesseling et al.9 described a good correlation, with an error of less than ±2%, between values obtained by this technique and thermodilution.
Determination of catecholamines and natriuretic peptidesTen ml of blood were collected in two different tubes: one containing EDTA and aprotinin (1.9 mg and 100 kIU/ml, respectively) for measurement of natriuretic peptides, and one heparinized tube containing 1.2 mg glutathione/ml for measurement of catecholamines.
Catecholamines were concentrated from plasma by liquid-liquid extraction and derivatized with the selective fluorescent agent 1,2-diphenylethylenediamine prior to chromatography. Optimal conditions for extraction, derivatization and chromatography have been investigated.11 Detection thresholds for this method are 0.3 pg for noradrenaline and adrenaline and 0.5 pg for dopamine. Atrial natriuretic peptide (ANP) was determined by radioimmunoassay (Nichols Institute) after extraction from plasma using Sep-Pak C18. BNP (brain natriuretic peptide) was measured by immunoradiometric assay (Shionoria), both without extraction.12 The intra-assay coefficient of variation of all methods used was in the range of <10%. All samples from each subject were analyzed in the same assay.
The samples were collected in supine rest and during the third minute of tilting.
Statistical analysisContinuous variables with normal distribution were expressed as mean and standard deviation. The Kruskal-Wallis test was used to compare means of independent samples and the Wilcoxon signed rank test and the Mann-Whitney test were used to compare changes in paired samples and for pairwise comparisons. A p value <0.05 was accepted for rejection of the null hypothesis for all comparisons. The statistical analysis was performed with SPSS version 14.0 (SPSS Inc., Chicago, IL, USA).
ResultsWe recruited 34 patients with orthostatic intolerance (12 with orthostatic neurogenic hypotension, 12 with neurally mediated syncope and 10 with POTS) and 12 healthy controls of comparable age (Tables 1–3).
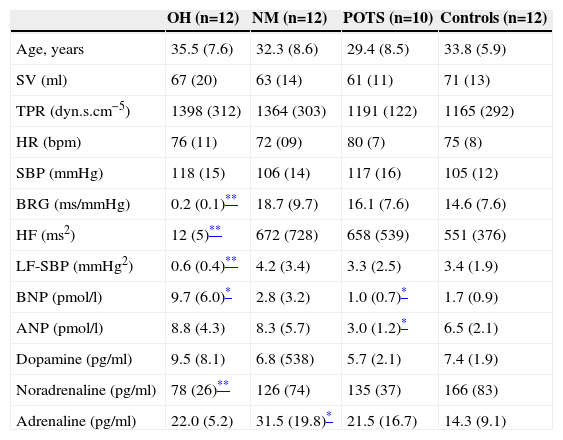
Autonomic, hemodynamic and hormonal variables in baseline supine position.
| OH (n=12) | NM (n=12) | POTS (n=10) | Controls (n=12) | |
|---|---|---|---|---|
| Age, years | 35.5 (7.6) | 32.3 (8.6) | 29.4 (8.5) | 33.8 (5.9) |
| SV (ml) | 67 (20) | 63 (14) | 61 (11) | 71 (13) |
| TPR (dyn.s.cm−5) | 1398 (312) | 1364 (303) | 1191 (122) | 1165 (292) |
| HR (bpm) | 76 (11) | 72 (09) | 80 (7) | 75 (8) |
| SBP (mmHg) | 118 (15) | 106 (14) | 117 (16) | 105 (12) |
| BRG (ms/mmHg) | 0.2 (0.1)** | 18.7 (9.7) | 16.1 (7.6) | 14.6 (7.6) |
| HF (ms2) | 12 (5)** | 672 (728) | 658 (539) | 551 (376) |
| LF-SBP (mmHg2) | 0.6 (0.4)** | 4.2 (3.4) | 3.3 (2.5) | 3.4 (1.9) |
| BNP (pmol/l) | 9.7 (6.0)* | 2.8 (3.2) | 1.0 (0.7)* | 1.7 (0.9) |
| ANP (pmol/l) | 8.8 (4.3) | 8.3 (5.7) | 3.0 (1.2)* | 6.5 (2.1) |
| Dopamine (pg/ml) | 9.5 (8.1) | 6.8 (538) | 5.7 (2.1) | 7.4 (1.9) |
| Noradrenaline (pg/ml) | 78 (26)** | 126 (74) | 135 (37) | 166 (83) |
| Adrenaline (pg/ml) | 22.0 (5.2) | 31.5 (19.8)* | 21.5 (16.7) | 14.3 (9.1) |
ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; BRG: baroreceptor gain; HF: high frequency component of heart rate variability; HR: heart rate in bpm; LF-SBP: low frequency component of systolic blood pressure variability; MAP: mean arterial pressure; NM: patients with neurally mediated syncope; OH: patients with orthostatic hypotension; POTS: patients with postural tachycardia syndrome; SV: stroke volume; TPR: total peripheral resistance.
All values are mean (SD).
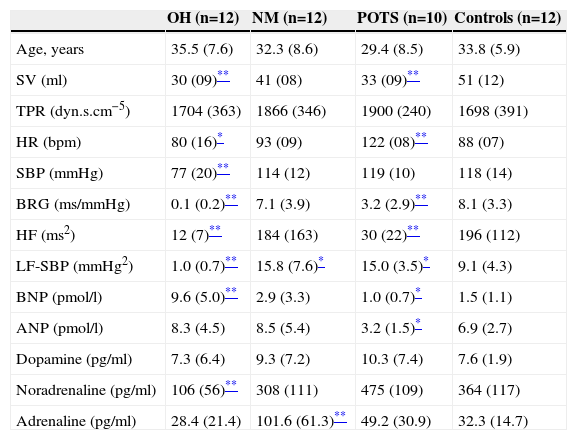
Autonomic, hemodynamic and hormonal variables during the first 10 min of orthostatic stress.
| OH (n=12) | NM (n=12) | POTS (n=10) | Controls (n=12) | |
|---|---|---|---|---|
| Age, years | 35.5 (7.6) | 32.3 (8.6) | 29.4 (8.5) | 33.8 (5.9) |
| SV (ml) | 30 (09)** | 41 (08) | 33 (09)** | 51 (12) |
| TPR (dyn.s.cm−5) | 1704 (363) | 1866 (346) | 1900 (240) | 1698 (391) |
| HR (bpm) | 80 (16)* | 93 (09) | 122 (08)** | 88 (07) |
| SBP (mmHg) | 77 (20)** | 114 (12) | 119 (10) | 118 (14) |
| BRG (ms/mmHg) | 0.1 (0.2)** | 7.1 (3.9) | 3.2 (2.9)** | 8.1 (3.3) |
| HF (ms2) | 12 (7)** | 184 (163) | 30 (22)** | 196 (112) |
| LF-SBP (mmHg2) | 1.0 (0.7)** | 15.8 (7.6)* | 15.0 (3.5)* | 9.1 (4.3) |
| BNP (pmol/l) | 9.6 (5.0)** | 2.9 (3.3) | 1.0 (0.7)* | 1.5 (1.1) |
| ANP (pmol/l) | 8.3 (4.5) | 8.5 (5.4) | 3.2 (1.5)* | 6.9 (2.7) |
| Dopamine (pg/ml) | 7.3 (6.4) | 9.3 (7.2) | 10.3 (7.4) | 7.6 (1.9) |
| Noradrenaline (pg/ml) | 106 (56)** | 308 (111) | 475 (109) | 364 (117) |
| Adrenaline (pg/ml) | 28.4 (21.4) | 101.6 (61.3)** | 49.2 (30.9) | 32.3 (14.7) |
ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; BRG: baroreceptor gain; HF: high frequency component of heart rate variability; HR: heart rate in bpm; LF-SBP: low frequency component of systolic blood pressure variability; MAP: mean arterial pressure; NM: patients with neurally mediated syncope; OH: patients with orthostatic hypotension; POTS: patients with postural tachycardia syndrome; SV: stroke volume; TPR: total peripheral resistance.
All values are mean (SD).
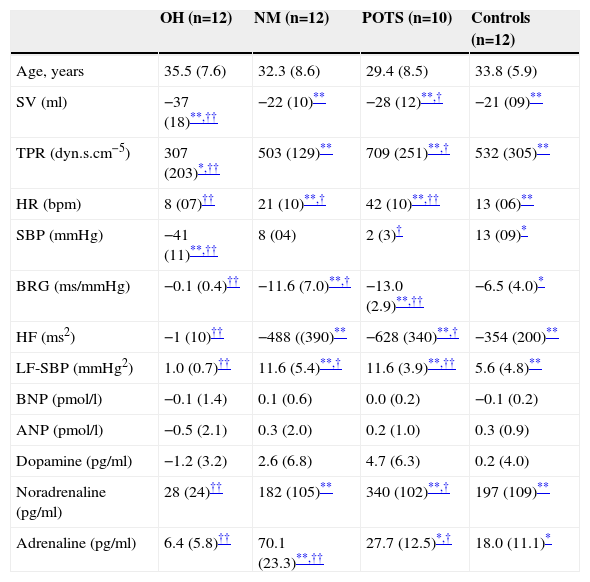
Autonomic, hemodynamic and hormonal changes from supine to standing in the different orthostatic intolerance groups and controls.
| OH (n=12) | NM (n=12) | POTS (n=10) | Controls (n=12) | |
|---|---|---|---|---|
| Age, years | 35.5 (7.6) | 32.3 (8.6) | 29.4 (8.5) | 33.8 (5.9) |
| SV (ml) | −37 (18)**,†† | −22 (10)** | −28 (12)**,† | −21 (09)** |
| TPR (dyn.s.cm−5) | 307 (203)*,†† | 503 (129)** | 709 (251)**,† | 532 (305)** |
| HR (bpm) | 8 (07)†† | 21 (10)**,† | 42 (10)**,†† | 13 (06)** |
| SBP (mmHg) | −41 (11)**,†† | 8 (04) | 2 (3)† | 13 (09)* |
| BRG (ms/mmHg) | −0.1 (0.4)†† | −11.6 (7.0)**,† | −13.0 (2.9)**,†† | −6.5 (4.0)* |
| HF (ms2) | −1 (10)†† | −488 ((390)** | −628 (340)**,† | −354 (200)** |
| LF-SBP (mmHg2) | 1.0 (0.7)†† | 11.6 (5.4)**,† | 11.6 (3.9)**,†† | 5.6 (4.8)** |
| BNP (pmol/l) | −0.1 (1.4) | 0.1 (0.6) | 0.0 (0.2) | −0.1 (0.2) |
| ANP (pmol/l) | −0.5 (2.1) | 0.3 (2.0) | 0.2 (1.0) | 0.3 (0.9) |
| Dopamine (pg/ml) | −1.2 (3.2) | 2.6 (6.8) | 4.7 (6.3) | 0.2 (4.0) |
| Noradrenaline (pg/ml) | 28 (24)†† | 182 (105)** | 340 (102)**,† | 197 (109)** |
| Adrenaline (pg/ml) | 6.4 (5.8)†† | 70.1 (23.3)**,†† | 27.7 (12.5)*,† | 18.0 (11.1)* |
ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; BRG: baroreceptor gain; HF: high frequency component of heart rate variability; HR: heart rate in bpm; LF-SBP: low frequency component of systolic blood pressure variability; MAP: mean arterial pressure; NM: patients with neurally mediated syncope; OH: patients with orthostatic hypotension; POTS: patients with postural tachycardia syndrome; SV: stroke volume; TPR: total peripheral resistance.
All values are mean (SD).
SV was similar between groups in supine position (Table 1). SV decreased in response to tilting in all groups, particularly in OH (−37±18 ml, p=0.001) and POTS patients (−28±12 ml, p=0.008). TPR was similar in all four groups during supine rest and rose in all groups in response to tilt. However this rise (p=0.04) was lower in OH patients compared to other groups (p<0.01) and was greater in patients with POTS (+709±251 dyn.s.cm−5, p=0.008) than in other groups. Supine HR was similar in all groups. In response to head-up tilting HR did not rise in patients with OH (+8±7 bpm, NS) but rose sharply in patients with POTS (+42±10 bpm, p=0.001) compared to healthy controls (+13±6 bpm, p=0.008). Supine systolic blood pressure (SBP) was similar among groups. SBP decreased markedly in patients with OH (−41±11 mmHg, p=0.002) in response to head-up tilting but did not change in NM syncope patients (+8±4 mmHg, NS) or POTS patients (+2±3 mmHg, NS).
Autonomic dataBRG was almost absent in OH patients (0.2±0.1 ms/mmHg in supine and 0.1±0.2 ms/mmHg in tilt position). At supine rest BRG was similar in POTS, NM syncope patients and healthy controls. After tilting, BRG in patients with POTS (−13.0±2.9 ms/mmHg, p=0.002) and NM (−11.6±7.0 ms/mmHg, p=0.007) decreased more than in healthy controls (−6.5±4.0 ms/mmHg, p=0.03), whereas vagal tone activity (HF) at different positions was similar in POTS, NM syncope and healthy controls (Tables 1–3). Low frequency of systolic blood pressure variability (LF-SBP) was almost absent in patients with OH (0.6±0.4 mmHg2 in supine and 1.0±0.7 mmHg2 in tilting position). It was similar in patients with NM syncope, POTS and in healthy controls in supine position. However in response to head-up tilt a large rise in LF-SBP occurred in patients with NM syncope (+11.6±5.4 mmHg2, p=0.004) and POTS patients (+11.6±3.9 mmHg2, p=0.003) as compared to healthy controls (+5.6±4.8 mmHg2 p=0.009).
Neurohormonal dataBrain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) did not change in response to tilt in any of the three patient groups or in controls. BNP was higher in patients with neurogenic OH (9.6±5.0 pmol/l, p=0.009) than in controls (1.5±1.1 pmol/l) and slightly lower in POTS patients (1.0±0.7 pmol/l, p=0.03). In patients with POTS, ANP in supine and tilt position was lower than in the other groups (p=0.03). Dopamine did not change with orthostatic stress in any group and levels in supine and tilt position were similar. As expected, patients with neurogenic OH had significant lower noradrenaline (NOR) in supine position (106±56 pg/ml, p=0.003) and a blunted, non-significant, response to orthostatic stress (+28±24 pg/ml, NS). POTS patients, NM patients and controls had a significant rise NOR after tilt. However patients with POTS compared to controls (197±109 pg/ml) had a more pronounced rise (340±102 pg/ml, p=0.03). Adrenaline was slightly elevated in patients with NM in supine position compared to the other groups and a large rise was observed during the first minutes of orthostatic stress and several minutes before the neurally mediated reflex started (+70±23 pg/ml, p=0.003). Adrenaline was similar in OH patients (22±5 pg/ml) and in controls (14±9 pg/ml) at supine rest but this parameter did not rise in response to tilting only (6±6 pg/ml in patients, NS, and 18±11 pg/ml in controls, p=0.04).
Tables 1–3 show detailed results.
DiscussionOur findings indicate that POTS patients (compared to other groups) had very low levels of ANP and BNP, suggesting a hypercontractile left ventricle and possibly hypovolemia.13,14 These observations were also recently described by Fedorowsky et al.,15 indicating that suppressed ANP levels predict POTS among patients with OI. BNP values in symptomatic FAP patients with OH were normal but higher than in other groups, probably implying that they already presented subtle subclinical cardiac dysfunction, not yet detectable by other measures of cardiac evaluation like LVEF assessed by echocardiography. Curiously, ANP and BNP did not change with postural challenge in any group, suggesting that these hormones depend more on left ventricular function than on the preload reduction observed during acute orthostatism or the volume depletion observed during prolonged orthostatism.16
Patients with NM syncope presented a large rise in adrenaline levels, several minutes before the development of the Bezold-Jarish reflex, which could have played a role in triggering the reflex. As expected, baseline plasma noradrenaline was very low in FAP patients and the response to tilt testing was markedly blunted, features already observed in patients with autonomic dysfunction. On the other hand, patients with POTS had normal noradrenaline values during supine position, but experienced a large rise during the initial tilting phase. This observation will probably affect the therapeutic assessment of these patients.13,15 Dopamine levels were similar among the different OI syndromes and did not change with orthostatic stress.
Cardiac output and stroke volume decreased with orthostatic stress. Moreover, after tilting, POTS patients had a similar decrease in cardiac output to other groups, but only because the larger reduction in stroke volume was compensated by an exaggerated rise in heart rate response.17 Orthostatic stress induced by head-up tilt was associated with an increase in total vascular resistance and heart rate response in all groups, but the increase was much less pronounced in patients with dysautonomia.18 As observed by others, our POTS patients had a large rise in HR compared to other OI groups.2,13 All groups had similar BP values during supine rest but tilting induced marked postural hypotension in patients with autonomic dysfunction. Systolic BP remained unchanged in patients with NM syncope in the initial phase of orthostatic stress and until the beginning of the reflex, which contrasts with the earlier changes observed in cerebrovascular dynamics.19
As expected, patients with autonomic insufficiency and OH had only residual autonomic activity (tonic and reflex), as HF of HRV and baroreceptor activity were almost undetectable. POTS patients had normal autonomic function while in supine position but showed a large increase in sympathetic activity and a profound reduction in vagal activity during orthostatic stress, suggesting a marked imbalance between the two components of autonomic tone2,13,14,16 with this posture. Patients with NM syncope had normal autonomic function in supine rest and initial tilting, but minutes before the neurocardiogenic response started, an abrupt fall in baroreceptor gain (vagal reflex activity) was observed, which could had been one of the triggers observed in this complex reflex.18 Sympathetic function, estimated by LF-SBP, was markedly reduced in dysautonomic patients. POTS patients had normal sympathetic activity while in supine rest but experienced a large rise during the initial phase of tilting, which could explain several of these patients’ symptoms after standing. Moreover, patients with NM syncope had only a small rise in sympathetic activity in the first minutes of tilt, as did normal controls.
In conclusion, we observed that although different orthostatic syndromes share similar symptoms, including blurred vision, syncope and dizziness particularly during orthostatic position, they have significantly different biochemical, autonomic and hemodynamic behaviors. Assessment of these differences could may be helpful for better diagnosis and could lead to more specific treatments.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.